Supramolecular-Covalent Peptides Self-Assembly: From Design to Regenerative Medicine and Beyond
Abstract
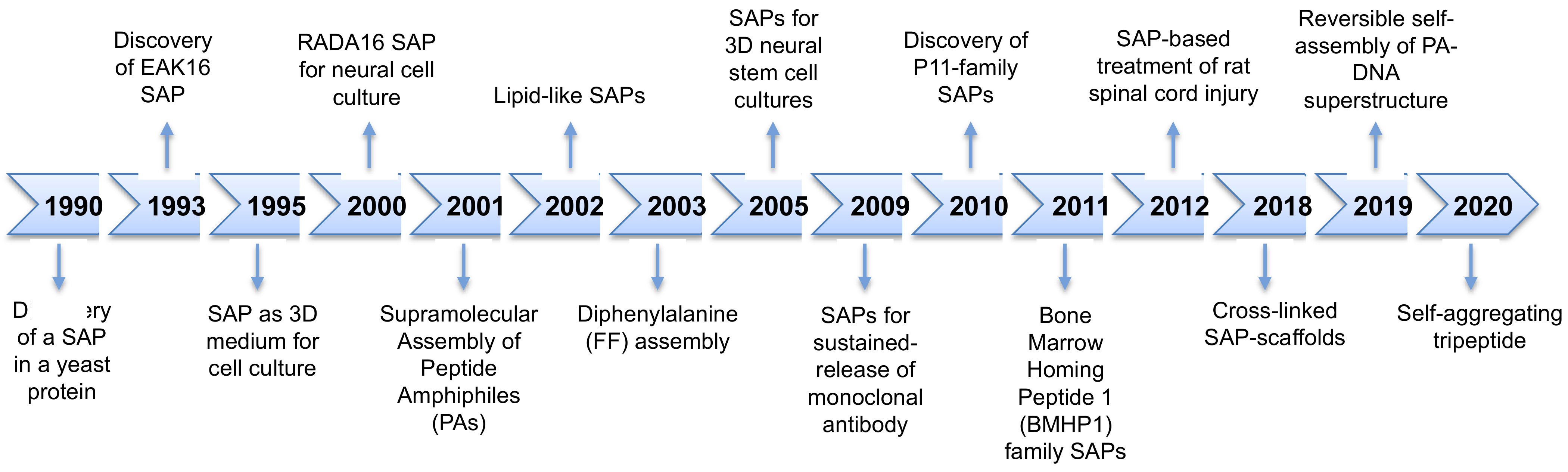
1. Introduction
2. Lysine Knots as a Molecular Fastener to Tune Supramolecular Peptides Stiffness
| Self-Assembly Peptides | Cross-Linker | Amino Acid Residues for Cross-Linking | Storage Modulus | Refs. |
|---|---|---|---|---|
| Ac-LDLKLDLKLDLK-CONH2 (LDLK12) LDLK12 functionalized with KLPGWSG, FAQRVPP | Multiple ramification of LDLK12 | - | 50–10,000 Pa | [50] |
| C16-V3A3E3 | Oligo-L-Lysines | - | 18–1276 Pa | [55] |
| 1,3-diene-palmitoyl-V3A3K3 | 1,3-diene-palmitic-acid | 1,3-diene | - | [56] |
| Collagen Mimetic Peptides (CMPs) | EDC/HOBt | Lysine-Aspartic acid Lysine-Glutamic acid | - | [57] |
| LDLK12 | EDC/Sulfo-NHS | Lysine-Aspartic acid | 1–2.2 kPa | [58] |
| Lauryl-VVAGKK-Am | Glutaraldehyde | Amine group (Lysine, Arginine) | 105 Pa | [59] |
| Ac-CGGLKLKLKLKLKLKGGC-CONH2 Ac-CGGCGGLKLKLKLKLKLKGGCGGC-CONH2 Ac-CGGCGGCGGLKLKLKLKLKLKGGCGGCGGC-CONH2 Ac-CGGCGGCGGCGGLKLKLKLKLKLKGGCGGCGGCGGC-CONH2 | Sulfo-SMCC | Lysine-Cysteine | 6–840 kPa | [60,61] |
| FYFCFYF | NH4HCO3 | Cysteine-Cysteine | 3360 Pa | [62] |
| Fmoc-FFF LDLK12 LDLK12 functionalized with KLPGWSG, FAQRVPP, SSLVND Branched-LDLK12 Biotin-GGGPFSSTKT Biotin-GGGAFSSTKT Biotin-GGGAFASTKT Biotin-GGGPFASTKT Biotin-GGGAFASAKA Ac-WGGGAFASTKT Ac-WGGGAFSSTKT | Genipin | Lysine-Lysine | 5 kPa–0.2 MPa | [63,64,65] |
| VKVKVKVKVDPPTKVYVKVKV-NH2 | Frémy’s salt | Tyrosine-Tyrosine | 25,470 Pa | [66] |
| Fmoc-FFY, Fmoc-FFGGGY Ac-YYGGGLDLKLDLKLDLK-CONH2 | Ru(bpy)3Cl2 | Tyrosine-Tyrosine | 26–106 kPa | [67,68] |
| SAP Sequence | Chemical Structures |
|---|---|
| Ac-LDLKLDLKLDLK |  |
| FAQRVPPGGGLDLKLDLKLDLK | 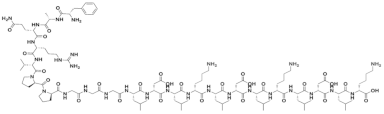 |
| KLPGWSGGGGLDLKLDLKLDLK |  |
| Branched-LDLKLDLKLDLK | 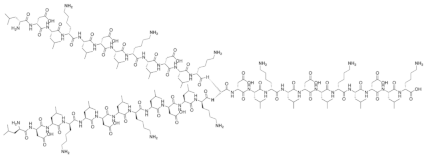 |
| SSLVNDGGGLDLKLDLKLDLK | 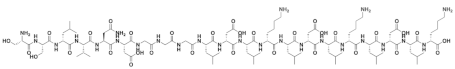 |
| Biotin-GGGPFSSTKT |  |
| Biotin-GGGAFSSTKT | 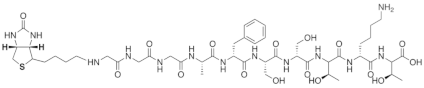 |
| Biotin-GGGAFASTKT |  |
| Biotin-GGGPFASTKT | 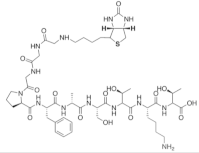 |
| Ac-WGGGAFASTKT | 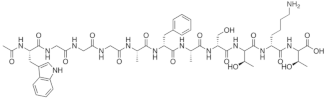 |
| Ac-WGGGAFSSTKT |  |
| Biotin-GGGAFASAKA | 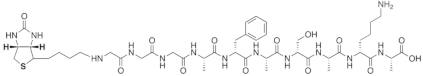 |
| Fmoc-FF |  |
| FYFCFYF | 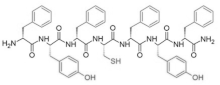 |
| C16-V3A3E3 | 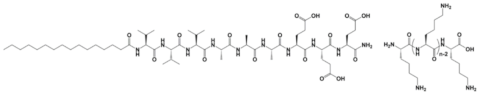 |
| 1,3-diene-palmitoyl-V3A3K3 |  |
3. Covalent Capture by Using Lysine-Aspartic Acid Pairs in Collagen Mimetic Peptides and Self-Assembling Peptides
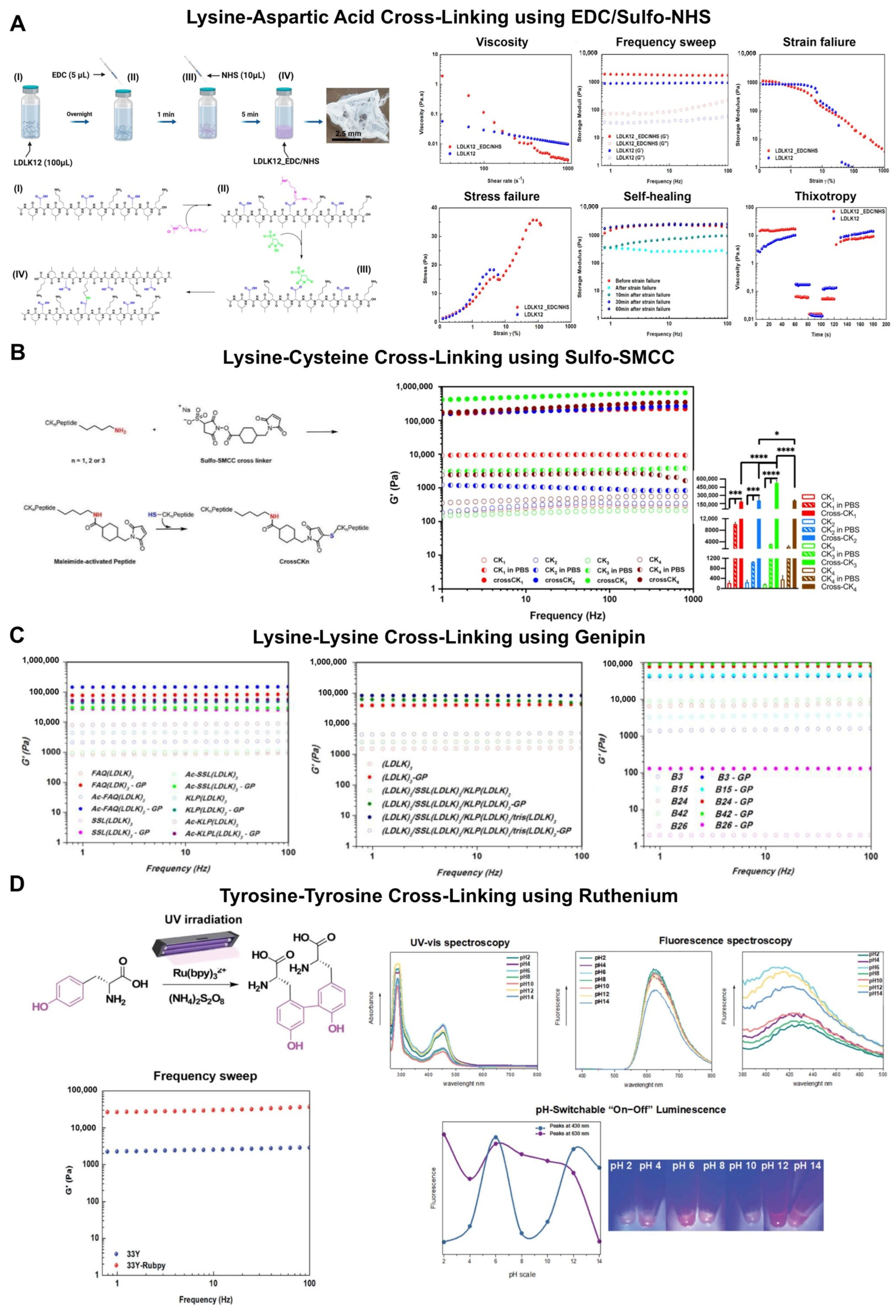
