Probing Biochemical Differences in Lipid Components of Human Cells by Means of ATR-FTIR Spectroscopy
Abstract
:1. Introduction
2. The Presence of Lipids in Human Cells
3. Materials and Methods
3.1. Lipid Commercial Samples
3.2. Attenuated Total Reflection–FourierTransform Infrared (ATR-FTIR) Spectroscopy
4. Results and Discussion
4.1. ATR-FTIR Spectra of Commercial Lipids
4.2. Ratiometric Analysis of ATR-FTIR Lipid Spectra
4.3. Analysis of Lipid Mixtures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fringeli, U.P.; Gunthard, H.H. Infrared membrane spectroscopy. Mol. Biol. Biochem. Biophys. 1981, 31, 270–332. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.K.; Tatulian, S.A. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q. Rev. Biophys. 1997, 30, 365–429. [Google Scholar] [CrossRef]
- Goormaghtigh, E.; Raussens, V.; Ruysschaert, J.-M. Attenuated total reflection infrared spectroscopy of proteins and lipids in biological membranes. Biochim. Biophys. Acta 1999, 1422, 105–185. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Koike, K. Lipid and membrane dynamics in biological tissues—Infrared spectroscopic studies. In Advances in Planar Lipid Bilayers and Liposomes; Elsevier Science Publisher: Amsterdam, The Netherlands, 2011; Volume 13, pp. 1–32. [Google Scholar]
- Derenne, A.; Claessens, T.; Conus, C.; Goormaghtigh, E. Infrared spectroscopy of membrane lipids. In Encyclopedia of Biophysics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1074–1081. [Google Scholar]
- Derenne, A.; Vandersleyen, O.; Goormaghtigh, E. Lipid quantification method using FTIR spectroscopy applied on cancer cell extracts. Biochim. Biophys. Acta 2014, 1841, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Dreissig, I.; Machill, S.; Salzer, R.; Krafft, C. Quantification of brain lipids by FTIR spectroscopy and partial least squares regression. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 71, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Beljebbar, A.; Amharref, N.; Lèvèques, A.; Dukic, S.; Venteo, L.; Schneider, L.; Pluot, M.; Manfait, M. Modeling and quantifying biochemical changes in C6 tumor gliomas by Fourier transform infrared imaging. Anal. Chem. 2008, 80, 8406–8415. [Google Scholar] [CrossRef] [PubMed]
- Beljebbar, A.; Dukic, S.; Amharref, N.; Bellefqih, S.; Manfait, M. Monitoring of biochemical changes through the C6 gliomas progression and invasion by Fourier transform infrared (FTIR) imaging. Anal. Chem. 2009, 81, 9247–9256. [Google Scholar] [CrossRef]
- Severcan, F.; Bozkurt, O.; Gurbanov, R.; Gorgulu, G. FT-IR spectroscopy in diagnosis of diabetes in rat animal model. J Biophotonics 2010, 3, 621–631. [Google Scholar] [CrossRef]
- Guleken, Z.; Bulut, H.; Depciuch, J.; Tarhan, N. Diagnosis of endometriosis using endometrioma volume and vibrational spectroscopy with multivariate methods as a noninvasive method. Acta A Mol. Biomol. Spectrosc. 2022, 264, 120246. [Google Scholar] [CrossRef]
