Effects of Ionic Liquids on Laccase from Trametes versicolor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
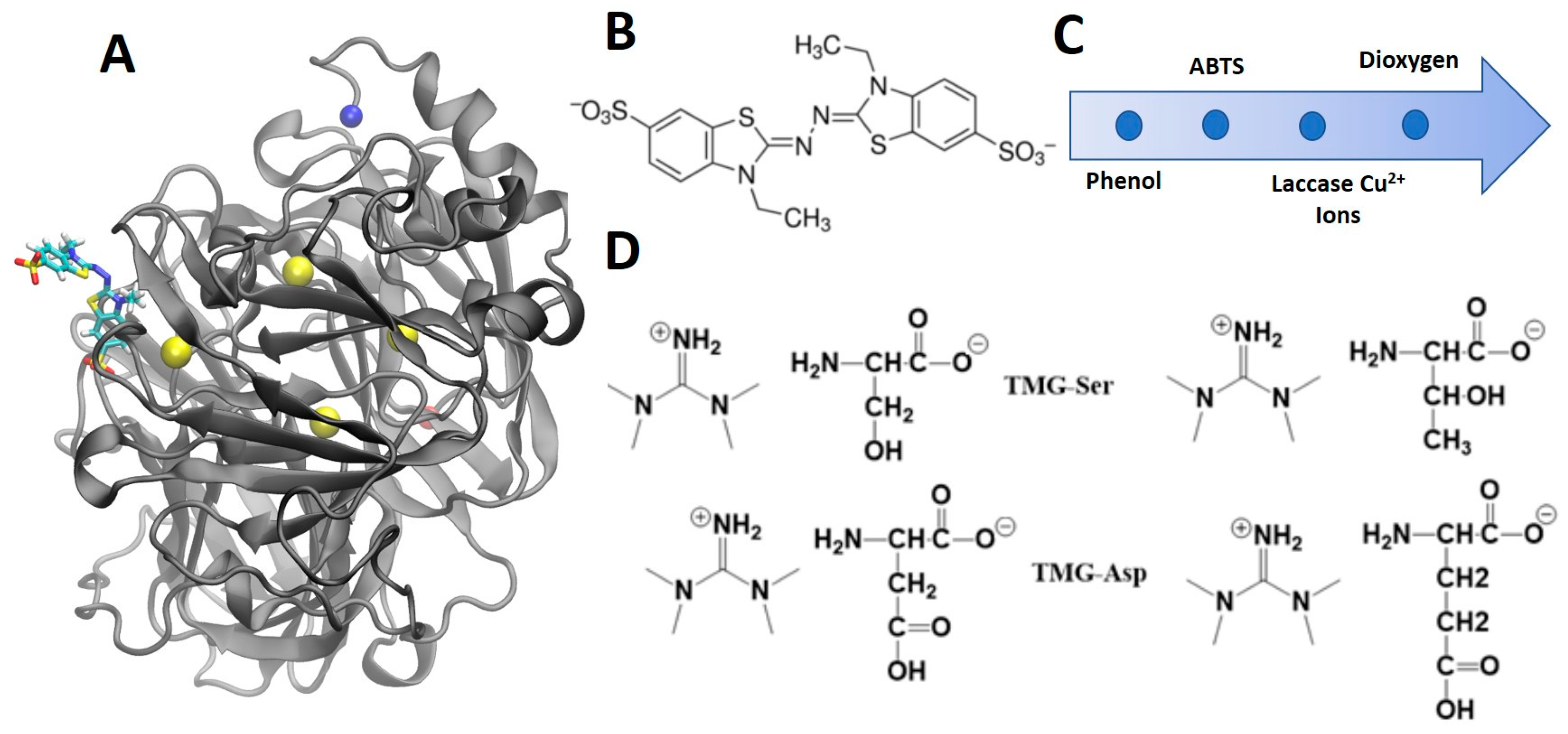
2.2. Laccase Purification
2.3. Enzymatic Assay
2.4. Fluorescence Spectroscopy
2.5. Molecular Dynamics Simulations
3. Results
3.1. Protein Sequence, Structure and Activity
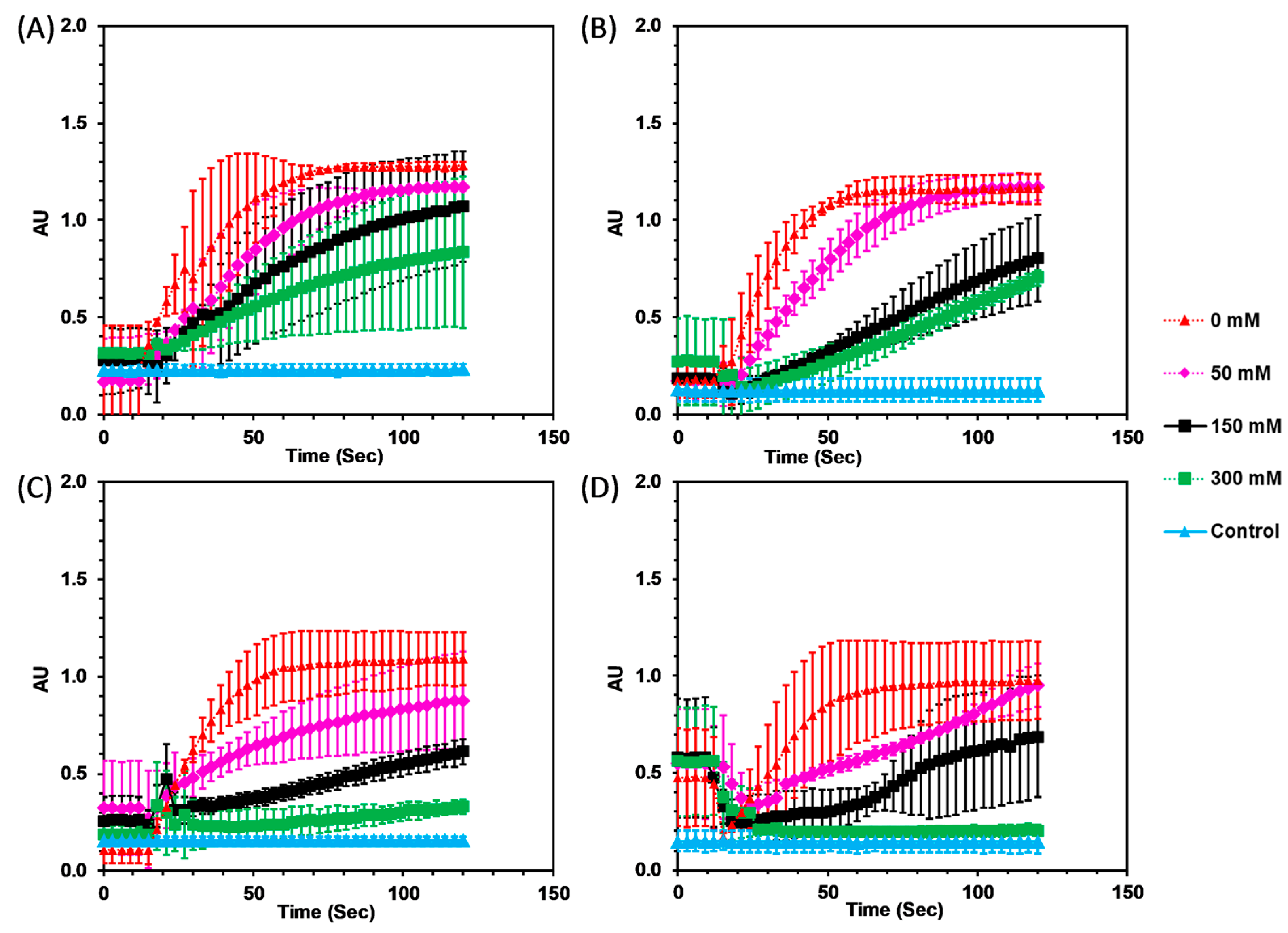
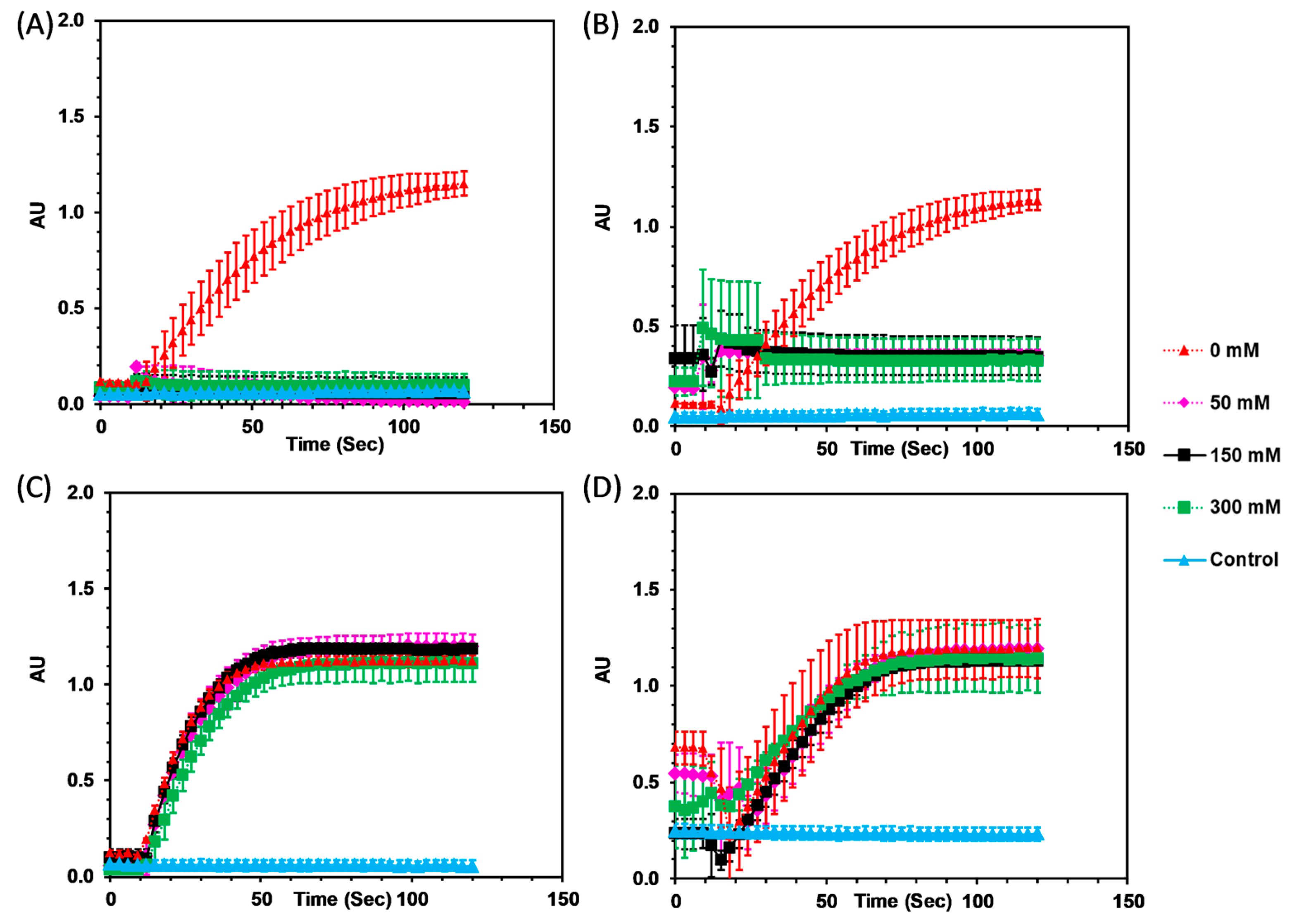
3.2. Enzymatic Activity
3.2.1. Imidazolium Ionic Liquids
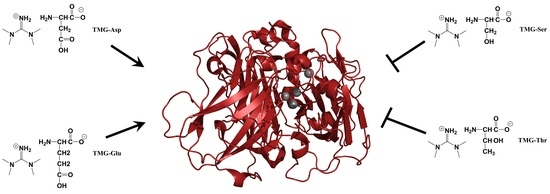
3.2.2. Amino Acid Ionic Liquids
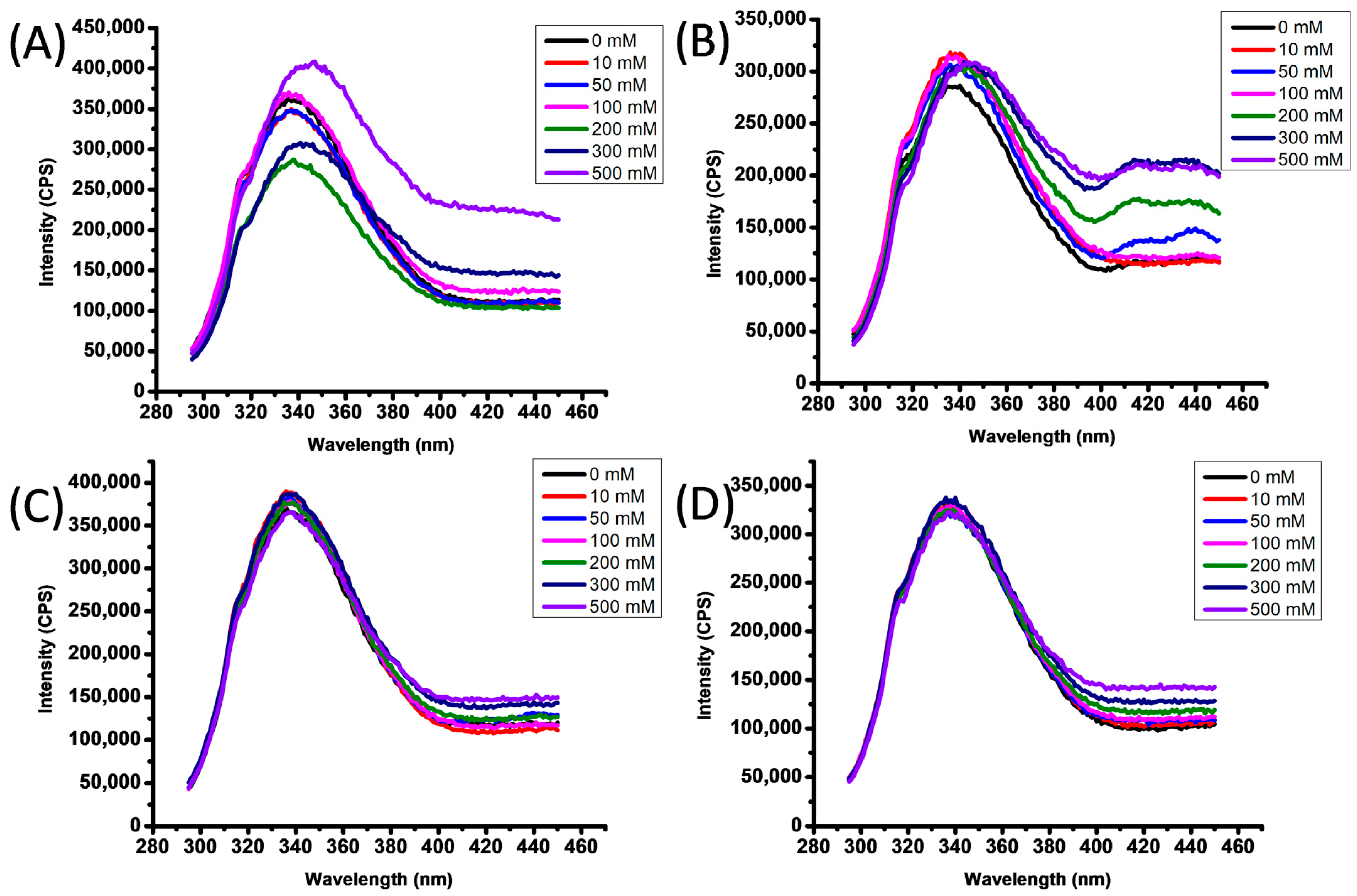
3.3. Laccase Intrinsic Fluorescence
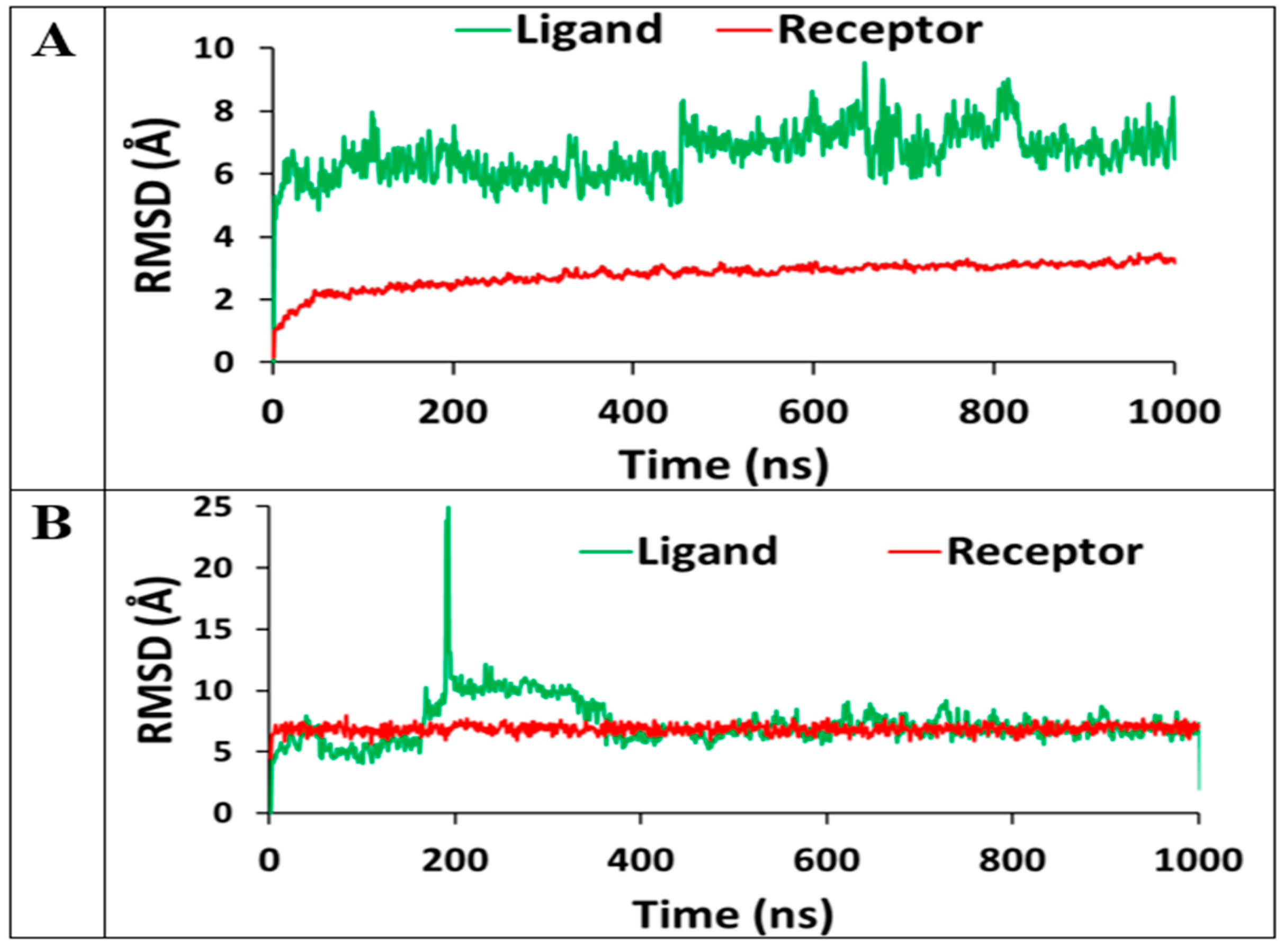
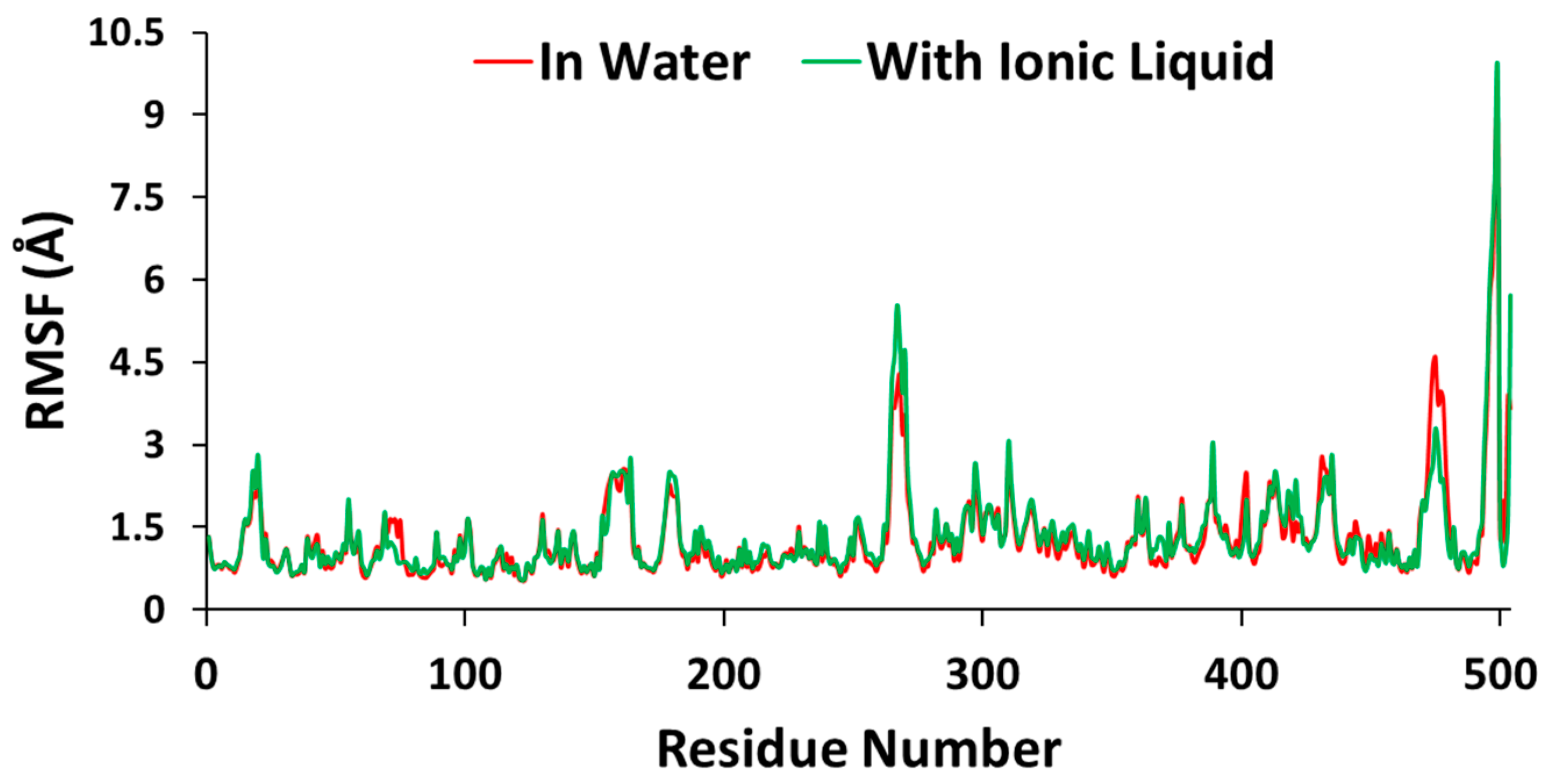
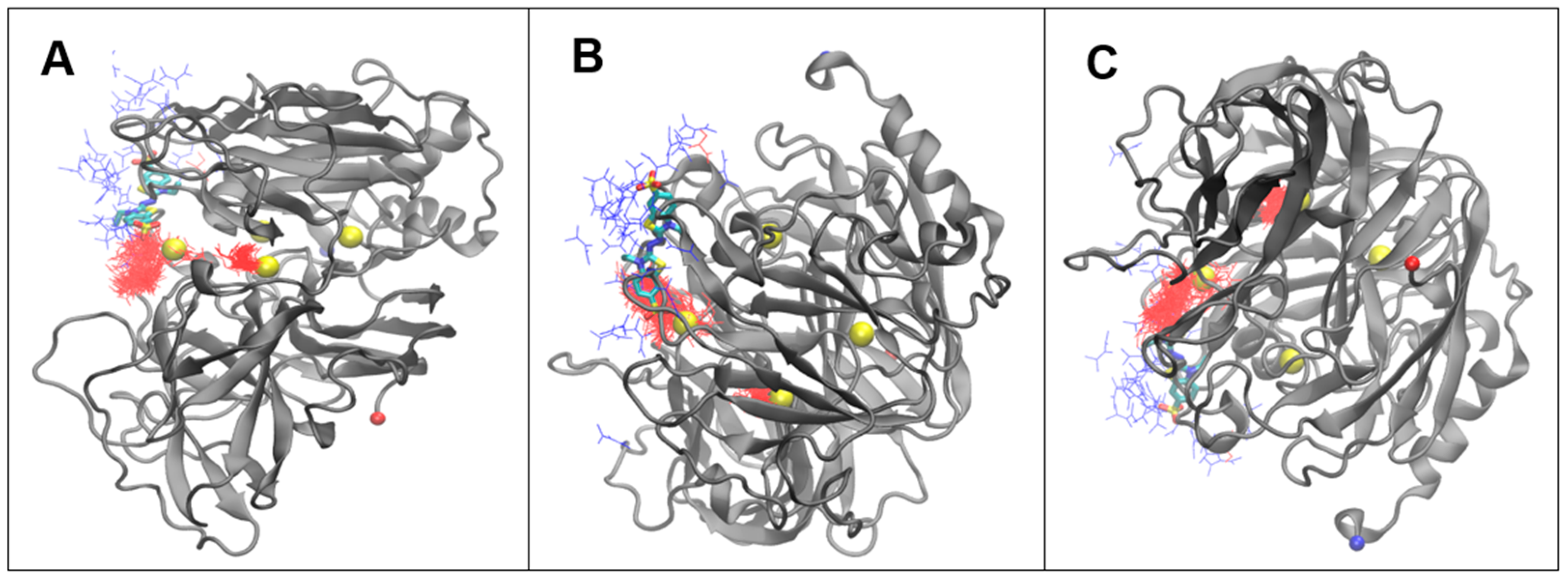
3.4. Molecular Dynamics Simulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Martins, V.L.; Torresi, R.M. Ionic liquids in electrochemical energy storage. Curr. Opin. Electrochem. 2018, 9, 26–32. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Li, J. Ionic liquids in surface electrochemistry. Phys. Chem. Chem. Phys. 2010, 12, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef]
- Baldrian, P.; Šnajdr, J. Production of ligninolytic enzymes by litter-decomposing fungi and their ability to decolorize synthetic dyes. Enzym. Microb. Technol. 2006, 39, 1023–1029. [Google Scholar] [CrossRef]
- Xu, F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: Correlation between activity and redox potentials as well as halide inhibition. Biochemistry 1996, 35, 7608–7614. [Google Scholar] [CrossRef]
