Microplastic and Extracellular Vesicle Interactions: Recent Studies on Human Health and Environment Risks
Abstract
1. Introduction
2. Microplastics: Environmental Prevalence and Biological Impact
2.1. Classification and Sources of Microplastics
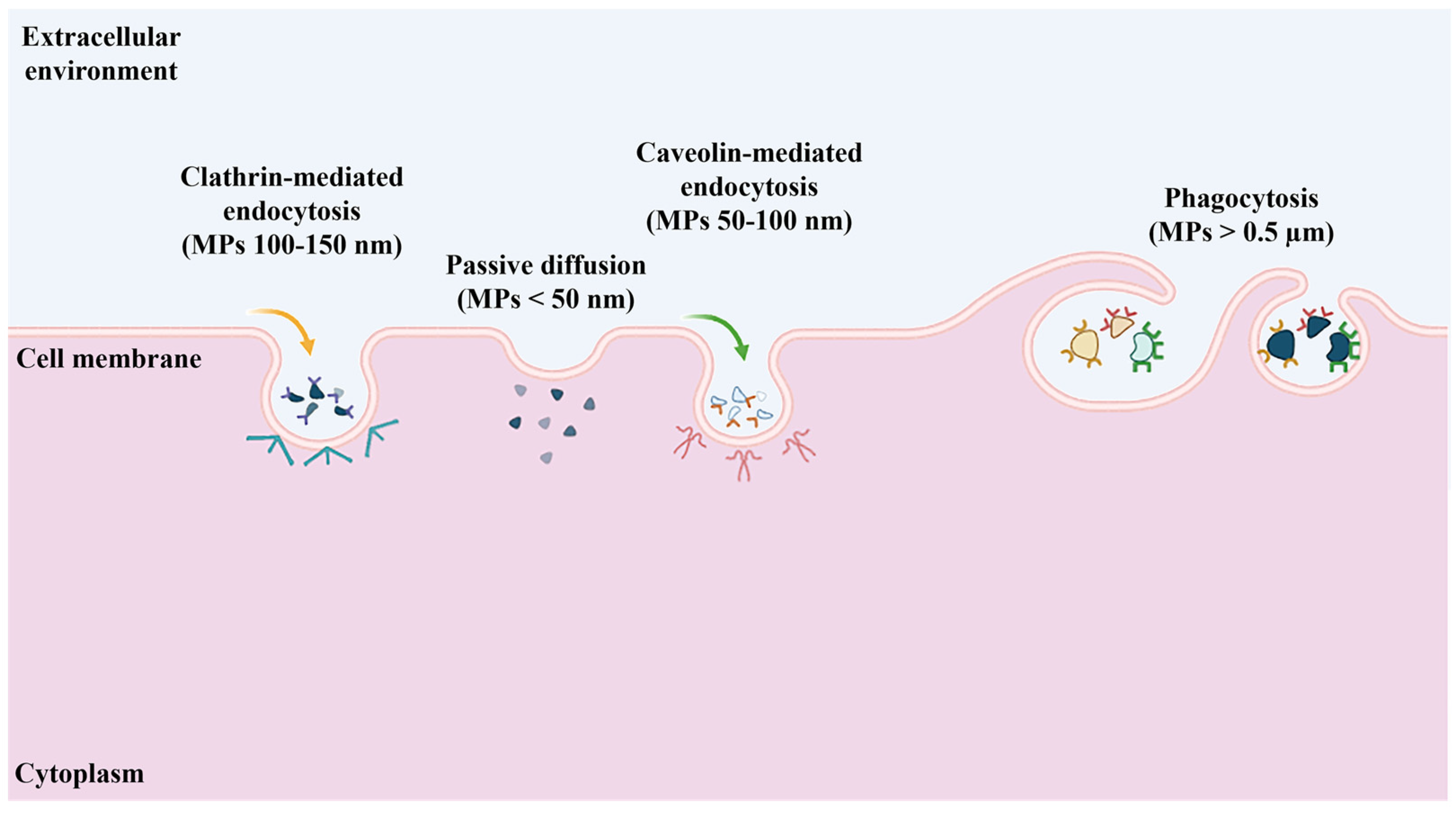
2.2. Biological Uptake of Microplastics
2.3. Impact of Microplastics on Cellular Health
3. Extracellular Vesicles: Biological Roles and Environmental Relevance
3.1. Extracellular Vesicles: Definition and Classification
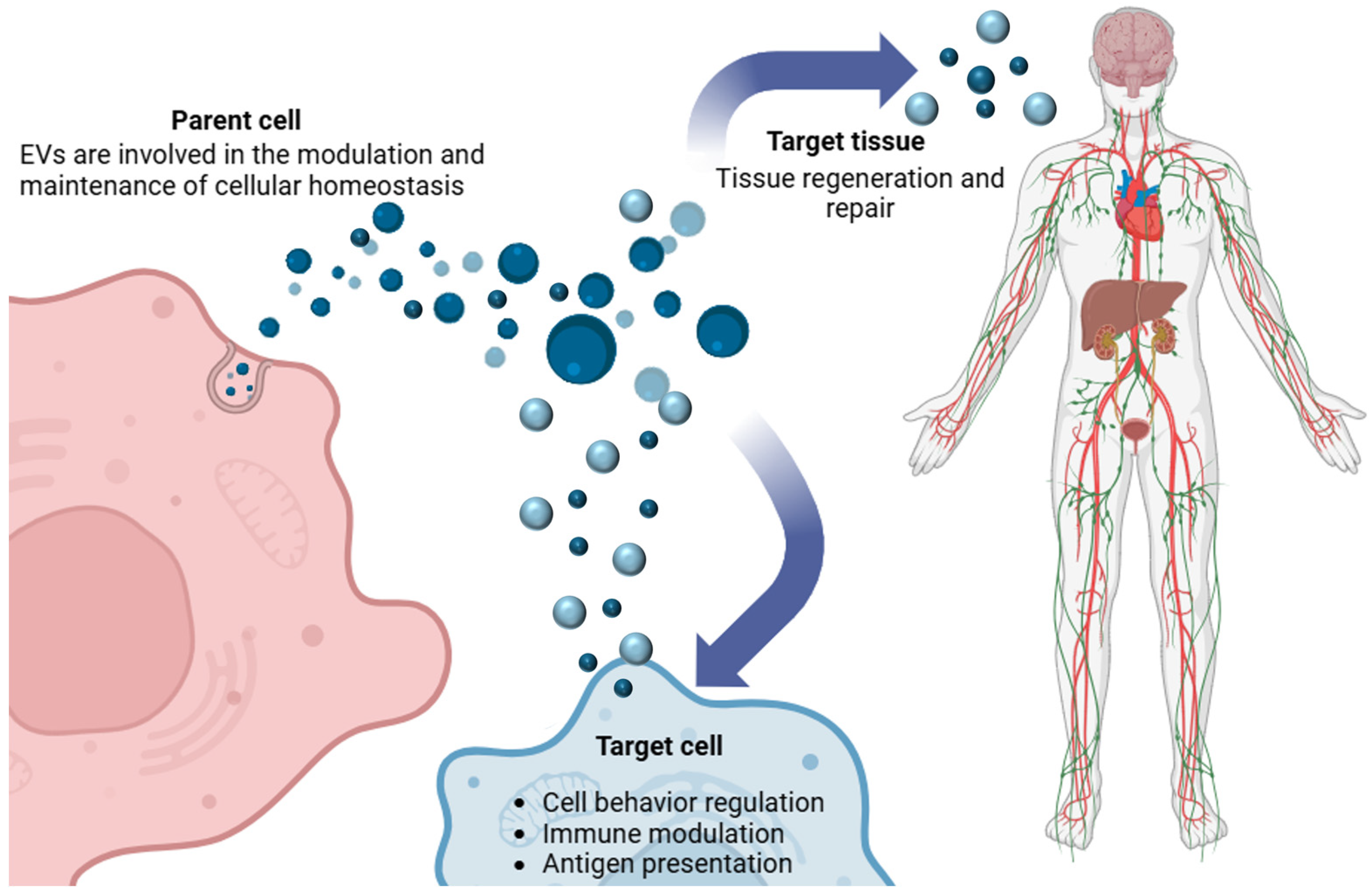
3.2. Biological Functions of Extracellular Vesicles
Intercellular Communication
3.3. Extracellular Vesicles in Environmental Pollution
4. Mechanisms of Microplastic–Extracellular Vesicle (MP–EV) Interaction
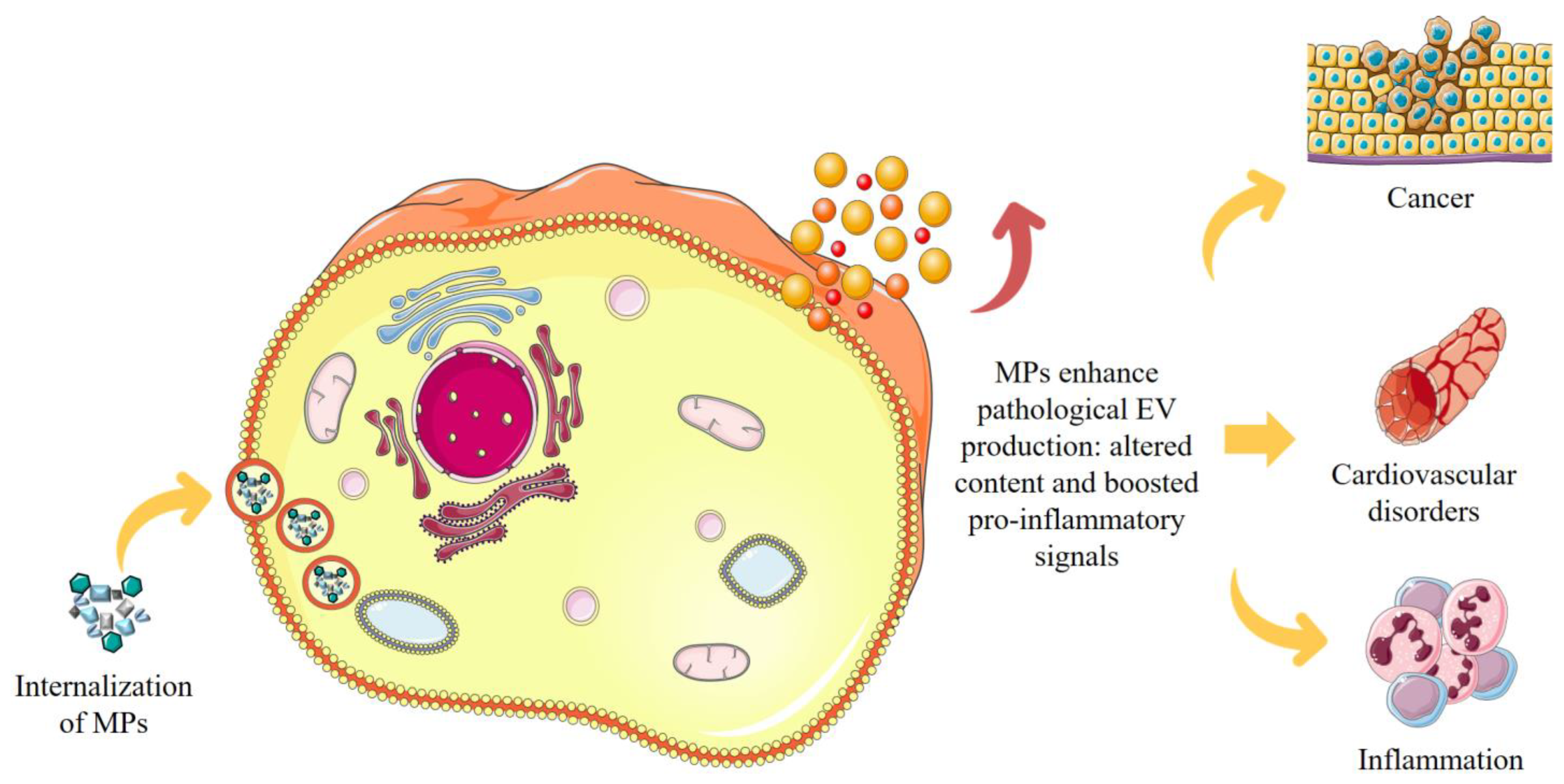
4.1. Impact of MP–EV Interaction on Cellular Function
4.2. MP–EV Interaction: Implications for Human Health
4.3. Evidence of MP Transport Mediated by EVs
5. Ecological Implications: Microplastic and Extracellular Vesicle Interaction in Ecosystems
5.1. MPs as Bioactive Pollutants in Ecosystems
5.2. Biological Amplification via EV-Mediated MP Transport
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Seijo, A.; Pereira, R. Chapter 3—Morphological and Physical Characterization of Microplastics. In Comprehensive Analytical Chemistry; Rocha-Santos, T.A.P., Duarte, A.C., Eds.; Characterization and Analysis of Microplastics; Elsevier: Amsterdam, The Netherlands, 2017; Volume 75, pp. 49–66. [Google Scholar]
- Caputo, F.; Vogel, R.; Savage, J.; Vella, G.; Law, A.; Della Camera, G.; Hannon, G.; Peacock, B.; Mehn, D.; Ponti, J.; et al. Measuring Particle Size Distribution and Mass Concentration of Nanoplastics and Microplastics: Addressing Some Analytical Challenges in the Sub-Micron Size Range. J. Colloid Interface Sci. 2021, 588, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An Overview on Separation, Identification and Characterization of Microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef]
- Gwenzi, W.; Simbanegavi, T.T.; Mahdi, H.I.; Azelee, N.I.W.; Muisa-Zikali, N.; Rangabhashiyam, S. Chapter 14—Occurrence and Ecological Health Risks of Microplastics. In Emerging Contaminants in the Terrestrial-Aquatic-Atmosphere Continuum; Gwenzi, W., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–270. ISBN 978-0-323-90051-5. [Google Scholar]
- Mamun, A.A.; Prasetya, T.A.E.; Dewi, I.R.; Ahmad, M. Microplastics in Human Food Chains: Food Becoming a Threat to Health Safety. Sci. Total Environ. 2023, 858, 159834. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M. How Microplastics Interact with Food Chain: A Short Overview of Fate and Impacts. J. Food Sci. Technol. 2024, 61, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Ziani, K.; Ioniță-Mîndrican, C.-B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.-T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Sampath, V.; Prunicki, M.; Aguilera, J.; Allen, H.; LaBeaud, D.; Veidis, E.; Barry, M.; Erny, B.; Patel, L.; et al. Characterization and Regulation of Microplastic Pollution for Protecting Planetary and Human Health. Environ. Pollut. 2022, 315, 120442. [Google Scholar] [CrossRef]
- Pilapitiya, P.N.T.; Ratnayake, A.S. The World of Plastic Waste: A Review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Ghosh, S.; Sinha, J.K.; Ghosh, S.; Vashisth, K.; Han, S.; Bhaskar, R. Microplastics as an Emerging Threat to the Global Environment and Human Health. Sustainability 2023, 15, 10821. [Google Scholar] [CrossRef]
- Xia, C.; Cai, L.; Lam, S.S.; Sonne, C. Microplastics Pollution: Economic Loss and Actions Needed. Eco Environ. Health 2023, 2, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Adhikary, S.; Bhattacharya, S.; Agarwal, R.; Ganguly, A.; Nanda, S.; Rajak, P. The Alarming Link between Environmental Microplastics and Health Hazards with Special Emphasis on Cancer. Life Sci. 2024, 355, 122937. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Lamba, M.; Pachar, A.K.; Yadav, S.; Acharya, A. Microplastics—A Growing Concern as Carcinogens in Cancer Etiology: Emphasis on Biochemical and Molecular Mechanisms. Cell Biochem. Biophys. 2024, 82, 3109–3121. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, S.; Kim, O.-H.; Kim, C.-H.; Kim, J.; Kim, J.-W.; Hong, S.; Lee, H.J. Polypropylene Microplastics Promote Metastatic Features in Human Breast Cancer. Sci. Rep. 2023, 13, 6252. [Google Scholar] [CrossRef] [PubMed]