- Gasper, R.; Dewelle, J.; Kiss, R.; Mijatovic, T.; Goormaghtigh, E. IR spectroscopy as a new tool for evidencing antitumor drug signatures. Biochim. Biophys. Acta 2009, 1788, 1263–1270. [Google Scholar] [CrossRef]
- Gasper, R.; Vandenbussche, G.; Goormaghtigh, E. Ouabain-induced modifications of prostate cancer cell lipidome investigated with mass spectrometry and FTIR spectroscopy. Biochim. Biophys. Acta 2011, 1808, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Lipiec, E.; Bambery, K.R.; Heraud, P.; Hirschmugl, C.; Lekki, J.; Kwiatek, W.M.; Tobin, M.J.; Vogel, C.; Whelan, D.; Wood, B.R. Synchrotron FTIR shows evidence of DNA damage and lipid accumulation in prostate adenocarcinoma PC-3 cells following proton irradiation. J. Mol. Struct. 2014, 1073, 134–141. [Google Scholar] [CrossRef]
- Ricciardi, V.; Portaccio, M.; Manti, L.; Lepore, M. An FTIR microspectroscopy ratiometric approach for monitoring X-ray irradiation effects on SH-SY5Y human neuroblastoma cells. Appl. Sci. 2020, 10, 2974. [Google Scholar] [CrossRef]
- Ricciardi, V.; Portaccio, M.; Perna, G.; Lasalvia, M.; Capozzi, V.; Cammarata, F.P.; Pisciotta, P.; Petringa, G.; Delfino, I.; Manti, L. FT-IR transflection micro-spectroscopy study on normal human breast cells after exposure to a proton beam. Appl. Sci. 2021, 11, 540. [Google Scholar] [CrossRef]
- Cakmak, G.; Miller, L.M.; Zorlu, F.; Severcan, F. Amifostine, a radioprotectant agent, protects rat brain tissue lipids against ionizing radiation induced damage: An FTIR microspectroscopic imaging study. Arch. Biochem. Biophys. 2012, 520, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, G.; Togan, I.; Severcan, F. 17β-Estradiol induced compositional, structural and functional changes in rainbow trout liver, revealed by FT-IR spectroscopy: A comparative study with nonylphenol. Aquat. Toxicol. 2006, 77, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Kamboj, V.K.; Ahlawat, P.; Kumar, V. Structural and molecular alterations in arsenic-induced hepatic oxidative stress in rats: A FTIR study. Toxicol. Environ. Chem. 2015, 97, 1408–1421. [Google Scholar] [CrossRef]
- Villalaín, J.; Ortiz, A.; Gómez-Fernández, J.C. Molecular interactions between sphingomyelin and phosphatidylcholine in phospholipid vesicles. Biochim. Biophys. Acta 1988, 941, 55–62. [Google Scholar] [CrossRef]
- Moore, D.J.; Rerek, M.E.; Mendelsohn, R. FTIR spectroscopy studies of the conformational order and phase behavior of ceramides. J. Phys. Chem. B 1997, 101, 8933–8940. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Navas-Iglesias, N.; Cuadros-Rodriguez, L. From lipid analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part I: Modern lipid analysis. Trends Anal. Chem. 2009, 28, 263–278. [Google Scholar] [CrossRef]