4. Chemically Cross-Linked Self-Assembling Peptide Scaffolds
5. Tyrosine Cross-Linking Boost the Mechanical Rigidity of Self-Assembling Peptide Scaffolds
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S. Self-assembling peptides: From a discovery in a yeast protein to diverse uses and beyond. Protein Sci. 2020, 29, 2281–2303. [Google Scholar] [CrossRef]
- Zhang, S.; Holmes, T.; Lockshin, C.; Rich, A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc. Natl. Acad. Sci. USA 1993, 90, 3334–3338. [Google Scholar] [CrossRef]
- Pugliese, R.; Gelain, F. Peptidic biomaterials: From self-assembling to regenerative medicine. Trends Biotechnol. 2017, 35, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Gelain, F.; Luo, Z.; Zhang, S. Self-assembling peptide EAK16 and RADA16 nanofiber scaffold hydrogel. Chem. Rev. 2020, 120, 13434–13460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, S. Designer self-assembling peptide materials. Macromol. Biosci. 2007, 7, 13–22. [Google Scholar] [CrossRef] [PubMed]
- La Manna, S.; Di Natale, C.; Onesto, V.; Marasco, D. Self-assembling peptides: From design to biomedical applications. Int. J. Mol. Sci. 2021, 22, 12662. [Google Scholar] [CrossRef]
- Hauser, C.A.; Zhang, S. Designer self-assembling peptide nanofiber biological materials. Chem. Soc. Rev. 2010, 39, 2780–2790. [Google Scholar] [CrossRef] [PubMed]
- Rauf, S.; Susapto, H.H.; Kahin, K.; Alshehri, S.; Abdelrahman, S.; Lam, J.H.; Asad, S.; Jadhav, S.; Sundaramurthi, D.; Gao, X.; et al. Self-assembling tetrameric peptides allow in situ 3D bioprinting under physiological conditions. J. Mater. Chem. B 2021, 9, 1069–1081. [Google Scholar] [CrossRef]
- Raspa, A.; Carminati, L.; Pugliese, R.; Fontana, F.; Gelain, F. Self-assembling peptide hydrogels for the stabilization and sustained release of active Chondroitinase ABC in vitro and in spinal cord injuries. J. Control. Release 2021, 330, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Johnson, M.E.; Oldenhuis, N.J.; Tiambeng, T.N.; Guan, Z. Structure-based design of dendritic peptide bolaamphiphiles for siRNA delivery. ACS Cent. Sci. 2015, 1, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, Z.; Xu, B. Supramolecular assemblies of peptides or nucleopeptides for gene delivery. Theranostics 2019, 9, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Raspa, A.; Gelain, F. Mimicking extracellular matrix via engineered nanostructured biomaterials for neural repair. Curr. Neuropharmacol. 2021, 19, 2110–2124. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.; Ingham, E.; Aggeli, A. Biomimetic self-assembling peptides as scaffolds for soft tissue engineering. Nanomedicine 2013, 8, 823–847. [Google Scholar] [CrossRef] [PubMed]
