Triglyceride Saturation in Patients at Risk of NASH and NAFLD: A Cross-Sectional Study
Abstract
:1. Introduction
2. Methods
2.1. Patient Group
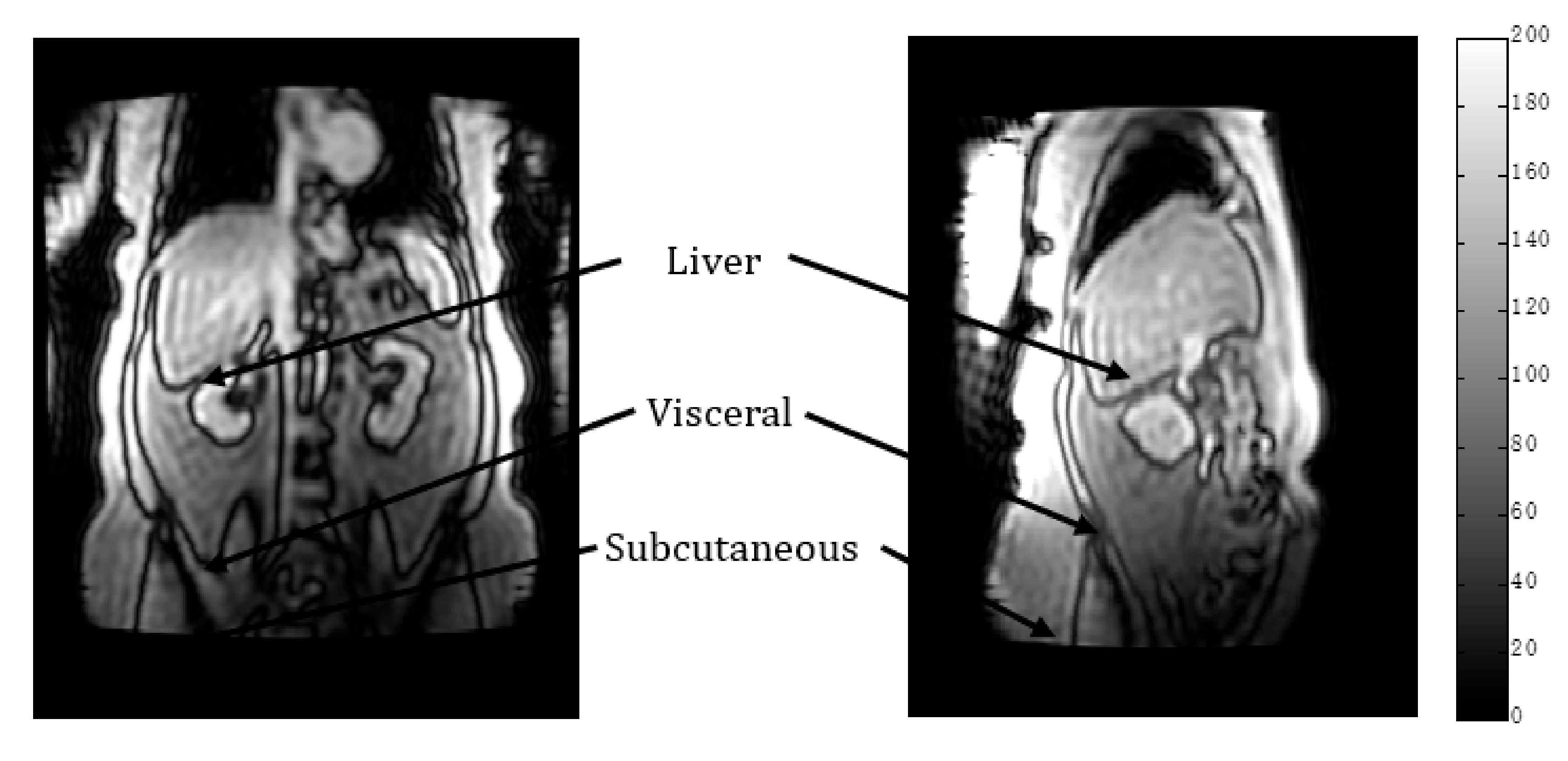
2.2. MR Methodology
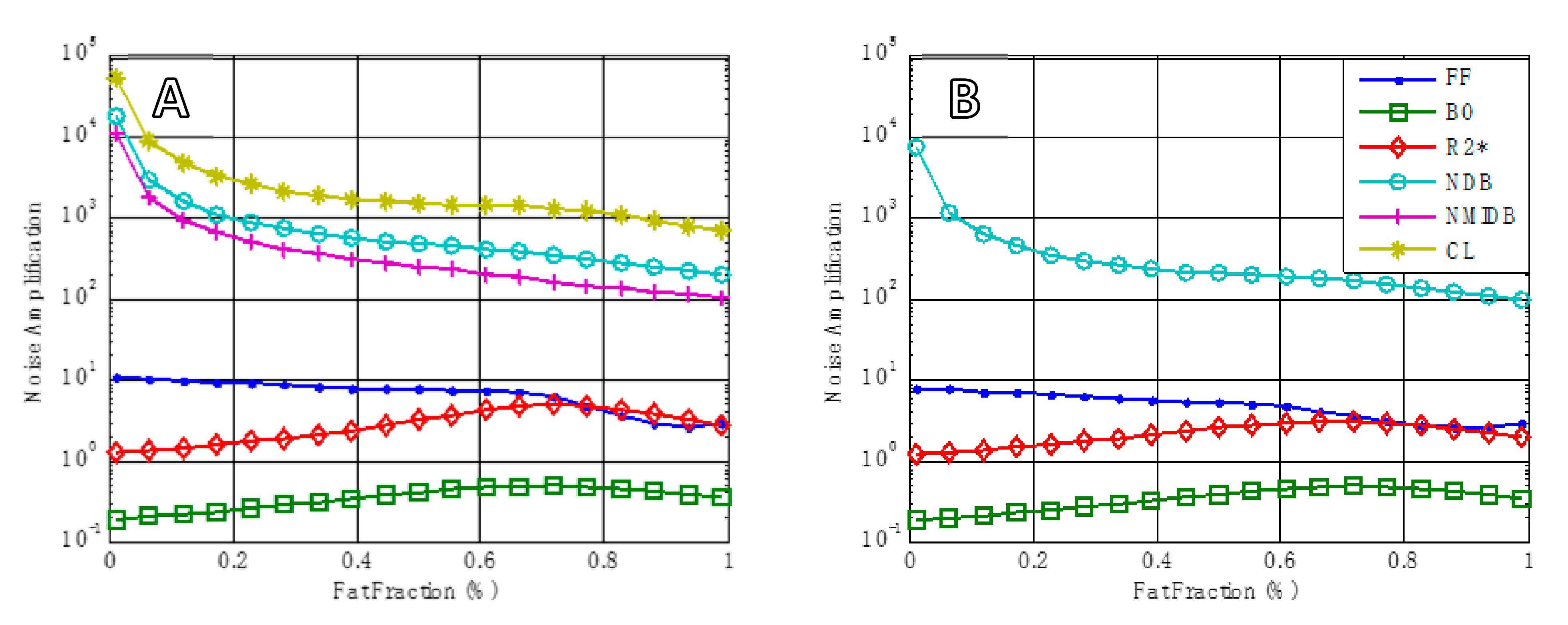
2.3. Statistical Tests
- (1)
- The following data were recorded:
- (a)
- Spectroscopy NDB: in subcutaneous tissue and visceral tissue.
- (b)
- Imaging NDB (pixel by pixel): regions of interest were drawn in subcutaneous tissue and visceral tissue; mean values were recorded.
- (c)
- Imaging (global): NDB.
- (d)
- Histology: NAS (NAFLD Activity Score) based on Ref [31].