- Navas-Iglesias, N.; Carrasco-Pancorbo, A.; Cuadros-Rodríguez, L. From lipids analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part II: Analytical lipidomics. Trends Anal. Chem. 2009, 28, 393–403. [Google Scholar] [CrossRef]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model. Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- De la Arada, I.; González-Ramírez, E.J.; Alonso, A.; Goñi, F.M.; Arrondo, J.-L.R. Exploring polar headgroup interactions between sphingomyelin and ceramide with infrared spectroscopy. Sci. Rep. 2020, 10, 17606. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.; Molendijk, J.; Shah, A.K.; Rahman, T.; Anderson, G.J.; Hill, M.M. Rapid Assessment of Lipidomics Sample Purity and Quantity Using Fourier-Transform Infrared Spectroscopy. Biomolecules 2022, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- Jurowski, K.; Kochan, K.; Walczak, J.; Barańska, M.; Piekoszewski, W.; Buszewski, B. Analytical techniques in lipidomics: State of the art. Crit. Rev. Anal. Chem. 2017, 47, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Butler, H.J.; Brennan, P.M.; Cameron, J.M.; Finlayson, D.; Hegarty, M.G.; Jenkinson, M.D.; Palmer, D.S.; Smith, B.R.; Baker, M.J. Development of high-throughput ATR-FTIR technology for rapid triage of brain cancer. Nat. Commun. 2019, 10, 4501. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wei, G.; Chen, W.; Lei, C.; Xu, C.; Guan, Y.; Ji, T.; Wang, F.; Liu, H. Fast and deep diagnosis using blood-based ATR-FTIR spectroscopy for digestive tract cancers. Biomolecules 2022, 12, 1815. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, T.; Mukherjee, R.; Ariese, F.; Somasundaram, K.; Umapathy, S. Raman and infra-red microspectroscopy: Towards quantitative evaluation for clinical research by ratiometric analysis. Chem. Soc. Rev. 2016, 45, 1879–1900. [Google Scholar] [CrossRef]
- Severcan, M.; Haris, P.I.; Severcan, F. Using artificially generated spectral data to improve protein secondary structure prediction from Fourier transform infrared spectra of proteins. Anal. Biochem. 2004, 332, 238–244. [Google Scholar] [CrossRef]
- Liu, W.; Luo, X.; Wang, J.; Li, Y.; Feng, F.; Zhao, M. Digestive behavior of unemulsified triglycerides with different chain lengths: In vitro dynamic and static simulated digestion models. LWT 2021, 149, 112006. [Google Scholar] [CrossRef]
- Christie, W.W. The composition, structure and function of lipids in the tissues of ruminant animals. Prog. Lipid Res. 1981, 95–191. [Google Scholar] [CrossRef]
- Cui, Z.; Houweling, M. Phosphatidylcholine and cell death. Biochim. Biophys. Acta 2002, 1585, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Deleault, N.R.; Piro, J.R.; Walsh, D.J.; Wang, F.; Ma, J.; Geoghegan, J.C.; Supattapone, S. Isolation of phosphatidylethanolamine as a solitary cofactor for prion formation in the absence of nucleic acids. Proc. Natl. Acad. Sci. USA 2012, 109, 8546–8551. [Google Scholar] [CrossRef] [PubMed]
