Sodium Channels Involved in the Initiation of Action Potentials in Invertebrate and Mammalian Neurons
Abstract
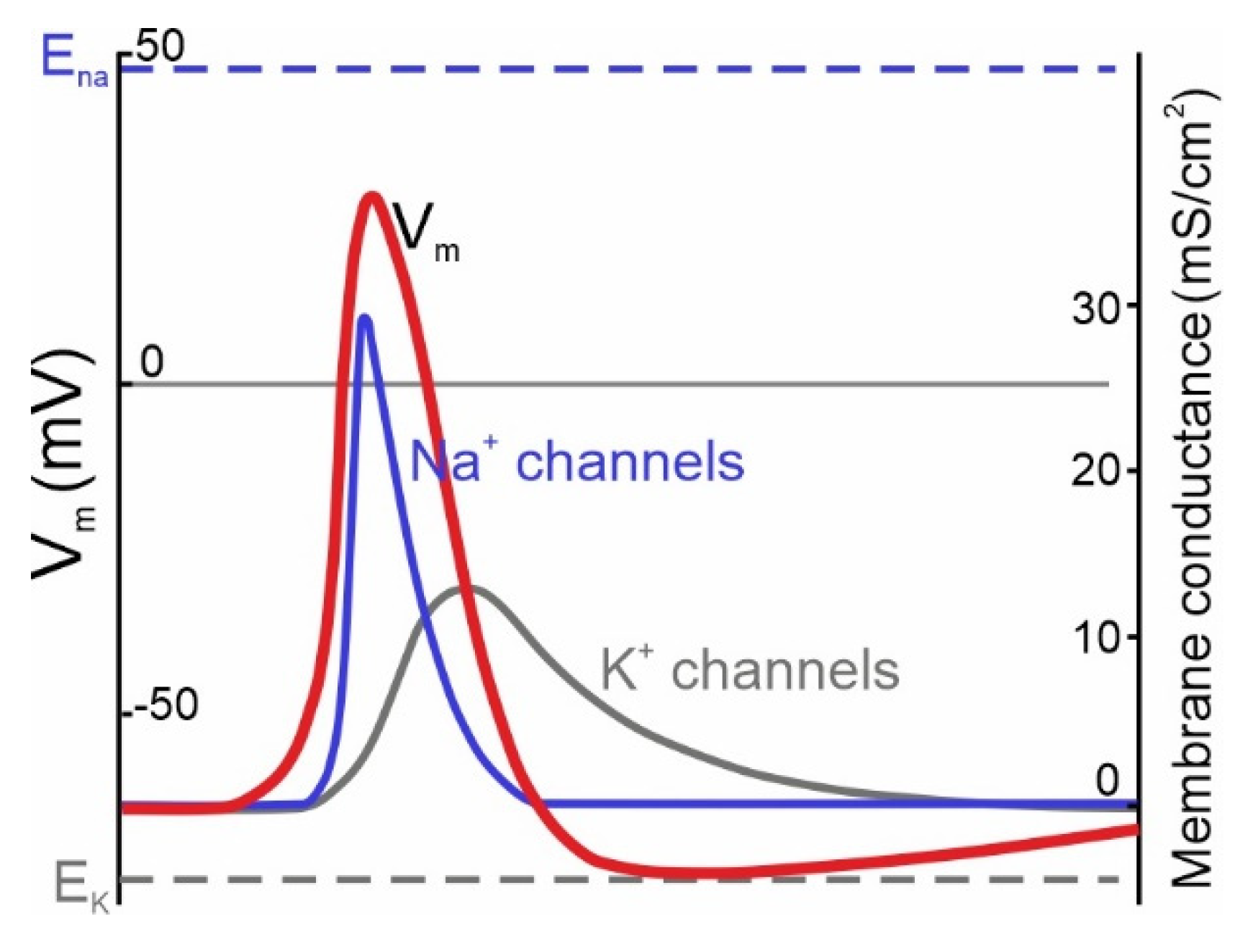
:1. Theoretical Role of NaV Channels in AP Initiation
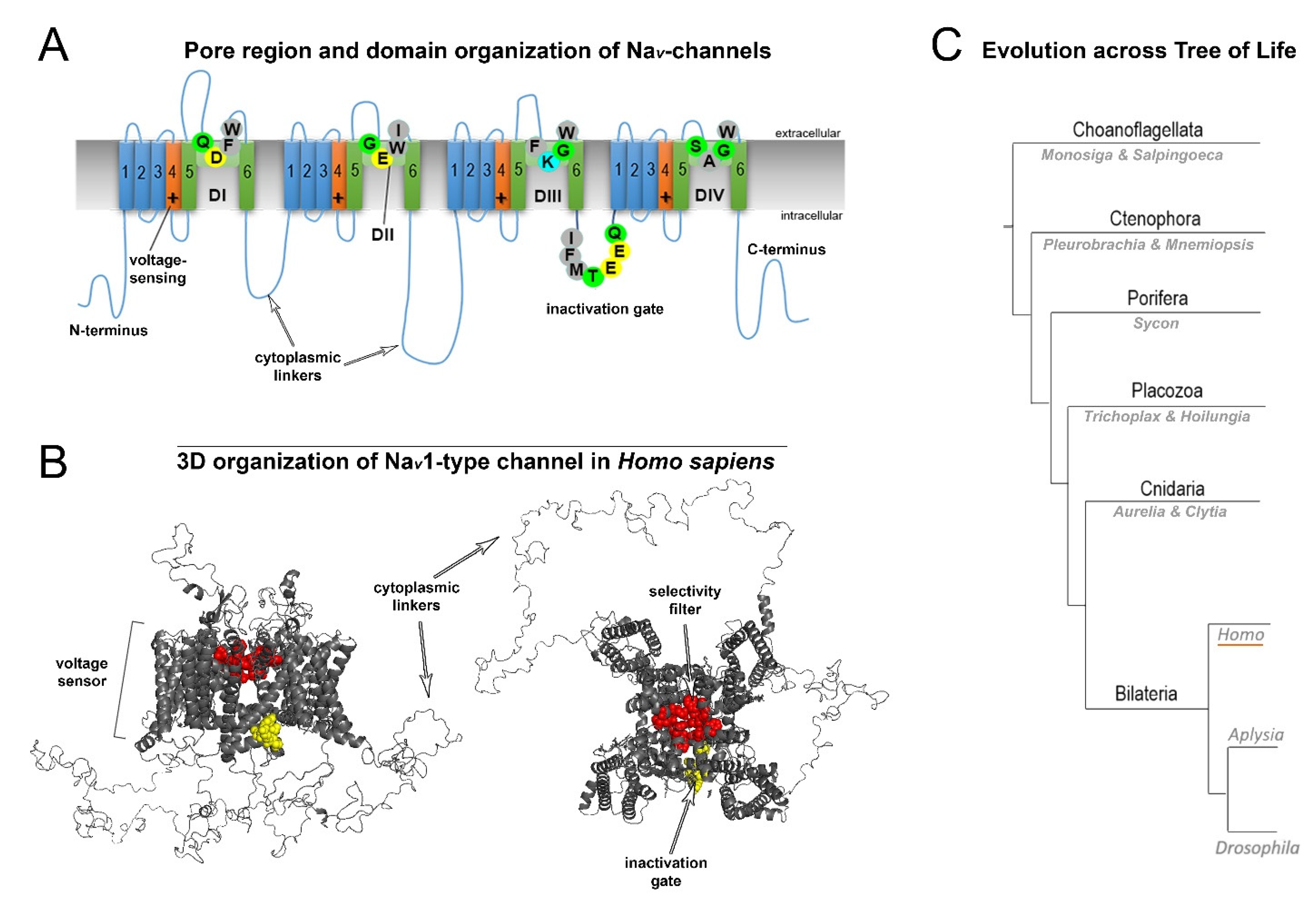
2. Normal Structure of Human NaV Channels and Their Pathological Mutations
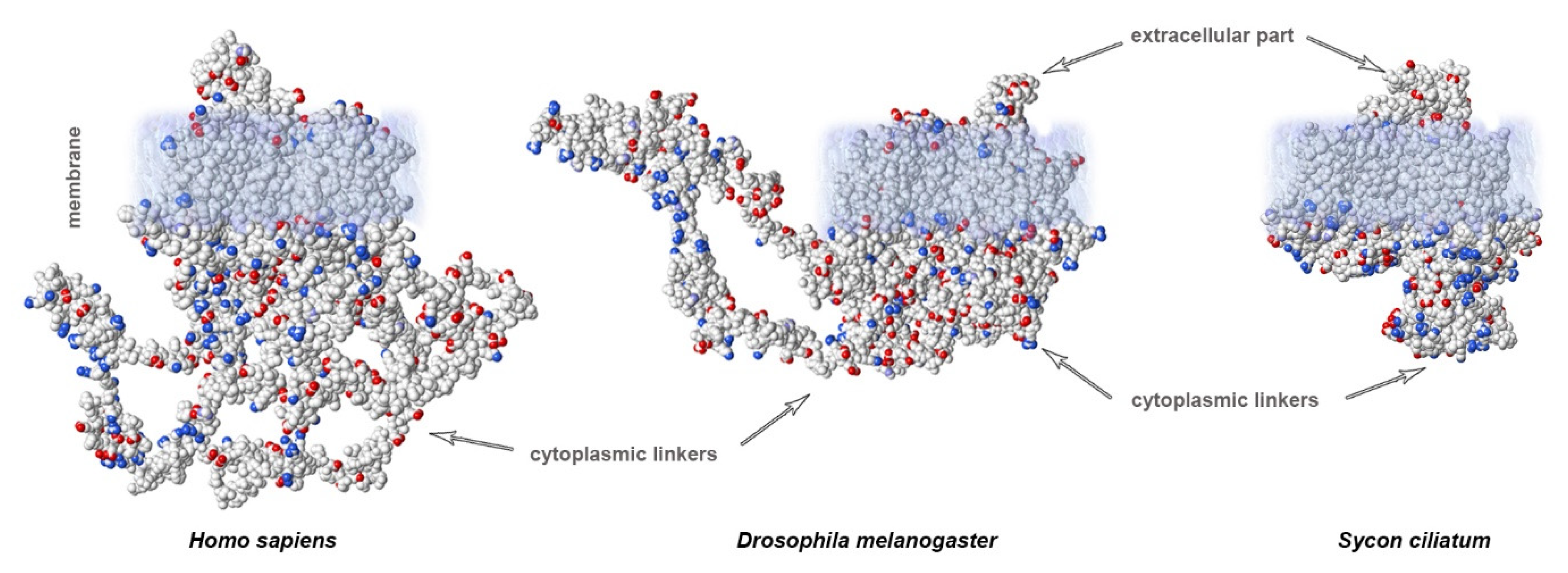
3. Variability of Linkers of NaV Channels in the Evolutionary Tree
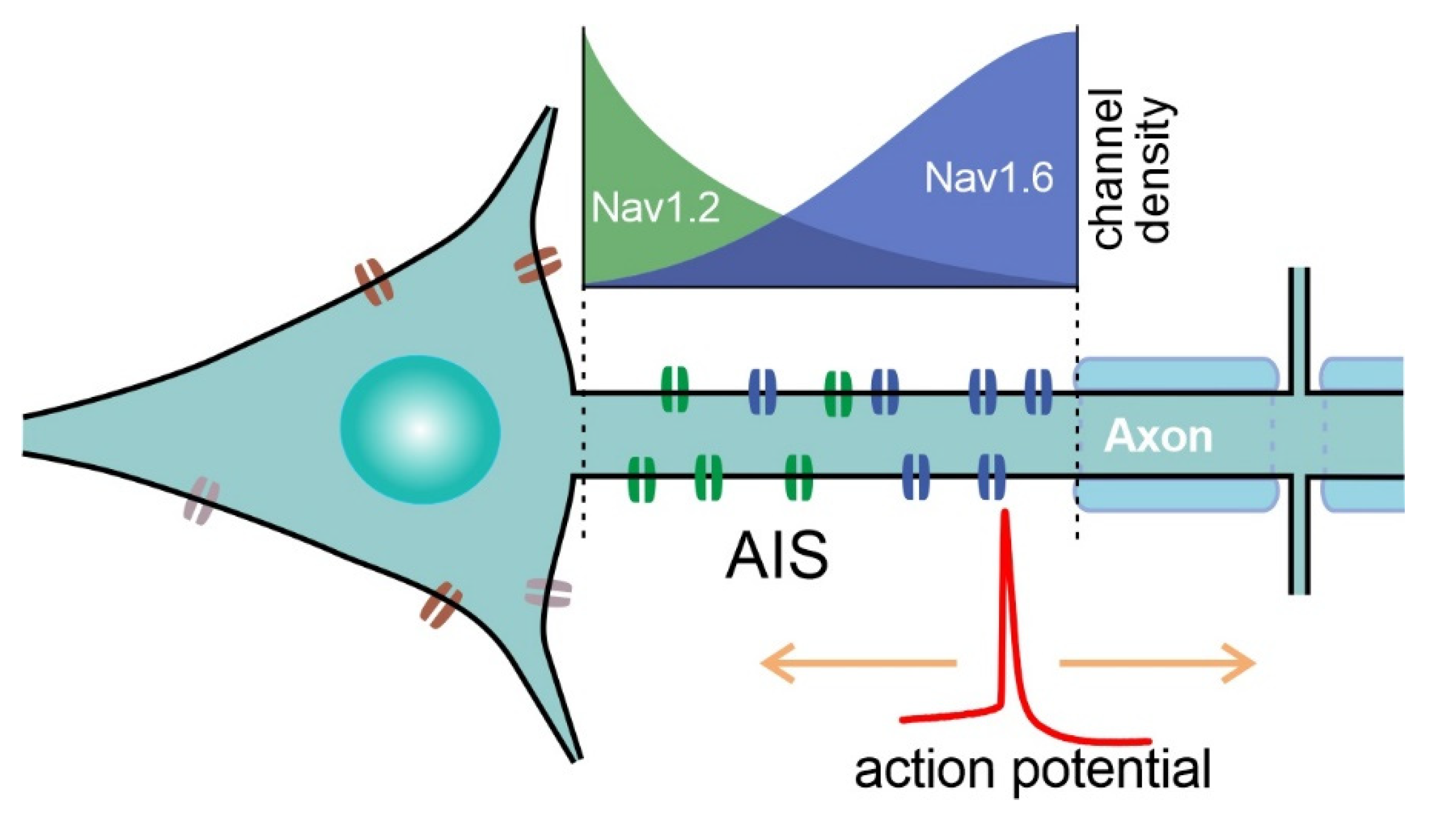
4. Sites of AP Initiation in Mammalian Neurons
5. Specific NaV Channels Involved in AP Initiation in the CNS of Mammals
6. Crucial Role of Nav1.6 in AP Initiation by Principal Neurons of the Cortex
7. NaV1.1 Function in GABA-ergic Neurons
8. Important NaV Channels of Peripheral Neurons
9. NaV Channels Underlying AP Initiation in Insects
10. Fast Sodium Currents in Mollusks
11. NaV Channels of Archaic Invertebrates and Photosynthetic Eucaryotes
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
- Kole, M.H.; Letzkus, J.J.; Stuart, G.J. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron 2007, 55, 633–647. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.A.; Shu, Y.; Yu, Y. Neurophysiology: Hodgkin and Huxley model—Still standing? Nature 2007, 445, E1–E2; discussion E2–E3. [Google Scholar] [CrossRef] [PubMed]
- Levitan, I.B.; Kaczmarek, L.K. The Neuron: Cell and Molecular Biology, 2nd ed.; Oxford University Press: New York, NY, USA, 1997; p. xii. 543p. [Google Scholar]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-gated sodium channels: Structure, function, pharmacology, and clinical indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef]
- Encinas, A.C.; Watkins, J.C.; Longoria, I.A.; Johnson, J.P., Jr.; Hammer, M.F. Variable patterns of mutation density among NaV1.1, NaV1.2 and NaV1.6 point to channel-specific functional differences associated with childhood epilepsy. PLoS ONE 2020, 15, e0238121. [Google Scholar] [CrossRef]
- Stuart, G.; Schiller, J.; Sakmann, B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J. Physiol. 1997, 505, 617–632. [Google Scholar] [CrossRef]