- Kehinde, S.A.; Fatokun, T.P.; Olajide, A.T.; Praveena, S.M.; Sokan-Adeaga, A.A.; Adekunle, A.P.; Fouad, D.; Papadakis, M. Impact of Polyethylene Microplastics Exposure on Kallikrein-3 Levels, Steroidal-Thyroidal Hormones, and Antioxidant Status in Murine Model: Protective Potentials of Naringin. Sci. Rep. 2024, 14, 23664. [Google Scholar] [CrossRef]
- Zheng, P.C.; Li, R.; Lai, K.P.; Zhang, X.X. Biological Exposure to Microplastics and Nanoplastics and Plastic Additives: Impairment of Glycolipid Metabolism and Adverse Effects on Metabolic Diseases. Environ. Sci. Pollut. Res. 2024, 31, 60778–60791. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Pelegrini, K.; Pereira, T.C.B.; Maraschin, T.G.; Teodoro, L.D.S.; Basso, N.R.D.S.; De Galland, G.L.B.; Ligabue, R.A.; Bogo, M.R. Micro- and Nanoplastic Toxicity: A Review on Size, Type, Source, and Test-Organism Implications. Sci. Total Environ. 2023, 878, 162954. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Zhang, Y.; Wang, C.; Wang, X.; Zhou, J.; Shen, M.; Zhao, Y.; Fu, Z.; Jin, Y. Maternal Exposure to Different Sizes of Polystyrene Microplastics during Gestation Causes Metabolic Disorders in Their Offspring. Environ. Pollut. 2019, 255, 113122. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, N.; Yang, X.; Xia, Y.; Wu, D. Effects Induced by Polyethylene Microplastics Oral Exposure on Colon Mucin Release, Inflammation, Gut Microflora Composition and Metabolism in Mice. Ecotoxicol. Environ. Saf. 2021, 220, 112340. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A. Advances in Microplastics Detection: A Comprehensive Review of Methodologies and Their Effectiveness. TrAC Trends Anal. Chem. 2024, 170, 117440. [Google Scholar] [CrossRef]
- Marcuello, C. Present and Future Opportunities in the Use of Atomic Force Microscopy to Address the Physico-Chemical Properties of Aquatic Ecosystems at the Nanoscale Level. Int. Aquat. Res. 2022, 14, 231–240. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Montegiove, N.; Di Michele, A.; Emiliani, C. Enhanced Stability of Long-Living Immobilized Recombinant β-d-N-Acetyl-Hexosaminidase A on Polylactic Acid (PLA) Films for Potential Biomedical Applications. J. Funct. Biomater. 2021, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Montegiove, N.; Di Michele, A.; Pellegrino, R.M.; Emiliani, C. HexA-Enzyme Coated Polymer Nanoparticles for the Development of a Drug-Delivery System in the Treatment of Sandhoff Lysosomal Storage Disease. J. Funct. Biomater. 2022, 13, 37. [Google Scholar] [CrossRef]
- Montegiove, N.; Calzoni, E.; Emiliani, C.; Cesaretti, A. Biopolymer Nanoparticles for Nose-to-Brain Drug Delivery: A New Promising Approach for the Treatment of Neurological Diseases. J. Funct. Biomater. 2022, 13, 125. [Google Scholar] [CrossRef]
- Zangi, A.R.; Amiri, A.; Borzouee, F.; Bagherifar, R.; Pazooki, P.; Hamishehkar, H.; Javadzadeh, Y. Immobilized Nanoparticles-Mediated Enzyme Therapy; Promising Way into Clinical Development. Discov. Nanoscale Res. Lett. 2023, 18, 55. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Cerrotti, G.; Buratta, S.; Latella, R.; Calzoni, E.; Cusumano, G.; Bertoldi, A.; Porcellati, S.; Emiliani, C.; Urbanelli, L. Hitting the Target: Cell Signaling Pathways Modulation by Extracellular Vesicles. Extracell. Vesicles Circ. Nucleic Acids 2024, 5, 627–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, F. Classification and Nomenclature of Extracellular Vesicles. In Extracellular Vesicles: From Bench to Bedside; Wang, Q., Zheng, L., Eds.; Springer Nature: Singapore, 2024; pp. 3–7. ISBN 978-981-9983-65-0. [Google Scholar]
- EL Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Mir, B.; Goettsch, C. Extracellular Vesicles as Delivery Vehicles of Specific Cellular Cargo. Cells 2020, 9, 1601. [Google Scholar] [CrossRef]
- Dellar, E.R.; Hill, C.; Melling, G.E.; Carter, D.R.F.; Baena-Lopez, L.A. Unpacking Extracellular Vesicles: RNA Cargo Loading and Function. J. Extracell. Biol. 2022, 1, e40. [Google Scholar] [CrossRef]
- Calzoni, E.; Argentati, C.; Cesaretti, A.; Montegiove, N.; Tortorella, I.; Bazzucchi, M.; Morena, F.; Martino, S.; Emiliani, C. RNA Modifications in Neurodegenerations. In Epitranscriptomics; Jurga, S., Barciszewski, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 23–77. ISBN 978-3-030-71612-7. [Google Scholar]
- Trianni, A.; Brossa, A.; Catalano, F.; Saraceni, A.; Bongiovanni, S.; Merlo, G.R.; Cottone, E.; Bovolin, P. Unraveling the molecular mechanisms of microplastics internalization and their intracellular impact. Eur. J. Histochem. 2024, 68, 47–48. [Google Scholar] [CrossRef]