- Emoto, K.; Kobayashi, T.; Yamaji, A.; Aizawa, H.; Yahara, I.; Inoue, K.; Umeda, M. Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc. Natl. Acad. Sci. USA 1996, 93, 12867–12872. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E.; Tasseva, G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta 2013, 1831, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Wellner, N.; Diep, T.A.; Janfelt, C.; Hansen, H.S. N-acylation of phosphatidylethanolamine and its biological functions in mammals. Biochim. Biophys. Acta 2013, 1831, 652–662. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Akbar, M.; Kim, Y.-S. Phosphatidylserine-dependent neuroprotective signaling promoted by docosahexaenoic acid. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2010, 82, 165–172. [Google Scholar] [CrossRef]
- Akbar, M.; Calderon, F.; Wen, Z.; Kim, H.-Y. Docosahexaenoic acid: A positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. USA 2005, 102, 10858–10863. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Akbar, M.; Lau, A.; Edsall, L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22: 6n-3): Role of phosphatidylserine in antiapoptotic effect. J. Biol. Chem. 2000, 275, 35215–35223. [Google Scholar] [CrossRef]
- Svennerholm, L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J. Lipid Res. 1968, 9, 570–579. [Google Scholar] [CrossRef]
- Mueller-Roeber, B.; Pical, C. Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol. 2002, 130, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.K.; Obeid, L.M.; Hannun, Y.A. Ceramide and the regulation of apoptosis and the stress response. Trends Cardiovasc. Med. 1996, 6, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Parveen, F.; Bender, D.; Law, S.-H.; Mishra, V.K.; Chen, C.-C.; Ke, L.-Y. Role of ceramidases in sphingolipid metabolism and human diseases. Cells 2019, 8, 1573. [Google Scholar] [CrossRef] [PubMed]
- Carpinteiro, A.; Dumitru, C.; Schenck, M.; Gulbins, E. Ceramide-induced cell death in malignant cells. Cancer Lett. 2008, 264, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Modrak, D.E.; Gold, D.V.; Goldenberg, D.M. Sphingolipid targets in cancer therapy. Mol. Cancer Ther. 2006, 5, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Beckham, T.H.; Cheng, J.C.; Marrison, S.T.; Norris, J.S.; Liu, X. Interdiction of sphingolipid metabolism to improve standard cancer therapies. Adv. Cancer Res. 2013, 117, 1–36. [Google Scholar]
- Henry, B.; Möller, C.; Dimanche-Boitrel, M.-T.; Gulbins, E.; Becker, K.A. Targeting the ceramide system in cancer. Cancer Lett. 2013, 332, 286–294. [Google Scholar] [CrossRef]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008, 20, 1010–1018. [Google Scholar] [CrossRef]
- Hajj, C.; Haimovitz-Friedman, A. Sphingolipids’ role in radiotherapy for prostate cancer. Handb. Exp. Pharmacol. 2013, 216, 115–130. [Google Scholar]
- Vit, J.-P.; Rosselli, F. Role of the ceramide-signaling pathways in ionizing radiation-induced apoptosis. Oncogene 2003, 22, 8645–8652. [Google Scholar] [CrossRef]