- Palmer, L.M.; Stuart, G.J. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci 2006, 26, 1854–1863. [Google Scholar] [CrossRef]
- Nikitin, E.S.; Malyshev, A.Y.; Balaban, P.M.; Volgushev, M.A. Physiological aspects of the use of the Hodgkin-Huxley model of action potential generation for neurons in invertebrates and vertebrates. Neurosci. Behav. Physiol. 2017, 47, 751–757. [Google Scholar] [CrossRef]
- Naundorf, B.; Wolf, F.; Volgushev, M. Unique features of action potential initiation in cortical neurons. Nature 2006, 440, 1060–1063. [Google Scholar] [CrossRef]
- Shu, Y.; Duque, A.; Yu, Y.; Haider, B.; McCormick, D.A. Properties of action-potential initiation in neocortical pyramidal cells: Evidence from whole cell axon recordings. J. Neurophysiol. 2007, 97, 746–760. [Google Scholar] [CrossRef]
- Jansen, R.F.; Pieneman, A.W.; Ter Maat, A. Spontaneous switching between ortho- and antidromic spiking as the normal mode of firing in the cerebral giant neurons of freely behaving Lymnaea stagnalis. J. Neurophysiol. 1996, 76, 4206–4209. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.A.; Monsivais, P.; Branco, T.; London, M.; Häusser, M. The site of action potential initiation in cerebellar Purkinje neurons. Nat. Neurosci. 2005, 8, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Foust, A.; Popovic, M.; Zecevic, D.; McCormick, D.A. Action potentials initiate in the axon initial segment and propagate through axon collaterals reliably in cerebellar Purkinje neurons. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 6891–6902. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, E.; Dannemeyer, M.; Feulner, B.; Enderlein, J.; Gutnick, M.J.; Wolf, F.; Neef, A. An axon initial segment is required for temporal precision in action potential encoding by neuronal populations. Sci. Adv. 2018, 4, eaau8621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hofmann, D.; Neef, A.; Wolf, F. Ultrafast population coding and axo-somatic compartmentalization. PLoS Comput. Biol. 2022, 18, e1009775. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Lenaeus, M.J.; Gamal El-Din, T.M. Structure and pharmacology of voltage-gated sodium and calcium channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Ilin, V.; Malyshev, A.; Wolf, F.; Volgushev, M. Fast computations in cortical ensembles require rapid initiation of action potentials. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Shirahata, E.; Iwasaki, H.; Takagi, M.; Lin, C.; Bennett, V.; Okamura, Y.; Hayasaka, K. Ankyrin-G regulates inactivation gating of the neuronal sodium channel, Nav1.6. J Neurophysiol 2006, 96, 1347–1357. [Google Scholar] [CrossRef]
- Vanoye, C.G.; Lossin, C.; Rhodes, T.H.; George, A.L., Jr. Single-channel properties of human NaV1.1 and mechanism of channel dysfunction in SCN1A-associated epilepsy. J. Gen. Physiol. 2005, 127, 1–14. [Google Scholar] [CrossRef]
- Kawai, T.; Hashimoto, M.; Eguchi, N.; Nishino, J.M.; Jinno, Y.; Mori-Kreiner, R.; Aspåker, M.; Chiba, D.; Ohtsuka, Y.; Kawanabe, A.; et al. Heterologous functional expression of ascidian Nav1 channels and close relationship with the evolutionary ancestor of vertebrate Nav channels. J. Biol. Chem. 2021, 296, 100783. [Google Scholar] [CrossRef]
- Billen, B.; Vassilevski, A.; Nikolsky, A.; Debaveye, S.; Tytgat, J.; Grishin, E. Unique bell-shaped voltage-dependent modulation of Na+ channel gating by novel insect-selective toxins from the spider Agelena orientalis. J. Biol. Chem. 2010, 285, 18545–18554. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nomura, Y.; Du, Y.; Dong, K. Differential effects of TipE and a TipE-homologous protein on modulation of gating properties of sodium channels from Drosophila melanogaster. PLoS ONE 2013, 8, e67551. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, K.E.; Chrachri, A.; Koester, J.A.; Wharam, S.; Taylor, A.R.; Wheeler, G.L.; Brownlee, C. A Novel Single-Domain Na(+)-Selective Voltage-Gated Channel in Photosynthetic Eukaryotes. Plant Physiol 2020, 184, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Staras, K.; Gyori, J.; Kemenes, G. Voltage-gated ionic currents in an identified modulatory cell type controlling molluscan feeding. Eur. J. Neurosci. 2002, 15, 109–119. [Google Scholar] [CrossRef]