- Imam, H.T.; Krasňan, V.; Rebroš, M.; Marr, A.C. Applications of Ionic Liquids in Whole-Cell and Isolated Enzyme Biocatalysis. Molecules 2021, 26, 4791. [Google Scholar] [CrossRef]
- Itoh, T. Ionic Liquids as Tool to Improve Enzymatic Organic Synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef]
- Itoh, T.; Takagi, Y. Laccase-Catalyzed Reactions in Ionic Liquids for Green Sustainable Chemistry. ACS Sustain. Chem. Eng. 2021, 9, 1443–1458. [Google Scholar] [CrossRef]
- Van Rantwijk, F.; Sheldon, R.A. Biocatalysis in Ionic Liquids. Chem. Rev. 2007, 107, 2757–2785. [Google Scholar] [CrossRef] [PubMed]
- Silvester, D.S. Recent advances in the use of ionic liquids for electrochemical sensing. Analyst 2011, 136, 4871–4882. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of ionic liquids to energy storage and conversion materials and devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef]
- Zhao, H.; Malhotra, S.V. Applications of ionic liquids in organic synthesis. Aldrichimica Acta 2002, 35, 75–83. [Google Scholar] [CrossRef]
- Baker, D. What has de novo protein design taught us about protein folding and biophysics? Protein Sci. 2019, 28, 678–683. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef]
- Dobson, C.M.; Šali, A.; Karplus, M. Protein folding: A perspective from theory and experiment. Angew. Chem. Int. Ed. 1998, 37, 868–893. [Google Scholar] [CrossRef]