- Koutsopoulos, S. Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: Progress, design guidelines, and applications. J. Biomed. Mater. Res. A 2016, 104, 1002–1016. [Google Scholar] [CrossRef]
- Tatman, P.D.; Muhonen, E.G.; Wickers, S.T.; Gee, A.O.; Kim, E.S.; Kim, D.H. Self-assembling peptides for stem cell and tissue engineering. Biomater. Sci. 2016, 4, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Tian, A.; Xue, J.; Sun, N. Advantages of self-assembled nano peptide hydrogels in biological tissue engineering. Curr. Protein Pept. Sci. 2022, 23, 395–401. [Google Scholar] [CrossRef]
- Yeh, J.I.; Du, S.; Tortajada, A.; Paulo, J.; Zhang, S. Peptergents: Peptide detergents that improve stability and functionality of a membrane protein, glycerol-3-phosphate dehydrogenase. Biochemistry 2005, 44, 16912–16919. [Google Scholar] [CrossRef] [PubMed]
- Kiley, P.; Zhao, X.; Vaughn, M.; Baldo, M.A.; Bruce, B.D.; Zhang, S. Self-assembling peptide detergents stabilize isolated photosystem I on a dry surface for an extended time. PLoS Biol. 2005, 3, e230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Nagai, Y.; Reeves, P.J.; Kiley, P.; Khorana, H.G.; Zhang, S. Designer short peptide surfactants stabilize G protein-coupled receptor bovine rhodopsin. Proc. Natl. Acad. Sci. USA 2006, 103, 17707–17712. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Tay, J.J.J. Advancement of peptide nanobiotechnology via emerging microfluidic technology. Micromachines 2019, 10, 627. [Google Scholar] [CrossRef]
- McCloskey, A.P.; Gilmore, B.F.; Laverty, G. Evolution of antimicrobial peptides to self-assembled peptides for biomaterial applications. Pathogens 2014, 3, 791–821. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.; Falanga, A.; Del Genio, V.; Galdiero, S. A new hope: Self-assembling peptides with antimicrobial activity. Pharmaceutics 2019, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Chen, W.T.; Sun, T.; Gao, Y.; Li, L.L.; Wang, H. Recent advances: Peptides and self-assembled peptide-nanosystems for antimicrobial therapy and diagnosis. Biomater. Sci. 2020, 8, 4975–4996. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, G.; Cui, X.; Chen, J.; Yu, Q.; Zong, C.; Zhao, Y.; Xu, M.; Zhou, S.; Xu, H. Mechanistic investigation of a self-assembling peptide against Escherichia coli. Langmuir 2020, 36, 9800–9809. [Google Scholar] [CrossRef] [PubMed]