- Nikitin, E.S.; Kiss, T.; Staras, K.; O’Shea, M.; Benjamin, P.R.; Kemenes, G. Persistent sodium current is a target for cAMP-induced neuronal plasticity in a state-setting modulatory interneuron. J. Neurophysiol. 2006, 95, 453–463. [Google Scholar] [CrossRef]
- Gilly, W.F.; Gillette, R.; McFarlane, M. Fast and slow activation kinetics of voltage-gated sodium channels in molluscan neurons. J. Neurophysiol. 1997, 77, 2373–2384. [Google Scholar] [CrossRef]
- Sole, L.; Tamkun, M.M. Trafficking mechanisms underlying Nav channel subcellular localization in neurons. Channels 2020, 14, 1–17. [Google Scholar] [CrossRef]
- Trimmer, J.S.; Rhodes, K.J. Localization of voltage-gated ion channels in mammalian brain. Annu. Rev. Physiol. 2004, 66, 477–519. [Google Scholar] [CrossRef]
- Hu, W.; Tian, C.; Li, T.; Yang, M.; Hou, H.; Shu, Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat. Neurosci. 2009, 12, 996–1002. [Google Scholar] [CrossRef]
- Wang, J.; Ou, S.-W.; Wang, Y.-J. Distribution and function of voltage-gated sodium channels in the nervous system. Channels 2017, 11, 534–554. [Google Scholar] [CrossRef]
- Katz, E.; Stoler, O.; Scheller, A.; Khrapunsky, Y.; Goebbels, S.; Kirchhoff, F.; Gutnick, M.J.; Wolf, F.; Fleidervish, I.A. Role of sodium channel subtype in action potential generation by neocortical pyramidal neurons. Proc. Natl. Acad. Sci. USA 2018, 115, E7184–E7192. [Google Scholar] [CrossRef] [PubMed]
- Zybura, A.; Hudmon, A.; Cummins, T.R. Distinctive properties and powerful neuromodulation of Nav1.6 sodium channels regulates neuronal excitability. Cells 2021, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Royeck, M.; Horstmann, M.T.; Remy, S.; Reitze, M.; Yaari, Y.; Beck, H. Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J. Neurophysiol. 2008, 100, 2361–2380. [Google Scholar] [CrossRef] [PubMed]
- Ierusalimsky, V.N.; Balaban, P.M.; Nikitin, E.S. Nav1.6 but not KCa3.1 channels contribute to heterogeneity in coding abilities and dynamics of action potentials in the L5 neocortical pyramidal neurons. Biochem. Biophys. Res. Commun. 2022, 615, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.R.; Cho, M.-H.; Ullian, E.M.; Isom, L.L.; Levinson, S.R.; Barres, B.A. Differential control of clustering of the sodium channels Nav1.2 and Nav1.6 at developing CNS nodes of Ranvier. Neuron 2001, 30, 105–119. [Google Scholar] [CrossRef]