- Bovolin, P.; Saraceni, A.; Cottone, E.; Mognetti, B.; Gallo, M.P.; Merlo, G.; Dal Bello, F. Microplastics and associated contaminants: Potential impact on cells and developing organisms. Eur. J. Histochem. 2023, 67, 5. [Google Scholar] [CrossRef]
- Mierzejewski, K.; Kurzyńska, A.; Golubska, M.; Całka, J.; Gałęcka, I.; Szabelski, M.; Paukszto, Ł.; Andronowska, A.; Bogacka, I. New Insights into the Potential Effects of PET Microplastics on Organisms via Extracellular Vesicle-Mediated Communication. Sci. Total Environ. 2023, 904, 166967. [Google Scholar] [CrossRef]
- Xu, Z.; Sui, Q.; Li, A.; Sun, M.; Zhang, L.; Lyu, S.; Zhao, W. How to Detect Small Microplastics (20–100 Μm) in Freshwater, Municipal Wastewaters and Landfill Leachates? A Trial from Sampling to Identification. Sci. Total Environ. 2020, 733, 139218. [Google Scholar] [CrossRef] [PubMed]
- Bandekar, M.; Abdolahpur Monikh, F.; Kekäläinen, J.; Tahvanainen, T.; Kortet, R.; Zhang, P.; Guo, Z.; Akkanen, J.; Leskinen, J.T.T.; Gomez-Gonzalez, M.A.; et al. Submicron Plastic Adsorption by Peat, Accumulation in Sphagnum Mosses and Influence on Bacterial Communities in Peatland Ecosystems. Environ. Sci. Technol. 2022, 56, 15661–15671. [Google Scholar] [CrossRef] [PubMed]
- Nigina; Sajith, V.; Nair, B.G. Microplastics and Nanoplastics in Terrestrial Systems. In Toxic Effects of Micro and Nanoplastics; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2024; pp. 163–213. ISBN 978-1-394-23816-3. [Google Scholar]
- Fu, W.; Min, J.; Jiang, W.; Li, Y.; Zhang, W. Separation, Characterization and Identification of Microplastics and Nanoplastics in the Environment. Sci. Total Environ. 2020, 721, 137561. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.I.; Liu, H.; Wang, D.; Lee, Y.H.; Lee, J.-S.; An, Y.-J. Critical Review of Environmental Impacts of Microfibers in Different Environmental Matrices. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 251, 109196. [Google Scholar] [CrossRef]
- Nur, A.; Setiyawan, A.S.; Oginawati, K.; Soewondo, P. Degradation of polyethylene terephthalate (pet) as secondary microplastics under three different environmental conditions. GEOMATE J. 2022, 22, 10–16. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Bahmid, N.A.; Salman, S.H.M.; Nawaz, A.; Walayat, N.; Shekhawat, G.K.; Gvozdenko, A.A.; Blinov, A.V.; Nagdalian, A.A. Chapter Eight—Migration of Microplastics from Plastic Packaging into Foods and Its Potential Threats on Human Health. In Advances in Food and Nutrition Research; Özogul, F., Ed.; Nano/micro-Plastics Toxicity on Food Quality and Food Safety; Academic Press: Cambridge, MA, USA, 2023; Volume 103, pp. 313–359. [Google Scholar]
- Lambert, S.; Wagner, M. Characterisation of Nanoplastics during the Degradation of Polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J.; et al. Current Progress on Plastic/Microplastic Degradation: Fact Influences and Mechanism. Environ. Pollut. 2022, 304, 119159. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in Wastewater Treatment Plants: Detection, Occurrence and Removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Sa’adu, I.; Farsang, A. Plastic Contamination in Agricultural Soils: A Review. Environ. Sci. Eur. 2023, 35, 13. [Google Scholar] [CrossRef]
- Porterfield, K.K.; Hobson, S.A.; Neher, D.A.; Niles, M.T.; Roy, E.D. Microplastics in Composts, Digestates, and Food Wastes: A Review. J. Environ. Qual. 2023, 52, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Alijagic, A.; Suljević, D.; Fočak, M.; Sulejmanović, J.; Šehović, E.; Särndahl, E.; Engwall, M. The Triple Exposure Nexus of Microplastic Particles, Plastic-Associated Chemicals, and Environmental Pollutants from a Human Health Perspective. Environ. Int. 2024, 188, 108736. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.A.; McKenna Neuman, C.L.; Aherne, J. Effects of Shape and Size on Microplastic Atmospheric Settling Velocity. Environ. Sci. Technol. 2023, 57, 11937–11947. [Google Scholar] [CrossRef]
- Sorolla-Rosario, D.; Llorca-Porcel, J.; Pérez-Martínez, M.; Lozano-Castelló, D.; Bueno-López, A. Study of Microplastics with Semicrystalline and Amorphous Structure Identification by TGA and DSC. J. Environ. Chem. Eng. 2022, 10, 106886. [Google Scholar] [CrossRef]