- Negahdaripour, M.; Golkar, N.; Hajighahramani, N.; Kianpour, S.; Nezafat, N.; Ghasemi, Y. Harnessing self-assembled peptide nanoparticles in epitope vaccine design. Biotechnol. Adv. 2017, 35, 575–596. [Google Scholar] [CrossRef]
- Zottig, X.; Al-Halifa, S.; Cote-Cyr, M.; Calzas, C.; Le Goffic, R.; Chevalier, C.; Archambault, D.; Bourgault, S. Self-assembled peptide nanorod vaccine confers protection against influenza A virus. Biomaterials 2021, 269, 120672. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Han, H.; Hudalla, G.A.; Wen, Y.; Pompano, R.R.; Collier, J.H. Thermal stability of self-assembled peptide vaccine materials. Acta Biomater. 2016, 30, 62–71. [Google Scholar] [CrossRef]
- Abudula, T.; Bhatt, K.; Eggermont, L.J.; O’Hare, N.; Memic, A.; Bencherif, S.A. Supramolecular self-assembled peptide-based vaccines: Current state and future perspectives. Front. Chem. 2020, 8, 598160. [Google Scholar] [CrossRef]
- Yokoi, H.; Kinoshita, T.; Zhang, S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc. Natl. Acad. Sci. USA 2005, 102, 8414–8419. [Google Scholar] [CrossRef]
- Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003, 21, 1171–1178. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Rodriguez, B.J.; Yang, R.; Yu, B.; Mei, D.; Li, J.; Tao, K.; Gazit, E. Microfabrication of peptide self-assemblies: Inspired by nature towards applications. Chem. Soc. Rev. 2022, 51, 6936–6947. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Hakala, T.A.; Schnaider, L.; Bernardes, G.J.; Gazit, E.; Knowles, T.P. Biomimetic peptide self-assembly for functional materials. Nat. Rev. Chem. 2020, 4, 615–634. [Google Scholar] [CrossRef]
- Galler, K.M.; Hartgerink, J.D.; Cavender, A.C.; Schmalz, G.; D’Souza, R.N. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng. Part A 2012, 18, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Afami, M.E.; El Karim, I.; About, I.; Krasnodembskaya, A.D.; Laverty, G.; Lundy, F.T. Multicomponent peptide hydrogels as an innovative platform for cell-based tissue engineering in the dental pulp. Pharmaceutics 2021, 13, 1575. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.; Grodzinsky, A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc. Natl. Acad. Sci. USA 2002, 99, 9996–10001. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Yuan, X.; Zhou, A.; Zhang, H.; Jiang, D. Designer functionalised self-assembling peptide nanofibre scaffolds for cartilage tissue engineering. Expert Rev. Mol. Med. 2014, 16, e12. [Google Scholar] [CrossRef]
- Semino, C.E. Self-assembling peptides: From bio-inspired materials to bone regeneration. J. Dent. Res. 2008, 87, 606–616. [Google Scholar] [CrossRef]