- Kaczmarek, L.K. The Na+ paradox: Reducing sodium currents increases excitability. Trends Neurosci. 2021, 44, 767–768. [Google Scholar] [CrossRef]
- Spratt, P.W.E.; Alexander, R.P.D.; Ben-Shalom, R.; Sahagun, A.; Kyoung, H.; Keeshen, C.M.; Sanders, S.J.; Bender, K.J. Paradoxical hyperexcitability from Na(V)1.2 sodium channel loss in neocortical pyramidal cells. Cell Rep. 2021, 36, 109483. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Eaton, M.; Wu, J.; Ma, Z.; Lai, S.; Park, A.; Ahmad, T.S.; Que, Z.; Lee, J.H.; et al. Severe deficiency of the voltage-gated sodium channel Na(V)1.2 elevates neuronal excitability in adult mice. Cell Rep. 2021, 36, 109495. [Google Scholar] [CrossRef]
- Blanchard, M.G.; Willemsen, M.H.; Walker, J.B.; Dib-Hajj, S.D.; Waxman, S.G.; Jongmans, M.C.; Kleefstra, T.; van de Warrenburg, B.P.; Praamstra, P.; Nicolai, J.; et al. De novo gain-of-function and loss-of-function mutations of SCN8A in patients with intellectual disabilities and epilepsy. J. Med. Genet. 2015, 52, 330–337. [Google Scholar] [CrossRef]
- Wang, J.; Ou, S.W.; Bai, Y.F.; Wang, Y.J.; Xu, Z.Q.D.; Luan, G.M. Multiple Nav1.5 isoforms are functionally expressed in the brain and present distinct expression patterns compared with cardiac Nav1.5. Mol. Med. Rep. 2017, 16, 719–729. [Google Scholar] [CrossRef]
- Duflocq, A.; Le Bras, B.; Bullier, E.; Couraud, F.; Davenne, M. Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Mol. Cell. Neurosci. 2008, 39, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tian, C.; Scalmani, P.; Frassoni, C.; Mantegazza, M.; Wang, Y.; Yang, M.; Wu, S.; Shu, Y. Action potential initiation in neocortical inhibitory interneurons. PLoS Biol. 2014, 12, e1001944. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, A.; Nusser, Z. Cell-type-dependent molecular composition of the axon initial segment. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 14329–14340. [Google Scholar] [CrossRef]