- Mortula, M.M.; Atabay, S.; Fattah, K.P.; Madbuly, A. Leachability of Microplastic from Different Plastic Materials. J. Environ. Manag. 2021, 294, 112995. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Seewoo, B.J.; Goodes, L.M.; Mofflin, L.; Mulders, Y.R.; Wong, E.V.; Toshniwal, P.; Brunner, M.; Alex, J.; Johnston, B.; Elagali, A.; et al. The Plastic Health Map: A Systematic Evidence Map of Human Health Studies on Plastic-Associated Chemicals. Environ. Int. 2023, 181, 108225. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-X.; Liu, R.; Hao, L.-T.; Liu, J.-F. Identification of Polystyrene Nanoplastics Using Surface Enhanced Raman Spectroscopy. Talanta 2021, 221, 121552. [Google Scholar] [CrossRef]
- Lv, L.; He, L.; Jiang, S.; Chen, J.; Zhou, C.; Qu, J.; Lu, Y.; Hong, P.; Sun, S.; Li, C. In Situ Surface-Enhanced Raman Spectroscopy for Detecting Microplastics and Nanoplastics in Aquatic Environments. Sci. Total Environ. 2020, 728, 138449. [Google Scholar] [CrossRef]
- Gillibert, R.; Balakrishnan, G.; Deshoules, Q.; Tardivel, M.; Magazzù, A.; Donato, M.G.; Maragò, O.M.; Lamy de La Chapelle, M.; Colas, F.; Lagarde, F.; et al. Raman Tweezers for Small Microplastics and Nanoplastics Identification in Seawater. Environ. Sci. Technol. 2019, 53, 9003–9013. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Le Juge, C.; Davranche, M.; El Hadri, H.; Grassl, B.; Reynaud, S.; Gigault, J. Nanoplastic Occurrence in a Soil Amended with Plastic Debris. Chemosphere 2021, 262, 127784. [Google Scholar] [CrossRef] [PubMed]
- Davranche, M.; Lory, C.; Juge, C.L.; Blancho, F.; Dia, A.; Grassl, B.; El Hadri, H.; Pascal, P.-Y.; Gigault, J. Nanoplastics on the Coast Exposed to the North Atlantic Gyre: Evidence and Traceability. NanoImpact 2020, 20, 100262. [Google Scholar] [CrossRef]
- Xu, G.; Cheng, H.; Jones, R.; Feng, Y.; Gong, K.; Li, K.; Fang, X.; Tahir, M.A.; Valev, V.K.; Zhang, L. Surface-Enhanced Raman Spectroscopy Facilitates the Detection of Microplastics <1 μm in the Environment. Environ. Sci. Technol. 2020, 54, 15594–15603. [Google Scholar] [CrossRef]
- Thomas, D.; Schütze, B.; Heinze, W.M.; Steinmetz, Z. Sample Preparation Techniques for the Analysis of Microplastics in Soil—A Review. Sustainability 2020, 12, 9074. [Google Scholar] [CrossRef]
- Barili, S.; Bernetti, A.; Sannino, C.; Montegiove, N.; Calzoni, E.; Cesaretti, A.; Pinchuk, I.; Pezzolla, D.; Turchetti, B.; Buzzini, P.; et al. Impact of PVC Microplastics on Soil Chemical and Microbiological Parameters. Environ. Res. 2023, 229, 115891. [Google Scholar] [CrossRef] [PubMed]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in Cosmetics: Environmental Issues and Needs for Global Bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Moore, F.; Keshavarzi, B.; Hopke, P.K.; Naidu, R.; Rahman, M.M.; Oleszczuk, P.; Karimi, J. PET-Microplastics as a Vector for Heavy Metals in a Simulated Plant Rhizosphere Zone. Sci. Total Environ. 2020, 744, 140984. [Google Scholar] [CrossRef]
- Liu, S.; Huang, J.; Zhang, W.; Shi, L.; Yi, K.; Yu, H.; Zhang, C.; Li, S.; Li, J. Microplastics as a Vehicle of Heavy Metals in Aquatic Environments: A Review of Adsorption Factors, Mechanisms, and Biological Effects. J. Environ. Manag. 2022, 302, 113995. [Google Scholar] [CrossRef]
- Weis, J.S.; Palmquist, K.H. Reality Check: Experimental Studies on Microplastics Lack Realism. Appl. Sci. 2021, 11, 8529. [Google Scholar] [CrossRef]
- Liu, K.; Li, Q.; Andrady, A.L.; Wang, X.; He, Y.; Li, D. Underestimated Activity-Based Microplastic Intake under Scenario-Specific Exposures. Environ. Sci. Ecotechnol. 2024, 18, 100316. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Khan, A.; Jia, Z. Recent Insights into Uptake, Toxicity, and Molecular Targets of Microplastics and Nanoplastics Relevant to Human Health Impacts. iScience 2023, 26, 106061. [Google Scholar] [CrossRef] [PubMed]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A Review on Microplastics and Nanoplastics in the Environment: Their Occurrence, Exposure Routes, Toxic Studies, and Potential Effects on Human Health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.I.; An, Y.-J. Post COVID-19 Pandemic: Biofragmentation and Soil Ecotoxicological Effects of Microplastics Derived from Face Masks. J. Hazard. Mater. 2021, 416, 126169. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Mikulewicz, M. Bioaccumulation. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 456–460. ISBN 978-0-12-386455-0. [Google Scholar]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- European Union. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment (Text with EEA Relevance). Off. J. Eur. Union 2019, 155, 1–19. [Google Scholar]
- EUR-Lex—L:2006:396:TOC—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ%3AL%3A2006%3A396%3ATOC (accessed on 11 December 2024).