- England, J.L.; Haran, G. Role of solvation effects in protein denaturation: From thermodynamics to single molecules and back. Annu. Rev. Phys. Chem. 2011, 62, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, I.; Mushegian, A. Modeling protein folding in vivo. Biol. Direct 2018, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.Y.; Jonnalagadda, K.S.; Paradis, N.; Vaden, T.D.; Wu, C.; Caputo, G.A. Effects of Ionic Liquids on Metalloproteins. Molecules 2021, 26, 514. [Google Scholar] [CrossRef]
- Zhao, H. Are ionic liquids kosmotropic or chaotropic? An evaluation of available thermodynamic parameters for quantifying the ion kosmotropicity of ionic liquids. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2006, 81, 877–891. [Google Scholar]
- Weingärtner, H.; Cabrele, C.; Herrmann, C. How ionic liquids can help to stabilize native proteins. Phys. Chem. Chem. Phys. 2012, 14, 415–426. [Google Scholar] [CrossRef]
- Fiebig, O.C.; Mancini, E.; Caputo, G.; Vaden, T.D. Quantitative Evaluation of Myoglobin Unfolding in the Presence of Guanidinium Hydrochloride and Ionic Liquids in Solution. J. Phys. Chem. B 2014, 118, 406–412. [Google Scholar] [CrossRef]
- Lee, J.Y.; Selfridge, K.M.; Kohn, E.M.; Vaden, T.D.; Caputo, G.A. Effects of Ionic Liquid Alkyl Chain Length on Denaturation of Myoglobin by Anionic, Cationic, and Zwitterionic Detergents. Biomolecules 2019, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; O’Malley, D.M.; Whetten, R.; Sederoff, R.R. A laccase associated with lignification in loblolly pine xylem. Science 1993, 260, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Kiefer-Meyer, M.