- Ogiwara, I.; Miyamoto, H.; Morita, N.; Atapour, N.; Mazaki, E.; Inoue, I.; Takeuchi, T.; Itohara, S.; Yanagawa, Y.; Obata, K.; et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: A circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 5903–5914. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, B.; Gao, N.; Li, H.; Wang, H.; Li, L.; Cui, W.; Zhang, L.; Sun, D.; Liu, F.; et al. Neddylation stabilizes Nav1.1 to maintain interneuron excitability and prevent seizures in murine epilepsy models. J. Clin. Invest. 2021, 131, e136956. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Paşca, S.P.; Portmann, T.; Goold, C.; Worringer, K.A.; Guan, W.; Chan, K.C.; Gai, H.; Vogt, D.; Chen, Y.J.; et al. A deleterious Nav1.1 mutation selectively impairs telencephalic inhibitory neurons derived from Dravet Syndrome patients. eLife 2016, 5, e13073. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Kalume, F.; Oakley, J.C. NaV1.1 channels and epilepsy. J. Physiol. 2010, 588, 1849–1859. [Google Scholar] [CrossRef]
- Kalume, F.; Yu, F.H.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: Implications for ataxia in severe myoclonic epilepsy in infancy. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 11065–11074. [Google Scholar] [CrossRef]
- Yu, F.H.; Mantegazza, M.; Westenbroek, R.E.; Robbins, C.A.; Kalume, F.; Burton, K.A.; Spain, W.J.; McKnight, G.S.; Scheuer, T.; Catterall, W.A. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat. Neurosci. 2006, 9, 1142–1149. [Google Scholar] [CrossRef]
- Amaya, F.; Wang, H.; Costigan, M.; Allchorne, A.J.; Hatcher, J.P.; Egerton, J.; Stean, T.; Morisset, V.; Grose, D.; Gunthorpe, M.J.; et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 12852–12860. [Google Scholar] [CrossRef]
- Klein, A.H.; Vyshnevska, A.; Hartke, T.V.; De Col, R.; Mankowski, J.L.; Turnquist, B.; Bosmans, F.; Reeh, P.W.; Schmelz, M.; Carr, R.W.; et al. Sodium channel Na(v)1.8 underlies TTX-resistant axonal action potential conduction in somatosensory C-fibers of distal cutaneous nerves. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 5204–5214. [Google Scholar] [CrossRef] [PubMed]