- The Resumed Session of UNEA-5 (UNEA-5.2). Available online: http://www.unep.org/environmentassembly/unea-5.2 (accessed on 11 December 2024).
- Oßmann, B.E. Microplastics in Drinking Water? Present State of Knowledge and Open Questions. Curr. Opin. Food Sci. 2021, 41, 44–51. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E.; Saluveer, M.; Sidaoui-Haddad, G. Microplastics in Fish and Fishmeal: An Emerging Environmental Challenge? Sci. Rep. 2021, 11, 2045. [Google Scholar] [CrossRef]
- Amran, N.H.; Zaid, S.S.M.; Mokhtar, M.H.; Manaf, L.A.; Othman, S. Exposure to Microplastics during Early Developmental Stage: Review of Current Evidence. Toxics 2022, 10, 597. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning Microplastics into Nanoplastics through Digestive Fragmentation by Antarctic Krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Expósito, N.; Rovira, J.; Sierra, J.; Gimenez, G.; Domingo, J.L.; Schuhmacher, M. Levels of Microplastics and Their Characteristics in Molluscs from North-West Mediterranean Sea: Human Intake. Mar. Pollut. Bull. 2022, 181, 113843. [Google Scholar] [CrossRef] [PubMed]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic Contamination of Tap Water, Beer, and Sea Salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in Bivalves Cultured for Human Consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Wu, J.; Lu, J.; Wu, J. Effect of Gastric Fluid on Adsorption and Desorption of Endocrine Disrupting Chemicals on Microplastics. Front. Environ. Sci. Eng. 2021, 16, 104. [Google Scholar] [CrossRef]
- Wagstaff, A.; Petrie, B. Enhanced Desorption of Fluoxetine from Polyethylene Terephthalate Microplastics in Gastric Fluid and Sea Water. Environ. Chem. Lett. 2022, 20, 975–982. [Google Scholar] [CrossRef]
- Jiménez-Arroyo, C.; Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. Simulated Gastrointestinal Digestion of Polylactic Acid (PLA) Biodegradable Microplastics and Their Interaction with the Gut Microbiota. Sci. Total Environ. 2023, 902, 166003. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A First Overview of Textile Fibers, Including Microplastics, in Indoor and Outdoor Environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C. Airborne Microplastics: Consequences to Human Health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, X.; Bi, R.; Guo, Q.; Yu, X.; Zeng, Q.; Huang, Z.; Liu, T.; Wu, H.; Chen, Y.; et al. Detection and Analysis of Microplastics in Human Sputum. Environ. Sci. Technol. 2022, 56, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Goros, R.A.; Dong, Z.; Meng, X.; Li, G.; Chen, W.; Liu, S.; Ma, J.; Zuo, Y.Y. Microplastics and Nanoplastics Impair the Biophysical Function of Pulmonary Surfactant by Forming Heteroaggregates at the Alveolar–Capillary Interface. Environ. Sci. Technol. 2023, 57, 21050–21060. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhang, Y.; Zhu, Y.; Song, B.; Zeng, G.; Hu, D.; Wen, X.; Ren, X. Recent Advances in Toxicological Research of Nanoplastics in the Environment: A Review. Environ. Pollut. 2019, 252, 511–521. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- Yacobi, N.R.; DeMaio, L.; Xie, J.; Hamm-Alvarez, S.F.; Borok, Z.; Kim, K.-J.; Crandall, E.D. Polystyrene Nanoparticle Trafficking across Alveolar Epithelium. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.M.; Mahamuni-Badiger, P.; Ingavale, R.R.; Patel, P.R.; Dhanavade, M.J. Usage of Microplastic Beads in Pharmaceuticals and Cosmetics Industry: A Review. In Microplastic Pollution; Shahnawaz, M., Adetunji, C.O., Dar, M.A., Zhu, D., Eds.; Springer Nature: Singapore, 2024; pp. 51–72. ISBN 978-981-9983-57-5. [Google Scholar]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, Quantity and Sorptive Properties of Microplastics Extracted from Cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Duis, K.; Coors, A. Microplastics in the Aquatic and Terrestrial Environment: Sources (with a Specific Focus on Personal Care Products), Fate and Effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S. Microplastic Pollution, a Threat to Marine Ecosystem and Human Health: A Short Review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef] [PubMed]
- Baroli, B. Penetration of Nanoparticles and Nanomaterials in the Skin: Fiction or Reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef]
- Damaj, S.; Trad, F.; Goevert, D.; Wilkesmann, J. Bridging the Gaps between Microplastics and Human Health. Microplastics 2024, 3, 46–66. [Google Scholar] [CrossRef]
- Wolff, C.M.; Singer, D.; Schmidt, A.; Bekeschus, S. Immune and Inflammatory Responses of Human Macrophages, Dendritic Cells, and T-Cells in Presence of Micro- and Nanoplastic of Different Types and Sizes. J. Hazard. Mater. 2023, 459, 132194. [Google Scholar] [CrossRef]
- Yang, W.; Jannatun, N.; Zeng, Y.; Liu, T.; Zhang, G.; Chen, C.; Li, Y. Impacts of Microplastics on Immunity. Front. Toxicol. 2022, 4, 956885. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Wang, D. Cellular Uptake, Transport, and Organelle Response After Exposure to Microplastics and Nanoplastics: Current Knowledge and Perspectives for Environmental and Health Risks. Rev. Environ. Contam. Toxicol. 2022, 260, 12. [Google Scholar] [CrossRef]
- Swanson, J.A. Shaping Cups into Phagosomes and Macropinosomes. Nat. Rev. Mol. Cell Biol. 2008, 9, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, H.; Shen, M.; Tao, J.; Chen, S.; Yin, L.; Zhou, W.; Wang, X.; Xiao, R.; Li, R. Recent Advances on the Transport of Microplastics/Nanoplastics in Abiotic and Biotic Compartments. J. Hazard. Mater. 2022, 438, 129515. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, K.; Zhang, B.; Ye, Y.; Zhang, Q.; Jiang, W. Cellular Internalization and Release of Polystyrene Microplastics and Nanoplastics. Sci. Total Environ. 2021, 779, 146523. [Google Scholar] [CrossRef]
- Sendra, M.; Saco, A.; Yeste, M.P.; Romero, A.; Novoa, B.; Figueras, A. Nanoplastics: From Tissue Accumulation to Cell Translocation into Mytilus Galloprovincialis Hemocytes. Resilience of Immune Cells Exposed to Nanoplastics and Nanoplastics plus Vibrio Splendidus Combination. J. Hazard. Mater. 2020, 388, 121788. [Google Scholar] [CrossRef]