-C.; Gomord, V.; O’Connell, A.; Halpin, C.; Faye, L. Cloning and sequence analysis of laccase-encoding cDNA clones from tobacco. Gene 1996, 178, 205–207. [Google Scholar] [CrossRef]
- LaFayette, P.R.; Eriksson, K.-E.L.; Dean, J.F. Characterization and heterologous expression of laccase cDNAs from xylem tissues of yellow-poplar (Liriodendron tulipifera). Plant Mol. Biol. 1999, 40, 23–35. [Google Scholar] [CrossRef]
- Ranocha, P.; McDougall, G.; Hawkins, S.; Sterjiades, R.; Borderies, G.; Stewart, D.; Cabanes-Macheteau, M.; Boudet, A.M.; Goffner, D. Biochemical characterization, molecular cloning and expression of laccases–a divergent gene family–in poplar. Eur. J. Biochem. 1999, 259, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Wuli, B.; Sederoff, R.; Whetten, R. Molecular cloning and expression of eight laccase cDNAs in loblolly pine (Pinus taeda). J. Plant Res. 2001, 114, 147–155. [Google Scholar] [CrossRef]
- Sterjiades, R.; Dean, J.F.; Eriksson, K.-E.L. Laccase from sycamore maple (Acer pseudoplatanus) polymerizes monolignols. Plant Physiol. 1992, 99, 1162–1168. [Google Scholar] [CrossRef]
- Couto, S.R.; Herrera, J.L.T. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef]
- Dabirmanesh, B.; Khajeh, K.; Ghazi, F.; Ranjbar, B.; Etezad, S.-M. A semi-rational approach to obtain an ionic liquid tolerant bacterial laccase through π-type interactions. Int. J. Biol. Macromol. 2015, 79, 822–829. [Google Scholar] [CrossRef]