- McDermott, L.A.; Weir, G.A.; Themistocleous, A.C.; Segerdahl, A.R.; Blesneac, I.; Baskozos, G.; Clark, A.J.; Millar, V.; Peck, L.J.; Ebner, D.; et al. Defining the functional role of Na(V)1.7 in human nociception. Neuron 2019, 101, 905–919.e908. [Google Scholar] [CrossRef] [PubMed]
- Middleton, S.J.; Perini, I.; Andreas, C.T.; Weir, G.A.; McCann, K.; Barry, A.M.; Marshall, A.; Lee, M.; Mayo, L.M.; Bohic, M.; et al. Nav1.7 is required for normal C-low threshold mechanoreceptor function in humans and mice. Brain J. Neurol. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.; McMahon, S.B. The physiological function of different voltage-gated sodium channels in pain. Nat. Rev. Neurosci. 2021, 22, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Fux, J.E.; Mehta, A.; Moffat, J.; Spafford, J.D. Eukaryotic voltage-gated sodium channels: On their origins, asymmetries, losses, diversification and adaptations. Front. Physiol. 2018, 9, 1406. [Google Scholar] [CrossRef]
- Cummins, T.R.; Aglieco, F.; Renganathan, M.; Herzog, R.I.; Dib-Hajj, S.D.; Waxman, S.G. Nav1.3 sodium channels: Rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 5952–5961. [Google Scholar] [CrossRef]
- Hains, B.C.; Klein, J.P.; Saab, C.Y.; Craner, M.J.; Black, J.A.; Waxman, S.G. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 8881–8892. [Google Scholar] [CrossRef]
- Vandael, D.H.; Ottaviani, M.M.; Legros, C.; Lefort, C.; Guérineau, N.C.; Allio, A.; Carabelli, V.; Carbone, E. Reduced availability of voltage-gated sodium channels by depolarization or blockade by tetrodotoxin boosts burst firing and catecholamine release in mouse chromaffin cells. J. Physiol. 2015, 593, 905–927. [Google Scholar] [CrossRef]
- Loughney, K.; Kreber, R.; Ganetzky, B. Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell 1989, 58, 1143–1154. [Google Scholar] [CrossRef]
- Dong, K. Insect sodium channels and insecticide resistance. Invertebr. Neurosci. 2007, 7, 17–30. [Google Scholar] [CrossRef]
- Thompson, A.J.; Verdin, P.S.; Burton, M.J.; Davies, T.G.E.; Williamson, M.S.; Field, L.M.; Baines, R.A.; Mellor, I.R.; Duce, I.R. The effects of knock-down resistance mutations and alternative splicing on voltage-gated sodium channels in Musca domestica and Drosophila melanogaster. Insect Biochem. Mol. Biol. 2020, 122, 103388. [Google Scholar] [CrossRef] [PubMed]