- Aureli, M.; Murdica, V.; Loberto, N.; Samarani, M.; Prinetti, A.; Bassi, R.; Sonnino, S. Exploring the link between ceramide and ionizing radiation. Glycoconj J. 2014, 31, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Corre, I.; Niaudet, C.; Paris, F. Plasma membrane signaling induced by ionizing radiation. Mutat. Res./Rev. Mutat. Res. 2010, 704, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, K.; Evans, T.; Hla, T. Sphingosine 1-phosphate signalling. Development 2014, 141, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J. Biol. Chem. 2007, 282, 2125–2129. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Sankala, H.; Hait, N.C.; Le Stunff, H.; Liu, H.; Toman, R.; Collier, C.; Zhang, M.; Satin, L.S.; Merrill, A.H. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 2005, 280, 37118–37129. [Google Scholar] [CrossRef] [PubMed]
- Sattler, K.; Levkau, B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc. Res. 2009, 82, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Maiti, A. The role of sphingosine-1-phosphate and ceramide-1-phosphate in inflammation and cancer. Mediat. Inflamm. 2017, 2017, 4806541. [Google Scholar] [CrossRef]
- Türker-Kaya, S.; Kına, A. Calorimetric and spectroscopic investigation of the interaction of chemotherapeutic agent carboplatin with sphingomyelin lipids. J. Therm. Anal. Calorim. 2021, 146, 2515–2522. [Google Scholar] [CrossRef]
- Ohanian, J.; Ohanian, V. Sphingolipids in mammalian cell signalling. Cell. Mol. Life Sci. 2001, 58, 2053–2068. [Google Scholar] [CrossRef]
- Lasch, P. Spectral pre-processing for biomedical vibrational spectroscopy and microspectroscopic imaging. Chem. Intell. Lab. Syst. 2012, 117, 100–114. [Google Scholar] [CrossRef]
- Abdelrazzak, A.B.; Hezma, A.; El-Bahy, G.S. ATR-FTIR spectroscopy probing of structural alterations in the cellular membrane of abscopal liver cells. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183726. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Faramarzi, B.; Moggio, M.; Diano, N.; Portaccio, M.; Lepore, M. A Brief Review of FT-IR Spectroscopy Studies of Sphingolipids in Human Cells. Biophysica 2023, 3, 158–180. [Google Scholar] [CrossRef]
- Albuquerque, M.; Guedes, I.; Alcantara, P., Jr.; Moreira, S. Infrared absorption spectra of Buriti (Mauritia flexuosa L.) oil. Vib. Spectrosc. 2003, 33, 127–131. [Google Scholar] [CrossRef]
- Krafft, C.; Neudert, L.; Simat, T.; Salzer, R. Near infrared Raman spectra of human brain lipids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Singh, V.K.; Kumar, V.; Khajuria, Y. Spectroscopic studies of cholesterol: Fourier transform infra-red and vibrational frequency analysis. Mater. Focus 2014, 3, 211–217. [Google Scholar] [CrossRef]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of multidimensional data processing approaches for Raman and infrared spectroscopy. EPJ Technol. Instrum. 2015, 2, 1–38. [Google Scholar] [CrossRef]
- Rohman, A.; Ghazali, M.A.I.B.; Windarsih, A.; Riyanto, S.; Yusof, F.M.; Mustafa, S. Comprehensive review on application of FTIR spectroscopy coupled with chemometrics for authentication analysis of fats and oils in the food products. Molecules 2020, 25, 5485. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef]