- Mari-Buye, N.; Luque, T.; Navajas, D.; Semino, C.E. Development of a three-dimensional bone-like construct in a soft self-assembling peptide matrix. Tissue Eng. Part A 2013, 19, 870–881. [Google Scholar] [CrossRef]
- Marchini, A.; Raspa, A.; Pugliese, R.; El Malek, M.A.; Pastori, V.; Lecchi, M.; Vescovi, A.L.; Gelain, F. Multifunctionalized hydrogels foster hNSC maturation in 3D cultures and neural regeneration in spinal cord injuries. Proc. Natl. Acad. Sci. USA 2019, 116, 7483–7492. [Google Scholar] [CrossRef]
- Tran, K.A.; Partyka, P.P.; Jin, Y.; Bouyer, J.; Fischer, I.; Galie, P.A. Vascularization of self-assembled peptide scaffolds for spinal cord injury repair. Acta Biomater. 2020, 104, 76–84. [Google Scholar] [CrossRef]
- Sahab Negah, S.; Oliazadeh, P.; Jahanbazi Jahan-Abad, A.; Eshaghabadi, A.; Samini, F.; Ghasemi, S.; Asghari, A.; Gorji, A. Transplantation of human meningioma stem cells loaded on a self-assembling peptide nanoscaffold containing IKVAV improves traumatic brain injury in rats. Acta Biomater. 2019, 92, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jung, Y.; Kim, S.H.; Sun, K.; Choi, J.; Kim, H.C.; Park, Y.; Kim, S.H. The enhancement of mature vessel formation and cardiac function in infarcted hearts using dual growth factor delivery with self-assembling peptides. Biomaterials 2011, 32, 6080–6088. [Google Scholar] [CrossRef] [PubMed]
- French, K.M.; Somasuntharam, I.; Davis, M.E. Self-assembling peptide-based delivery of therapeutics for myocardial infarction. Adv. Drug Deliv. Rev. 2016, 96, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Gelain, F.; Luo, Z.; Rioult, M.; Zhang, S. Self-assembling peptide scaffolds in the clinic. NPJ Regen. Med. 2021, 6, 9. [Google Scholar] [CrossRef]
- Gallo, E.; Rosa, E.; Diaferia, C.; Rossi, F.; Tesauro, D.; Accardo, A. Systematic overview of soft materials as a novel frontier for MRI contrast agents. RSC Adv. 2020, 10, 27064–27080. [Google Scholar] [CrossRef]
- Gallo, E.; Diaferia, C.; Gregorio, E.D.; Morelli, G.; Gianolio, E.; Accardo, A. Peptide-based soft hydrogels modified with gadolinium complexes as MRI contrast agents. Pharmaceuticals 2020, 13, 19. [Google Scholar] [CrossRef]
- Diaferia, C.; Gianolio, E.; Accardo, A. Peptide-based building blocks as structural elements for supramolecular Gd-containing MRI contrast agents. J. Pept. Sci. 2019, 25, e3157. [Google Scholar] [CrossRef]
- Diaferia, C.; Gianolio, E.; Accardo, A.; Morelli, G. Gadolinium containing telechelic PEG-polymers end-capped by di-phenylalanine motives as potential supramolecular MRI contrast agents. J. Pept. Sci. 2017, 23, 122–130. [Google Scholar] [CrossRef]