- Jolivalt, C.; Madzak, C.; Brault, A.; Caminade, E.; Malosse, C.; Mougin, C. Expression of laccase IIIb from the white-rot fungus Trametes versicolor in the yeast Yarrowia lipolytica for environmental applications. Appl. Microbiol. Biotechnol. 2005, 66, 450–456. [Google Scholar] [CrossRef]
- Saoudi, O.; Ghaouar, N.; Othman, T. Fluorescence study of laccase from Trametes versicolor under the effects of pH, chemical denaturants and ionic liquids. J. Mol. Liq. 2017, 225, 56–63. [Google Scholar] [CrossRef]
- Tavares, A.P.M.; Pereira, J.A.N.; Xavier, A.M.R.B. Effect of ionic liquids activation on laccase from Trametes versicolor: Enzymatic stability and activity. Eng. Life Sci. 2012, 12, 648–655. [Google Scholar] [CrossRef]
- Tavares, A.P.M.; Rodriguez, O.; Macedo, E.A. Ionic liquids as alternative co-solvents for laccase: Study of enzyme activity and stability. Biotechnol. Bioeng. 2008, 101, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H. Effect of ions and other compatible solutes on enzyme activity, and its implication for biocatalysis using ionic liquids. J. Mol. Catal. B Enzym. 2005, 37, 16–25. [Google Scholar] [CrossRef]
- DeStefano, I.; DeStefano, G.; Paradis, N.J.; Patel, R.; Clark, A.K.; Gogoj, H.; Singh, G.; Jonnalagadda, K.S.; Patel, A.Y.; Wu, C.; et al. Thermodynamic destabilization of azurin by four different tetramethylguanidinium amino acid ionic liquids. Int. J. Biol. Macromol. 