- Ricciardi, V.; Lasalvia, M.; Perna, G.; Portaccio, M.; Delfino, I.; Lepore, M.; Capozzi, V.; Manti, L. Vibrational spectroscopies for biochemical investigation of X-ray exposure effects on SH-SY5Y human neuroblastoma cells. Radiat. Env. Biophys. 2023, 62, 289–305. [Google Scholar] [CrossRef]
- Ray, T.K.; Skipski, V.P.; Barclay, M.; Essner, E.; Archibald, F.M. Lipid composition of rat liver plasma membranes. J Biol Chem. 1969, 244, 5528–5536. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Phospholipid synthesis and transport in mammalian cells. Traffic 2015, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Moggio, M.; Errico, S.; Diano, N.; Lepore, M. FTIR spectroscopy for evaluation and monitoring of lipid extraction efficiency for murine liver tissues analysis. Eng. Proc. 2021, 10, 9. [Google Scholar]
- Faramarzi, B.; Moggio, M.; Cardamuro, V.; Portaccio, M.; Diano, N.; Manti, L.; Lepore, M. An FTIR spectroscopy investigation on different methods of lipid extraction from HepG2 cells. Eng. Proc. 2022, 27, 39. [Google Scholar]

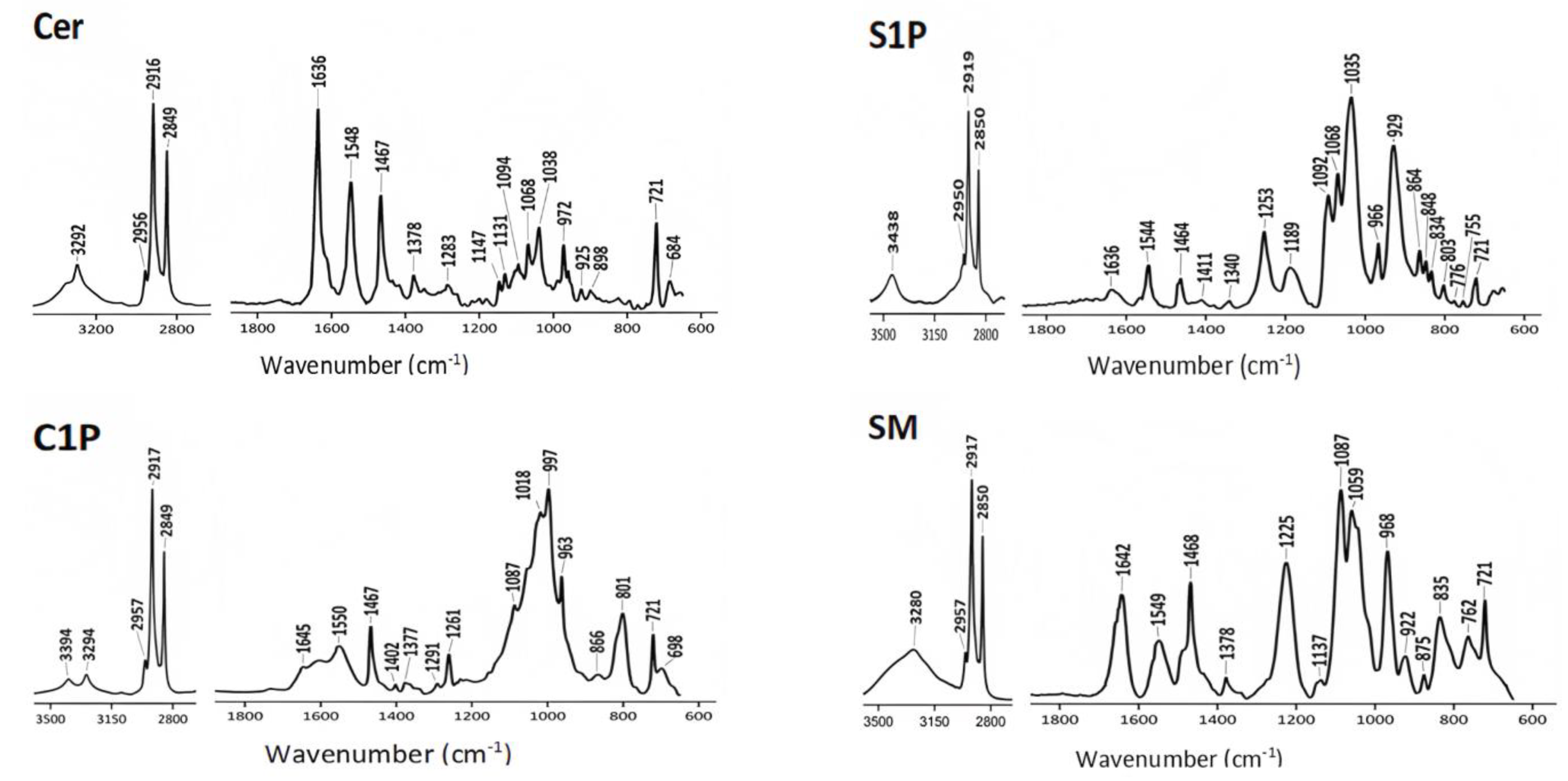
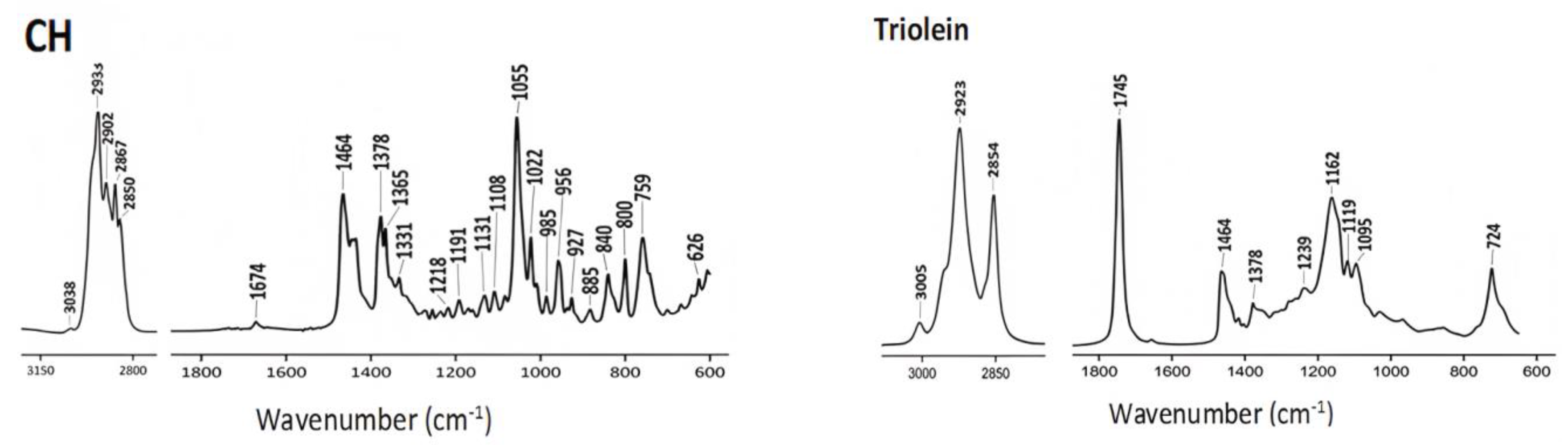


| Commercial Lipids | Structure |
|---|---|
| Phosphatidylcholine (PC) |  |
| Phosphatidylethanolamine (PE) |  |
| Phosphatidylserine (PS) |  |
| Phosphatidylinositol (PI) |  |
| Ceramide (Cer) |  |
| Sphingosine 1-phosphate (S1P) |  |
| Ceramide-1-phosphate (C1P) |  |
| Sphingomyelin (SM) |  |
| Cholesterol (CH) |  |
| Triolein |  |
| Area Ratio | Baseline Points (cm−1) | Indication | PI | SM | |
|---|---|---|---|---|---|
| (1) | CH2as/Lipid | (2900–2948)/ (2830–3027) | Chain length of lipids | 0.49 | 0.46 |
| (2) | CH3as/Lipid | (2943–2968)/ (2830–3027) | Methyl concentration | 0.11 | 0.09 |
| (3) | Olefinic=CH/ Lipid | (2992–3030)/ (2830–3027) | Concentration unsaturated fatty acids | 0.035 | 0.045 |
| (4) | Carbonyl C=O/Lipid | (1717–1753)/ (2830–3027) | Carbonyl content | 0.27 | 0.007 |
| (5) | CH2as/CH3as | (2900–2948)/ (2943–2968) | Length of hydrocarbon chain | 4.43 | 5.16 |
| (6) | CH2s + CH2as/Olefinic=CH | (2855 + 2920)/ (2992–3030) | Saturation level of lipids | 0.42 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portaccio, M.; Faramarzi, B.; Lepore, M. Probing Biochemical Differences in Lipid Components of Human Cells by Means of ATR-FTIR Spectroscopy. Biophysica 2023, 3, 524-538. https://doi.org/10.3390/biophysica3030035
Portaccio M, Faramarzi B, Lepore M. Probing Biochemical Differences in Lipid Components of Human Cells by Means of ATR-FTIR Spectroscopy. Biophysica. 2023; 3(3):524-538. https://doi.org/10.3390/biophysica3030035
Chicago/Turabian StylePortaccio, Marianna, Bahar Faramarzi, and Maria Lepore. 2023. "Probing Biochemical Differences in Lipid Components of Human Cells by Means of ATR-FTIR Spectroscopy" Biophysica 3, no. 3: 524-538. https://doi.org/10.3390/biophysica3030035
APA StylePortaccio, M., Faramarzi, B., & Lepore, M. (2023). Probing Biochemical Differences in Lipid Components of Human Cells by Means of ATR-FTIR Spectroscopy. Biophysica, 3(3), 524-538. https://doi.org/10.3390/biophysica3030035