- Diaferia, C.; Gianolio, E.; Palladino, P.; Arena, F.; Boffa, C.; Morelli, G.; Accardo, A. Peptide materials obtained by aggregation of polyphenylalanine conjugates as gadolinium-based magnetic resonance imaging contrast agents. Adv. Funct. Mater. 2015, 25, 7003–7016. [Google Scholar] [CrossRef]
- Pugliese, R.; Fontana, F.; Marchini, A.; Gelain, F. Branched peptides integrate into self-assembled nanostructures and enhance biomechanics of peptidic hydrogels. Acta Biomater. 2018, 66, 258–271. [Google Scholar] [CrossRef]
- Antonovaite, N.; Beekmans, S.V.; Hol, E.M.; Wadman, W.J.; Iannuzzi, D. Regional variations in stiffness in live mouse brain tissue determined by depth-controlled indentation mapping. Sci. Rep. 2018, 8, 12517. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Natalello, A.; Sanii, B.; Vasita, R.; Saracino, G.; Zuckermann, R.N.; Doglia, S.M.; Gelain, F. Synthesis and characterization of designed BMHP1-derived self-assembling peptides for tissue engineering applications. Nanoscale 2013, 5, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Marchini, A.; Favoino, C.; Gelain, F. Multi-functionalized self-assembling peptides as reproducible 3D cell culture systems enabling differentiation and survival of various human neural stem cell lines. Front. Neurosci. 2020, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, M.P.; Sato, K.; Palmer, L.C.; Stupp, S.I. Supramolecular assembly of peptide amphiphiles. Acc. Chem. Res. 2017, 50, 2440–2448. [Google Scholar] [CrossRef]
- Godbe, J.M.; Freeman, R.; Burbulla, L.F.; Lewis, J.; Krainc, D.; Stupp, S.I. Gelator length precisely tunes supramolecular hydrogel stiffness and neuronal phenotype in 3D culture. ACS Biomater. Sci. Eng. 2020, 6, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Ji, W.; Palmer, L.C.; Weber, B.; Barz, M.; Stupp, S.I. Programmable assembly of peptide amphiphile via noncovalent-to-covalent bond conversion. J. Am. Chem. Soc. 2017, 139, 8995–9000. [Google Scholar] [CrossRef]
- Hulgan, S.A.H.; Jalan, A.A.; Li, I.C.; Walker, D.R.; Miller, M.D.; Kosgei, A.J.; Xu, W.; Phillips, G.N., Jr.; Hartgerink, J.D. Covalent capture of collagen triple helices using lysine-aspartate and lysine-glutamate pairs. Biomacromolecules 2020, 21, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Gelain, F. Cross-linked self-assembling peptides and their post-assembly functionalization via one-pot and in situ gelation system. Int. J. Mol. Sci. 2020, 21, 4261. [Google Scholar] [CrossRef]