2021, 180, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Procacci, P.; Berne, B.J. Multiple time scale methods for constant pressure molecular dynamics simulations of molecular systems. Mol. Phys. 1994, 83, 255–272. [Google Scholar] [CrossRef]
- Kohn, E.M.; Lee, J.Y.; Calabro, A.; Vaden, T.D.; Caputo, G.A. Heme Dissociation from Myoglobin in the Presence of the Zwitterionic Detergent N,N-Dimethyl-N-Dodecylglycine Betaine: Effects of Ionic Liquids. Biomolecules 2018, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Barkley, M.D. Toward Understanding Tryptophan Fluorescence in Proteins. Biochemistry 1998, 37, 9976–9982. [Google Scholar] [CrossRef] [PubMed]
- Vivian, J.T.; Callis, P.R. Mechanisms of Tryptophan Fluorescence Shifts in Proteins. Biophys. J. 2001, 80, 2093–2109. [Google Scholar] [CrossRef]
- Kelly, S.M.; Price, N.C. The use of circular dichroism in the investigation of protein structure and function. Curr. Protein Pept. Sci. 2000, 1, 349–384. [Google Scholar] [CrossRef]
- Eftink, M.R. The use of fluorescence methods to monitor unfolding transitions in proteins. Biophys. J. 1994, 66, 482–501. [Google Scholar] [CrossRef]
- Acharyya, A.; DiGiuseppi, D.; Stinger, B.L.; Schweitzer-Stenner, R.; Vaden, T.D. Structural Destabilization of Azurin by Imidazolium Chloride Ionic Liquids in Aqueous Solution. J. Phys. Chem. B 2019, 123, 6933–6945. [Google Scholar] [CrossRef]