- (2)
- The following correlations were performed:
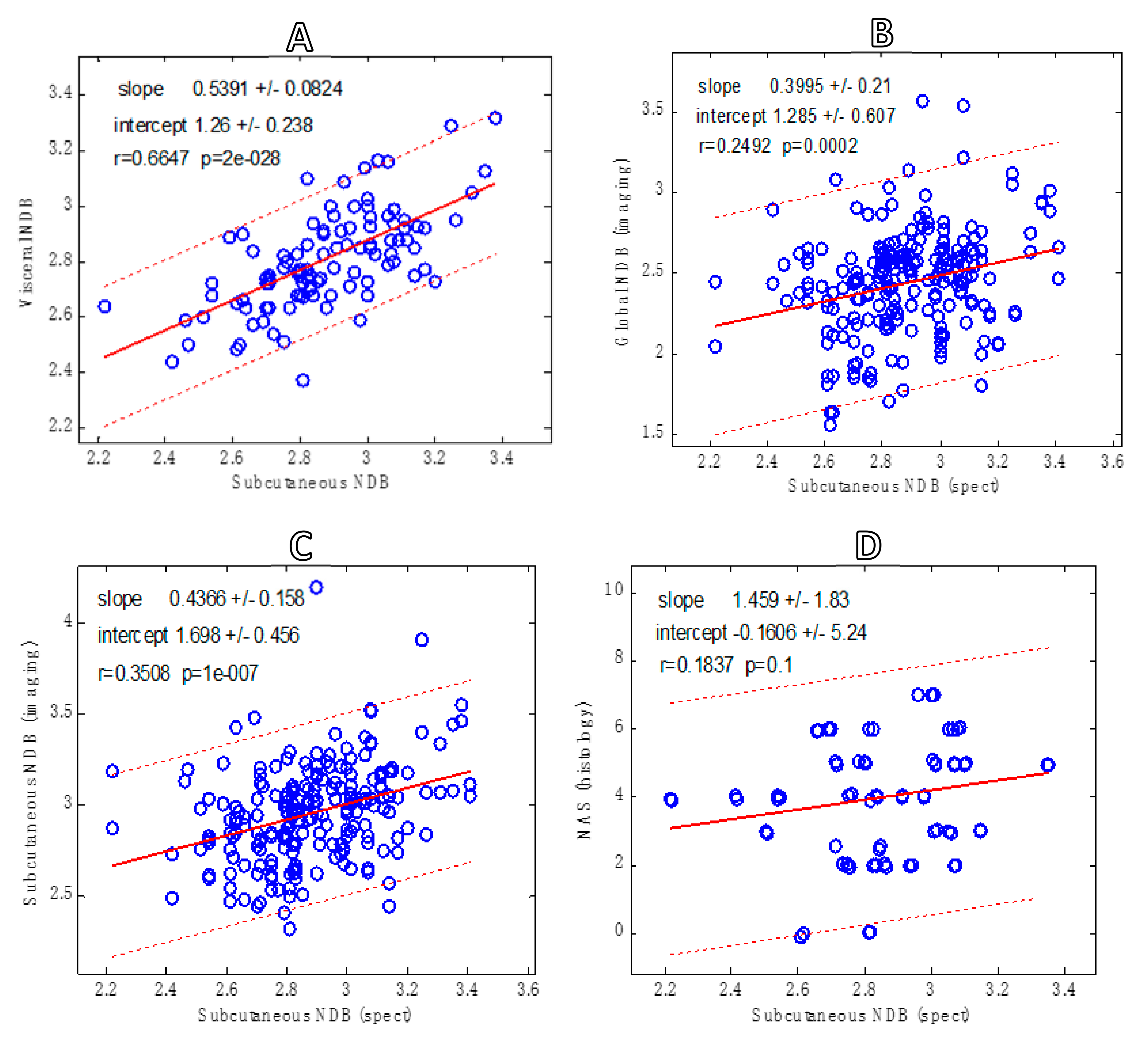
- (a)
- Spectroscopy: NDB in subcutaneous versus visceral tissue.
- (b)
- Imaging: global NDB versus subcutaneous.
- (c)
- Spectroscopy NDB versus imaging NDB (subcutaneous).
- (d)
- Spectroscopy NDB versus NAS.
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bydder, M.; Yokoo, T.; Hamilton, G.; Middleton, M.S.; Chavez, A.D.; Schwimmer, J.; Lavine, J.E.; Sirlin, C. Relaxation effects in the quantification of fat using gradient echo imaging. Magn. Reson. Imaging 2008, 26, 347–359. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Shimakawa, A.; McKenzie, C.; Brodsky, E.; Brittain, J.H.; Reeder, S. Multiecho water-fat separation and simultaneous R2 estimation with multifrequency fat spectrum modeling. Magn. Reson. Med. 2008, 60, 1122–1134. [Google Scholar] [CrossRef] [Green Version]
- Reeder, S.B.; Hu, H.H.; Sirlin, C. Proton density fat-fraction: A standardized mr-based biomarker of tissue fat concentration. J. Magn. Reson. Imaging 2012, 36, 1011–1014. [Google Scholar] [CrossRef]
- Bashir, M.R.; Zhong, X.; Nickel, M.D.; Fananapazir, G.; Kannengiesser, S.A.R.; Kiefer, B.; Dale, B.M. Quantification of Hepatic Steatosis With a Multistep Adaptive Fitting MRI Approach: Prospective Validation Against MR Spectroscopy. Am. J. Roentgenol. 2015, 204, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Reeder, S.B.; Sirlin, C.B.; Loomba, R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology 2018, 68, 763–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoo, T.; Serai, S.D.; Pirasteh, A.; Bashir, M.R.; Hamilton, G.; Hernando, D.; Hu, H.H.; Hetterich, H.; Kühn, J.-P.; Kukuk, G.M.; et al. Linearity, Bias, and Precision of Hepatic Proton Density Fat Fraction Measurements by Using MR Imaging: A Meta-Analysis. Radiology 2018, 286, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.B.; Cruite, I.; Hamilton, G.; Sirlin, C.B. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J. Magn. Reson Imaging 2011, 34, 729–749. [Google Scholar] [CrossRef]
- Ren, J.; Dimitrov, I.; Sherry, D.; Malloy, C.R. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J. Lipid Res. 2008, 49, 2055–2062. Available online: http://www.jlr.org/lookup/doi/10.1194/jlr.D800010-JLR200 (accessed on 23 September 2021). [CrossRef] [Green Version]