- Khalily, M.A.; Goktas, M.; Guler, M.O. Tuning viscoelastic properties of supramolecular peptide gels via dynamic covalent crosslinking. Org. Biomol. Chem. 2015, 13, 1983–1987. [Google Scholar] [CrossRef]
- Pugliese, R.; Marchini, A.; Saracino, G.A.A.; Zuckermann, R.N. Cross-linked self-assembling peptide scaffolds. Nano Res. 2018, 11, 586–602. [Google Scholar] [CrossRef]
- Ciulla, M.G.; Pugliese, R.; Gelain, F. Boosted cross-linking and characterization of high-performing self-assembling peptides. Nanomaterials 2022, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Diaferia, C.; Rosa, E.; Balasco, N.; Sibillano, T.; Morelli, G.; Giannini, C.; Vitagliano, L.; Accardo, A. The introduction of a cysteine residue modulates the mechanical properties of aromatic-based solid aggregates and self-supporting hydrogels. Chemistry 2021, 27, 14886–14898. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Margheritelli, S.; Toumia, Y.; Paradossi, G.; Bordi, F.; Sennato, S.; Palocci, C. Biosynthesis and characterization of cross-linked fmoc peptide-based hydrogels for drug delivery applications. Gels 2015, 1, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Toumia, Y.; Cerroni, B.; Pandolfi, D.; Paradossi, G.; Palocci, C. Biofabrication of genipin-crosslinked peptide hydrogels and their use in the controlled delivery of naproxen. New Biotechnol. 2017, 37, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Moretti, L.; Maiuri, M.; Romanazzi, T.; Cerullo, G.; Gelain, F. Superior mechanical and optical properties of a heterogeneous library of cross-linked biomimetic self-assembling peptides. Mater. Des. 2020, 194, 108901. [Google Scholar] [CrossRef]
- Fichman, G.; Schneider, J.P. Utilizing fremy’s salt to increase the mechanical rigidity of supramolecular peptide-based gel networks. Front. Bioeng. Biotechnol. 2020, 8, 594258. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Qin, M.; Cao, Y.; Wang, W. Photo-cross-linking approach to engineering small tyrosine-containing peptide hydrogels with enhanced mechanical stability. Langmuir 2013, 29, 13299–13306. [Google Scholar] [CrossRef]
- Pugliese, R.; Montuori, M.; Gelain, F. Bioinspired photo-crosslinkable self-assembling peptides with pH-switchable “on–off” luminescence. Nanoscale Adv. 2022, 4, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Prins, L.J.; Scrimin, P. Covalent capture: Merging covalent and noncovalent synthesis. Angew. Chem. Int. Ed. 2009, 48, 2288–2306. [Google Scholar] [CrossRef]
- Montemurro, F.; De Maria, C.; Orsi, G.; Ghezzi, L.; Tine, M.R.; Vozzi, G. Genipin diffusion and reaction into a gelatin matrix for tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 473–480. [Google Scholar] [CrossRef]