- Kiss, A.L.; Botos, E. Endocytosis via Caveolae: Alternative Pathway with Distinct Cellular Compartments to Avoid Lysosomal Degradation? J. Cell. Mol. Med. 2009, 13, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Roux, A. Mechanisms of Clathrin-Mediated Endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Q.; Sun, J.; Mao, Y.; Liu, X.; Que, S.; Yu, W.; Kan, Y. The Adsorption and Desorption Behavior of Bisphenol A on Five Microplastics Under Simulated Gastrointestinal Conditions. Water Air Soil Pollut. 2023, 234, 106. [Google Scholar] [CrossRef]
- Liu, X.; Gharasoo, M.; Shi, Y.; Sigmund, G.; Hüffer, T.; Duan, L.; Wang, Y.; Ji, R.; Hofmann, T.; Chen, W. Key Physicochemical Properties Dictating Gastrointestinal Bioaccessibility of Microplastics-Associated Organic Xenobiotics: Insights from a Deep Learning Approach. Environ. Sci. Technol. 2020, 54, 12051–12062. [Google Scholar] [CrossRef]
- Mattioda, V.; Benedetti, V.; Tessarolo, C.; Oberto, F.; Favole, A.; Gallo, M.; Martelli, W.; Crescio, M.I.; Berio, E.; Masoero, L.; et al. Pro-Inflammatory and Cytotoxic Effects of Polystyrene Microplastics on Human and Murine Intestinal Cell Lines. Biomolecules 2023, 13, 140. [Google Scholar] [CrossRef]
- Pulvirenti, E.; Ferrante, M.; Barbera, N.; Favara, C.; Aquilia, E.; Palella, M.; Cristaldi, A.; Conti, G.O.; Fiore, M. Effects of Nano and Microplastics on the Inflammatory Process: In Vitro and In Vivo Studies Systematic Review. Front. Biosci. 2022, 27, 287. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Kim, D.; Kim, H.-Y.; Jeong, S.W.; Lee, S.-G.; Kim, H.-C.; Lee, Y.-J.; Kwon, M.K.; Hwang, J.-S.; Han, J.E.; et al. Microglial Phagocytosis of Polystyrene Microplastics Results in Immune Alteration and Apoptosis in Vitro and in Vivo. Sci. Total Environ. 2022, 807, 150817. [Google Scholar] [CrossRef] [PubMed]
- Das, A. The Emerging Role of Microplastics in Systemic Toxicity: Involvement of Reactive Oxygen Species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef]
- Ding, P.; Xiang, C.; Yao, Q.; Li, X.; Zhang, J.; Yin, R.; Zhang, L.; Li, A.J.; Hu, G. Aged Polystyrene Microplastics Exposure Affects Apoptosis via Inducing Mitochondrial Dysfunction and Oxidative Stress in Early Life of Zebrafish. J. Environ. Manag. 2024, 367, 121995. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, S.; Yin, L.; Pu, Y.; Liang, G. Recent Consequences of Micro-Nanaoplastics (MNPLs) in Subcellular/Molecular Environmental Pollution Toxicity on Human and Animals. Ecotoxicol. Environ. Saf. 2023, 249, 114385. [Google Scholar] [CrossRef]
- Ma, S.; Xiao, Y.; Zhang, X.; Xu, Y.; Zhu, K.; Zhang, K.; Li, X.; Zhou, H.; Chen, G.; Guo, X. Dietary Exposure to Polystyrene Microplastics Exacerbates Liver Damage in Fulminant Hepatic Failure via ROS Production and Neutrophil Extracellular Trap Formation. Sci. Total Environ. 2024, 907, 167403. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shen, J.; Lin, L.; Chen, J.; Wang, L.; Deng, X.; Wu, X.; Lin, Z.; Zhang, Y.; Yu, R.; et al. Exposure to Irregular Microplastic Shed from Baby Bottles Activates the ROS/NLRP3/Caspase-1 Signaling Pathway, Causing Intestinal Inflammation. Environ. Int. 2023, 181, 108296. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, H.; Huang, Y.; Zhang, Y.; Ren, H.; Fang, M.; Wang, Q.; Chen, W.; Hale, R.C.; Galloway, T.S.; et al. Long-Term Exposure to Environmentally Relevant Doses of Large Polystyrene Microplastics Disturbs Lipid Homeostasis via Bowel Function Interference. Environ. Sci. Technol. 2022, 56, 15805–15817. [Google Scholar] [CrossRef]
- Raposo, G.; Stahl, P.D. Extracellular Vesicles: A New Communication Paradigm? Nat. Rev. Mol. Cell Biol. 2019, 20, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Urbanelli, L.; Magini, A.; Buratta, S.; Brozzi, A.; Sagini, K.; Polchi, A.; Tancini, B.; Emiliani, C. Signaling Pathways in Exosomes Biogenesis, Secretion and Fate. Genes 2013, 4, 152–170. [Google Scholar] [CrossRef]
- Latella, R.; Calzoni, E.; Urbanelli, L.; Cerrotti, G.; Porcellati, S.; Emiliani, C.; Buratta, S.; Tancini, B. Isolation of Extracellular Vesicles from Agri-Food Wastes: A Novel Perspective in the Valorization of Agri-Food Wastes and By-Products. Foods 2024, 13, 1492. [Google Scholar] [CrossRef] [PubMed]
- Marzan, A.L.; Nedeva, C.; Mathivanan, S. Extracellular Vesicles in Metabolism and Metabolic Diseases. In New Frontiers: Extracellular Vesicles; Mathivanan, S., Fonseka, P., Nedeva, C., Atukorala, I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 393–410. ISBN 978-3-030-67171-6. [Google Scholar]
- Takahashi, Y.; Takakura, Y. Extracellular Vesicle-Based Therapeutics: Extracellular Vesicles as Therapeutic Targets and Agents. Pharmacol. Ther. 2023, 242, 108352. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Altarejos, P.; Moreno-Manzano, V.; Felipo, V. Pathological and Therapeutic Effects of Extracellular Vesicles in Neurological and Neurodegenerative Diseases. Neural Regen. Res. 2024, 19, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, F.; Hanachi, P.; Montaseri, A. Extracellular Vesicles of Immune Cells; Immunomodulatory Impacts and Therapeutic Potentials. Clin. Immunol. 2023, 248, 109237. [Google Scholar] [CrossRef]
- Veziroglu, E.M.; Mias, G.I. Characterizing Extracellular Vesicles and Their Diverse RNA Contents. Front. Genet. 2020, 11, 700. [Google Scholar] [CrossRef]
- Silva, A.M.; Teixeira, J.H.; Almeida, M.I.; Gonçalves, R.M.; Barbosa, M.A.; Santos, S.G. Extracellular Vesicles: Immunomodulatory Messengers in the Context of Tissue Repair/Regeneration. Eur. J. Pharm. Sci. 2017, 98, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Bahram Sangani, N.; Gomes, A.R.; Curfs, L.M.G.; Reutelingsperger, C.P. The Role of Extracellular Vesicles during CNS Development. Prog. Neurobiol. 2021, 205, 102124. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jiang, X.; He, Y.; Zheng, X. Metabolic Landscape in Venous Thrombosis: Insights into Molecular Biology and Therapeutic Implications. Ann. Med. 2024, 56, 2401112. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, Y.; Jo, H.A.; Lee, J.; Song, Y.S. Non-Coding RNAs Shuttled via Exosomes Reshape the Hypoxic Tumor Microenvironment. J. Hematol. Oncol. 2020, 13, 67. [Google Scholar] [CrossRef]