- Hamilton, G.; Yokoo, T.; Bydder, M.; Cruite, I.; Schroeder, M.E.; Sirlin, C.B.; Middleton, M.S. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed. 2011, 24, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, I.; Douglas, D.; Ren, J.; Smith, N.B.; Webb, A.G.; Sherry, D.; Malloy, C.R. In vivo determination of human breast fat composition by 1H magnetic resonance spectroscopy at 7 T. Magn. Reson. Med. 2012, 67, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, G.; Smith, D.L., Jr.; Bydder, M.; Nayak, K.; Hu, H.H. MR properties of brown and white adipose tissues. J. Magn. Reson. Imaging 2011, 34, 468–473. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, G.; Bs, A.N.S.; Middleton, M.S.; Bs, C.A.H.; Wolfson, T.; Gamst, A.C.; Loomba, R.; Sirlin, C.B. In vivo triglyceride composition of abdominal adipose tissue measured by 1 H MRS at 3T. J. Magn. Reson. Imaging 2017, 45, 1455–1463. [Google Scholar] [CrossRef] [Green Version]
- Bydder, M.; Girard, O.; Hamilton, G. Mapping the double bonds in triglycerides. Magn. Reson. Imaging 2011, 29, 1041–1046. [Google Scholar] [CrossRef]
- Peterson, P.; Månsson, S. Simultaneous quantification of fat content and fatty acid composition using MR imaging. Magn. Reson. Med. 2013, 69, 688–697. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Valentić, S.; Šestan, M.; Turk Wensveen, T.; Polić, B. Interactions between adipose tissue and the immune system in health and malnutrition. Semin. Immunol. 2015, 27, 322–333. [Google Scholar] [CrossRef]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef] [PubMed]
- Victor, T.A.; Bergman, A.; Knop, R.H. Detecting fatty acids of dietary origin in normal and cancerous human breast tissue by 13C nuclear magnetic resonance spectroscopy. Br. J. Cancer 1993, 68, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darabi, M.; Pezeshkian, M.; Noori, M.; Najjarpour-Jabbari, H.; Abolfathi, A.; Darabi, M.; Darabi, M.; Shaaker, M.; Shahmohammadi, G. Fatty acid composition of epicardial and subcutaneous human adipose tissue. Metab. Syndr. Relat. Disord. 2009, 7, 125–132. [Google Scholar]
- Lundbom, J.; Hakkarainen, A.; Söderlund, S.; Westerbacka, J.; Lundbom, N.; Taskinen, M.-R. Long-TE 1H MRS suggests that liver fat is more saturated than subcutaneous and visceral fat. NMR Biomed. 2010, 24, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Pond, C.M. The Fats of Life; Cambridge University Press: Cambridge, UK, 1998; Available online: https://www.cambridge.org/core/product/identifier/9780511584633/type/book (accessed on 23 September 2021).