- Field, L.M.; Emyr Davies, T.G.; O’Reilly, A.O.; Williamson, M.S.; Wallace, B.A. Voltage-gated sodium channels as targets for pyrethroid insecticides. Eur. Biophys. J. 2017, 46, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Romanova, D.Y.; Smirnov, I.V.; Nikitin, M.A.; Kohn, A.B.; Borman, A.I.; Malyshev, A.Y.; Balaban, P.M.; Moroz, L.L. Sodium action potentials in placozoa: Insights into behavioral integration and evolution of nerveless animals. Biochem. Biophys. Res. Commun. 2020, 532, 120–126. [Google Scholar] [CrossRef] [PubMed]
| Channel/Current | Threshold: Approx. mV | Peak, mV | Time to ½ Peak, ms | References |
|---|---|---|---|---|
| NaV1.6/AnkG | −45 | −10 | ~0.25 | [19] |
| NaV1.2 | −40 | −5 | ~0.3 | [19] |
| NaV1.1 | −40 | 0 | ~0.28 | [20] |
| Ciona NaV1a | −30 | 5 | ~0.5 | [21] |
| Insect DmNaV1/tipE | −45 | −5 | ~1.1–1.2 | [22,23] |
| Plant EukCatB | −90 | −40 | ~0.6 | [24] |
| Lymnaea INaT | −55 | −35 | ~2.5 | [25,26] |
| Loligo INaT | −35 | −5 | ~0.6 | [27] |
| Aplysia INaT | −30 | 0 | ~0.9 | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanova, D.Y.; Balaban, P.M.; Nikitin, E.S. Sodium Channels Involved in the Initiation of Action Potentials in Invertebrate and Mammalian Neurons. Biophysica 2022, 2, 184-193. https://doi.org/10.3390/biophysica2030019
Romanova DY, Balaban PM, Nikitin ES. Sodium Channels Involved in the Initiation of Action Potentials in Invertebrate and Mammalian Neurons. Biophysica. 2022; 2(3):184-193. https://doi.org/10.3390/biophysica2030019
Chicago/Turabian StyleRomanova, Daria Y, Pavel M Balaban, and Evgeny S Nikitin. 2022. "Sodium Channels Involved in the Initiation of Action Potentials in Invertebrate and Mammalian Neurons" Biophysica 2, no. 3: 184-193. https://doi.org/10.3390/biophysica2030019
APA StyleRomanova, D. Y., Balaban, P. M., & Nikitin, E. S. (2022). Sodium Channels Involved in the Initiation of Action Potentials in Invertebrate and Mammalian Neurons. Biophysica, 2(3), 184-193. https://doi.org/10.3390/biophysica2030019