- Zhong, D.; Pal, S.K.; Zhang, D.; Chan, S.I.; Zewail, A.H. Femtosecond dynamics of rubredoxin: Tryptophan solvation and resonance energy transfer in the protein. Proc. Natl. Acad. Sci. USA 2002, 99, 13. [Google Scholar] [CrossRef]
- Ghirlanda, G.; Lear, J.D.; Ogihara, N.L.; Eisenberg, D.; DeGrado, W.F. A Hierarchic Approach to the Design of Hexameric Helical Barrels. J. Mol. Biol. 2002, 319, 243–253. [Google Scholar] [CrossRef]
- Jones, J.D.; Gierasch, L.M. Effect of charged residue substitutions on the membrane-interactive properties of signal sequences of the Escherichia coli LamB protein. Biophys. J. 1994, 67, 1534–1545. [Google Scholar] [CrossRef][Green Version]
- Caputo, G.A.; London, E. Position and ionization state of Asp in the core of membrane-inserted alpha helices control both the equilibrium between transmembrane and nontransmembrane helix topography and transmembrane helix positioning. Biochemistry 2004, 43, 8794–8806. [Google Scholar] [CrossRef]
- Enguita, F.J.; Marçal, D.; Martins, L.O.; Grenha, R.; Henriques, A.O.; Lindley, P.F.; Carrondo, M.A. Substrate and Dioxygen Binding to the Endospore Coat Laccase from Bacillus subtilis*. J. Biol. Chem. 2004, 279, 23472–23476. [Google Scholar] [CrossRef]
- Ferraroni, M.; Myasoedova, N.M.; Schmatchenko, V.; Leontievsky, A.A.; Golovleva, L.A.; Scozzafava, A.; Briganti, F. Crystal structure of a blue laccase from Lentinus tigrinus: Evidences for intermediates in the molecular oxygen reductive splitting by multicopper oxidases. BMC Struct. Biol. 