- Kalinec, G.M.; Gao, L.; Cohn, W.; Whitelegge, J.P.; Faull, K.F.; Kalinec, F. Extracellular Vesicles From Auditory Cells as Nanocarriers for Anti-Inflammatory Drugs and Pro-Resolving Mediators. Front. Cell. Neurosci. 2019, 13, 530. [Google Scholar] [CrossRef]
- de Freitas, R.C.C.; Hirata, R.D.C.; Hirata, M.H.; Aikawa, E. Circulating Extracellular Vesicles As Biomarkers and Drug Delivery Vehicles in Cardiovascular Diseases. Biomolecules 2021, 11, 388. [Google Scholar] [CrossRef]
- Carnino, J.M.; Hao Kwok, Z.; Jin, Y. Extracellular Vesicles: A Novel Opportunity for Precision Medicine in Respiratory Diseases. Front. Med. 2021, 8, 661679. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, C.M.; Baccarelli, A.A.; Wu, H. Environmental Exposures and Extracellular Vesicles: Indicators of Systemic Effects and Human Disease. Curr. Environ. Health Rep. 2022, 9, 465–476. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Santonocito, R.; Vitale, A.M.; Scalia, F.; Marino Gammazza, A.; Campanella, C.; Bucchieri, F.; Cappello, F.; Caruso Bavisotto, C. Air Pollution: Role of Extracellular Vesicles-Derived Non-Coding RNAs in Environmental Stress Response. Cells 2023, 12, 1498. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Long, L.; Huang, Q. Extracellular Vesicles in Toxicological Studies: Key Roles in Communication between Environmental Stress and Adverse Outcomes. J. Appl. Toxicol. 2020, 40, 1166–1182. [Google Scholar] [CrossRef]
- Liu, S.; Costa, M.; Ortiz, A. Chapter 12—Effects of Metals on Extracellular Vesicle Signaling. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 279–298. ISBN 978-0-12-823292-7. [Google Scholar]
- Neven, K.Y.; Nawrot, T.S.; Bollati, V. Extracellular Vesicles: How the External and Internal Environment Can Shape Cell-To-Cell Communication. Curr. Environ. Health Rep. 2017, 4, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, X.; Lin, H.; Zhao, M.; Liu, W.; Li, W.; Han, L.; Ma, Q.; Dong, C.; Li, Y.; et al. Adipose Mesenchymal Stem Cell-Derived Antioxidative Extracellular Vesicles Exhibit Anti-Oxidative Stress and Immunomodulatory Effects under PM2.5 Exposure. Toxicology 2021, 447, 152627. [Google Scholar] [CrossRef]
- Sun, J.; Yang, S.; Zhou, G.-J.; Zhang, K.; Lu, Y.; Jin, Q.; Lam, P.K.S.; Leung, K.M.Y.; He, Y. Release of Microplastics from Discarded Surgical Masks and Their Adverse Impacts on the Marine Copepod Tigriopus japonicus. Environ. Sci. Technol. Lett. 2021, 8, 1065–1070. [Google Scholar] [CrossRef]
- Bruno, A.; Dovizio, M.; Milillo, C.; Aruffo, E.; Pesce, M.; Gatta, M.; Chiacchiaretta, P.; Di Carlo, P.; Ballerini, P. Orally Ingested Micro- and Nano-Plastics: A Hidden Driver of Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 3079. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, Y.; Bai, L.; Cui, J. Microplastics: An Often-Overlooked Issue in the Transition from Chronic Inflammation to Cancer. J. Transl. Med. 2024, 22, 959. [Google Scholar] [CrossRef]
- Donisi, I.; Colloca, A.; Anastasio, C.; Balestrieri, M.L.; D’Onofrio, N. Micro(Nano)Plastics: An Emerging Burden for Human Health. Int. J. Biol. Sci. 2024, 20, 5779–5792. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and Directions in Studying Cell–Cell Communication by Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Mantile, F.; Franco, P.; Stoppelli, M.P.; Liguori, G.L. Chapter Two—Biological Role and Clinical Relevance of Extracellular Vesicles as Key Mediators of Cell Communication in Cancer. In Advances in Biomembranes and Lipid Self-Assembly; Bongiovanni, A., Pocsfalvi, G., Manno, M., Kralj-Iglič, V., Eds.; Advances in Biomembranes and Lipid Self-Assembly; Academic Press: Cambridge, MA, USA, 2021; Volume 33, pp. 37–117. [Google Scholar]
- Wang, Y.; Xu, X.; Jiang, G. Microplastics Exposure Promotes the Proliferation of Skin Cancer Cells but Inhibits the Growth of Normal Skin Cells by Regulating the Inflammatory Process. Ecotoxicol. Environ. Saf. 2023, 267, 115636. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Chen, Y.; Qu, H.; Sun, J.; Wang, T.; Ma, Y.; Yuan, Y.; Bian, J.; Liu, Z. Microplastics Exacerbate Cadmium-Induced Kidney Injury by Enhancing Oxidative Stress, Autophagy, Apoptosis, and Fibrosis. Int. J. Mol. Sci. 2022, 23, 14411. [Google Scholar] [CrossRef]
- Deiuliis, J.A. MicroRNAs as Regulators of Metabolic Disease: Pathophysiologic Significance and Emerging Role as Biomarkers and Therapeutics. Int. J. Obes. 2016, 40, 88–101. [Google Scholar] [CrossRef]
- Yu, T.; Ma, P.; Wu, D.; Shu, Y.; Gao, W. Functions and Mechanisms of microRNA-31 in Human Cancers. Biomed. Pharmacother. 2018, 108, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Huang, C.C.-Y.; Zheng, C.-M.; Liu, W.-C.; Lee, Y.-H.; Chiu, H.-W. Polystyrene Microplastic-Induced Extracellular Vesicles Cause Kidney-Related Effects in the Crosstalk between Tubular Cells and Fibroblasts. Ecotoxicol. Environ. Saf. 2024, 273, 116098. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Weng, Y.; Shen, Q.; Zhao, Y.; Luo, T.; Xiao, Y.; Yang, G.; Jin, Y. Nano- and Micro-Polystyrene Plastics Interfered the Gut Barrier Function Mediated by Exosomal miRNAs in Rats. Environ. Pollut. 2023, 335, 122275. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Lin, P.; Wu, Z.; Lu, Z.; Ma, L.; Dong, X.; He, L.; Dai, Z.; Zhou, C.; Hong, P.; et al. Exosomal miRNA Analysis Provides New Insights into Exposure to Nanoplastics and Okadaic Acid. Sci. Total Environ. 2023, 905, 167010. [Google Scholar] [CrossRef] [PubMed]
- Contursi, A.; Sacco, A.; Grande, R.; Dovizio, M.; Patrignani, P. Platelets as Crucial Partners for Tumor Metastasis: From Mechanistic Aspects to Pharmacological Targeting. Cell. Mol. Life Sci. 2017, 74, 3491–3507. [Google Scholar] [CrossRef]
- Ballerini, P.; Contursi, A.; Bruno, A.; Mucci, M.; Tacconelli, S.; Patrignani, P. Inflammation and Cancer: From the Development of Personalized Indicators to Novel Therapeutic Strategies. Front. Pharmacol. 2022, 13, 838079. [Google Scholar] [CrossRef]