- Gelain, F.; Bottai, D.; Vescovi, A.; Zhang, S. Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS ONE 2006, 1, e119. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Bonhome-Espinosa, A.B.; Vizcaino, G.; Rodriguez, I.A.; Duran-Herrera, D.; Lopez-Lopez, M.T.; Sanchez-Montesinos, I.; Alaminos, M.; Sanchez-Quevedo, M.C.; Carriel, V. Generation of genipin cross-linked fibrin-agarose hydrogel tissue-like models for tissue engineering applications. Biomed. Mater. 2018, 13, 025021. [Google Scholar] [CrossRef] [PubMed]
- Schek, R.M.; Michalek, A.J.; Iatridis, J.C. Genipin-crosslinked fibrin hydrogels as a potential adhesive to augment intervertebral disc annulus repair. Eur. Cells Mater. 2011, 21, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Dare, E.V.; Griffith, M.; Poitras, P.; Kaupp, J.A.; Waldman, S.D.; Carlsson, D.J.; Dervin, G.; Mayoux, C.; Hincke, M.T. Genipin cross-linked fibrin hydrogels for in vitro human articular cartilage tissue-engineered regeneration. Cells Tissues Organs 2009, 190, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.H.; Narayanan, A.; Hett, E.C. Understanding and applying tyrosine biochemical diversity. Mol. Biosyst. 2014, 10, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Partlow, B.P.; Applegate, M.B.; Omenetto, F.G.; Kaplan, D.L. Dityrosine cross-linking in designing biomaterials. ACS Biomater. Sci. Eng. 2016, 2, 2108–2121. [Google Scholar] [CrossRef]
- Jang, H.S.; Lee, J.H.; Park, Y.S.; Kim, Y.O.; Park, J.; Yang, T.Y.; Jin, K.; Lee, J.; Park, S.; You, J.M.; et al. Tyrosine-mediated two-dimensional peptide assembly and its role as a bio-inspired catalytic scaffold. Nat. Commun. 2014, 5, 3665. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, R. Supramolecular-Covalent Peptides Self-Assembly: From Design to Regenerative Medicine and Beyond. Biophysica 2022, 2, 324-339. https://doi.org/10.3390/biophysica2040030
Pugliese R. Supramolecular-Covalent Peptides Self-Assembly: From Design to Regenerative Medicine and Beyond. Biophysica. 2022; 2(4):324-339. https://doi.org/10.3390/biophysica2040030
Chicago/Turabian StylePugliese, Raffaele. 2022. "Supramolecular-Covalent Peptides Self-Assembly: From Design to Regenerative Medicine and Beyond" Biophysica 2, no. 4: 324-339. https://doi.org/10.3390/biophysica2040030
APA StylePugliese, R. (2022). Supramolecular-Covalent Peptides Self-Assembly: From Design to Regenerative Medicine and Beyond. Biophysica, 2(4), 324-339. https://doi.org/10.3390/biophysica2040030