2007, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Baba, A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006, 55, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Picarazzi, F.; Vicenti, I.; Saladini, F.; Zazzi, M.; Mori, M. Targeting the RdRp of Emerging RNA Viruses: The Structure-Based Drug Design Challenge. Molecules 2020, 25, 5695. [Google Scholar] [CrossRef] [PubMed]
- Tainer, J.A.; Getzoff, E.D.; Richardson, J.S.; Richardson, D.C. Structure and mechanism of copper, zinc superoxide dismutase. Nature 1983, 306, 284–287. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.Y.; Clark, A.K.; Paradis, N.J.; Amin, M.; Vaden, T.D.; Wu, C.; Caputo, G.A. Effects of Ionic Liquids on Laccase from Trametes versicolor. Biophysica 2021, 1, 429-444. https://doi.org/10.3390/biophysica1040031
Patel AY, Clark AK, Paradis NJ, Amin M, Vaden TD, Wu C, Caputo GA. Effects of Ionic Liquids on Laccase from Trametes versicolor. Biophysica. 2021; 1(4):429-444. https://doi.org/10.3390/biophysica1040031
Chicago/Turabian StylePatel, Aashka Y., Austin K. Clark, Nicholas J. Paradis, Meeraj Amin, Timothy D. Vaden, Chun Wu, and Gregory A. Caputo. 2021. "Effects of Ionic Liquids on Laccase from Trametes versicolor" Biophysica 1, no. 4: 429-444. https://doi.org/10.3390/biophysica1040031
APA StylePatel, A. Y., Clark, A. K., Paradis, N. J., Amin, M., Vaden, T. D., Wu, C., & Caputo, G. A. (2021). Effects of Ionic Liquids on Laccase from Trametes versicolor. Biophysica, 1(4), 429-444. https://doi.org/10.3390/biophysica1040031