- Kim, N.; Park, J.H.; Lee, I.; Jung, G.S.; Lee, J.H.; Lee, M.J.; Im, W.; Cho, S.; Choi, Y.S. Investigation of Cell-to-Cell Transfer of Polystyrene Microplastics through Extracellular Vesicle-Mediated Communication. Biochem. Biophys. Res. Commun. 2024, 734, 150719. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, K.; Sidoryk-Węgrzynowicz, M.; Dąbrowska-Bouta, B.; Sulkowski, G.; Strużyńska, L. Plastic Nanoparticles Interfere with Extracellular Vesicle Pathway in Primary Astrocytes. Ecotoxicol. Environ. Saf. 2024, 286, 117180. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, C.; Li, H.; Zhang, P.; Liu, X. The Impact of Microplastic-Microbe Interactions on Animal Health and Biogeochemical Cycles: A Mini-Review. Sci. Total Environ. 2021, 773, 145697. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zhou, S.; Zhang, L.; Ding, S. The Effects of Three Different Microplastics on Enzyme Activities and Microbial Communities in Soil. Water Environ. Res. 2021, 93, 24–32. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Zhang, Z.; Grossart, H.-P.; Gadd, G.M. Microplastics Provide New Microbial Niches in Aquatic Environments. Appl. Microbiol. Biotechnol. 2020, 104, 6501–6511. [Google Scholar] [CrossRef]
- Zhu, L.; Xie, C.; Chen, L.; Dai, X.; Zhou, Y.; Pan, H.; Tian, K. Transport of Microplastics in the Body and Interaction with Biological Barriers, and Controlling of Microplastics Pollution. Ecotoxicol. Environ. Saf. 2023, 255, 114818. [Google Scholar] [CrossRef] [PubMed]
- Athey, S.N.; Albotra, S.D.; Gordon, C.A.; Monteleone, B.; Seaton, P.; Andrady, A.L.; Taylor, A.R.; Brander, S.M. Trophic Transfer of Microplastics in an Estuarine Food Chain and the Effects of a Sorbed Legacy Pollutant. Limnol. Oceanogr. Lett. 2020, 5, 154–162. [Google Scholar] [CrossRef]
- Botterell, Z.L.R.; Beaumont, N.; Cole, M.; Hopkins, F.E.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability of Microplastics to Marine Zooplankton: Effect of Shape and Infochemicals. Environ. Sci. Technol. 2020, 54, 12024–12033. [Google Scholar] [CrossRef]
- Malafeev, K.V.; Apicella, A.; Incarnato, L.; Scarfato, P. Understanding the Impact of Biodegradable Microplastics on Living Organisms Entering the Food Chain: A Review. Polymers 2023, 15, 3680. [Google Scholar] [CrossRef]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of Microplastic Debris throughout the Marine Ecosystem. Nat. Ecol. Evol. 2017, 1, 0116. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Trevisan, R.; Ranasinghe, P.; Merrill, G.B.; Santos, J.; Hong, A.; Edward, W.C.; Jayasundara, N.; Somarelli, J.A. A Growing Crisis for One Health: Impacts of Plastic Pollution across Layers of Biological Function. Front. Mar. Sci. 2022, 9, 980705. [Google Scholar] [CrossRef]
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and Biomagnification of Microplastics in Marine Organisms: A Review and Meta-Analysis of Current Data. PLoS ONE 2020, 15, e0240792. [Google Scholar] [CrossRef] [PubMed]

| MP Type | MP Size | Environmental Source | References |
|---|---|---|---|
| PS | ˂50 nm | River water | [58] |
| PS | 100 nm, 500 nm, and 10 μm | Seawater | [59] |
| PE | 2 μm | Seawater | [60] |
| PS, PE, PVC | 20–150 nm | Agricultural soil | [61] |
| PVC | 10–500 nm | Sand | [62] |
| PS | 200–400 nm | Sand | [62] |
| PS | ˂450 nm | Air | [63] |
| EV Size Range | EV Major Category | EV Protein Markers |
|---|---|---|
| 500–2000 nm | Apoptotic bodies | Histone |
| 50–1000 nm | Microvesicles | CD40 |
| 40–120 nm | Exosomes | CD9, CD63, CD81, TSG101, and ALIX |
| Type of MP | EV Biological Source | MP Size | Molecular Alterations Induced by the MP–EV Complex | References |
|---|---|---|---|---|
| PS | Human melanoma cells | Nanoscale (<100 nm) | Alters signaling pathways (mitochondrial function, apoptosis, and autophagy). | [155] |
| Fluorescent PS | Zebrafish kidney cells | 50–500 nm | Gene expression changes (oxidative stress response and immune modulation). | [121] |
| PET | Pig serum (gilts exposed to PET MPs) | Microscale (<1 µm) | Alters miRNA expression linked to obesity, insulin resistance, and metabolic syndrome. | [38] |
| PS | Mouse kidney cells | Nanoscale | Induces ROS, cellular stress, and renal fibrosis markers. | [159] |
| PS | Rat intestinal cells | Nanoscale (<100 nm) | Alters intestinal barrier function and increases apoptosis and oxidative stress. | [160] |
| PS and oleic acid | Gastric adenocarcinoma (AGS) cells | Nanoscale | Modifies miRNA composition in exosomes indicating potential toxicity. | [161] |
| MPs and NPs | Platelets | Nano/micron | Release of lipids (TXA2, PGE2, 12S-HETE), proteins (growth factors, proteases, cytokines), and miRNA-rich EVs. Promotion of chronic inflammation, and increased risk of intestinal tumorigenesis. | [150,162,163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzoni, E.; Montegiove, N.; Cesaretti, A.; Bertoldi, A.; Cusumano, G.; Gigliotti, G.; Emiliani, C. Microplastic and Extracellular Vesicle Interactions: Recent Studies on Human Health and Environment Risks. Biophysica 2024, 4, 724-746. https://doi.org/10.3390/biophysica4040047
Calzoni E, Montegiove N, Cesaretti A, Bertoldi A, Cusumano G, Gigliotti G, Emiliani C. Microplastic and Extracellular Vesicle Interactions: Recent Studies on Human Health and Environment Risks. Biophysica. 2024; 4(4):724-746. https://doi.org/10.3390/biophysica4040047
Chicago/Turabian StyleCalzoni, Eleonora, Nicolò Montegiove, Alessio Cesaretti, Agnese Bertoldi, Gaia Cusumano, Giovanni Gigliotti, and Carla Emiliani. 2024. "Microplastic and Extracellular Vesicle Interactions: Recent Studies on Human Health and Environment Risks" Biophysica 4, no. 4: 724-746. https://doi.org/10.3390/biophysica4040047
APA StyleCalzoni, E., Montegiove, N., Cesaretti, A., Bertoldi, A., Cusumano, G., Gigliotti, G., & Emiliani, C. (2024). Microplastic and Extracellular Vesicle Interactions: Recent Studies on Human Health and Environment Risks. Biophysica, 4(4), 724-746. https://doi.org/10.3390/biophysica4040047