- Simonsen, N.; Van’t Veer, P.; Strain, J.J.; Martin-Moreno, J.M.; Huttunen, J.K.; Navajas, J.F.C.; Martin, B.C.; Thamm, M.; Kardinaal, A.F.M.; Kok, F.J.; et al. Adipose tissue omega-3 and omega-6 fatty acid content and breast cancer in the euramic study. Am. J. Epidemiol. 1998, 147, 342–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, S.B.; Horvat, J.V.; Hancu, I.; Bs, O.M.S.; Bernard-Davila, B.; Weber, M.; Oh, J.H.; Marino, M.A.; Avendano, D.; Leithner, D.; et al. Quantitative in vivo proton MR spectroscopic assessment of lipid metabolism: Value for breast cancer diagnosis and prognosis. J. Magn. Reson. Imaging 2019, 50, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Greco, A.; Capristo, E.; Gniuli, D.; De Gaetano, A.; Gasbarrini, G. Insulin resistance directly correlates with increased saturated fatty acids in skeletal muscle triglycerides. Metabolism 2000, 49, 220–224. [Google Scholar] [CrossRef]
- Iggman, D.; Ärnlöv, J.; Vessby, B.; Cederholm, T.; Sjögren, P.; Risérus, U. Adipose tissue fatty acids and insulin sensitivity in elderly men. Diabetology 2010, 53, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Leporq, B.; Lambert, S.; Ronot, M.; Vilgrain, V.; Van Beers, B. Simultaneous MR quantification of hepatic fat content, fatty acid composition, transverse relaxation time and magnetic susceptibility for the diagnosis of non-alcoholic steatohepatitis. NMR Biomed. 2017, 30, e3766. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.L.; Haus, J.M.; Malin, S.K.; Flask, C.A.; McCullough, A.J.; Kirwan, J.P. Non-invasive assessment of hepatic lipid subspecies matched with non-alcoholic fatty liver disease phenotype. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Bydder, M.; Hamilton, G.; Yokoo, T.; Sirlin, C.B. Optimal phased-array combination for spectroscopy. Magn. Reson. Imaging 2008, 26, 847–850. [Google Scholar] [CrossRef] [Green Version]
- Vanhamme, L.; van den Boogaart, A.; Van Huffel, S. Improved Method for Accurate and Efficient Quantification of MRS Data with Use of Prior Knowledge Leentje. J Magn. Reson. 1997, 129, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Naressi, A.; Couturier, C.; Devos, J.M.; Janssen, M.; Mangeat, C.; De Beer, R.; Graveron-Demilly, D. Java-based graphical user interface for the MRUI quantitation package. Magn. Reson. Mater. Phys. Biol. Med. 2001, 12, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Berglund, J.; Ahlström, H.; Kullberg, J. Model-based mapping of fat unsaturation and chain length by chemical shift imaging-phantom validation and in vivo feasibility. Magn. Reson. Med. 2012, 68, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Peterson, P.; Trinh, L.; Månsson, S. Quantitative 1 H MRI and MRS of fatty acid composition. Magn. Reson. Med. 2021, 85, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.; Schlein, A.N.; Wolfson, T.; Cunha, G.M.; Fowler, K.J.; Middleton, M.S.; Loomba, R.; Sirlin, C.B. The relationship between liver triglyceride composition and proton density fat fraction as assessed by 1 H MRS. NMR Biomed. 2020, 33, e4286. [Google Scholar] [CrossRef] [PubMed]
- Bydder, M.; Hamilton, G.; De Rochefort, L.; Desai, A.; Heba, E.R.; Loomba, R.; Schwimmer, J.B.; Szeverenyi, N.M.; Sirlin, C.B. Sources of systematic error in proton density fat fraction (PDFF) quantification in the liver evaluated from magnitude images with different numbers of echoes. NMR Biomed. 2018, 31, e3843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bydder, M.; Ghodrati, V.; Gao, Y.; Robson, M.D.; Yang, Y.; Hu, P. Constraints in estimating the proton density fat fraction. Magn. Reson. Imaging 2020, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bydder, M.; Chavez, T.; Lam, J.; Henderson, W.; Pinto, N.; Chavarria, R.; Pham, A.D.; Loomba, R.; Schwimmer, J.; Sirlin, C.; et al. Triglyceride Saturation in Patients at Risk of NASH and NAFLD: A Cross-Sectional Study. Biophysica 2022, 2, 8-15. https://doi.org/10.3390/biophysica2010002
Bydder M, Chavez T, Lam J, Henderson W, Pinto N, Chavarria R, Pham AD, Loomba R, Schwimmer J, Sirlin C, et al. Triglyceride Saturation in Patients at Risk of NASH and NAFLD: A Cross-Sectional Study. Biophysica. 2022; 2(1):8-15. https://doi.org/10.3390/biophysica2010002
Chicago/Turabian StyleBydder, Mark, Tanya Chavez, Jessica Lam, Walter Henderson, Nick Pinto, Roxana Chavarria, Alex D. Pham, Rohit Loomba, Jeff Schwimmer, Claude Sirlin, and et al. 2022. "Triglyceride Saturation in Patients at Risk of NASH and NAFLD: A Cross-Sectional Study" Biophysica 2, no. 1: 8-15. https://doi.org/10.3390/biophysica2010002
APA StyleBydder, M., Chavez, T., Lam, J., Henderson, W., Pinto, N., Chavarria, R., Pham, A. D., Loomba, R., Schwimmer, J., Sirlin, C., & Hamilton, G. (2022). Triglyceride Saturation in Patients at Risk of NASH and NAFLD: A Cross-Sectional Study. Biophysica, 2(1), 8-15. https://doi.org/10.3390/biophysica2010002





