Chiral Dualism as a Unifying Principle in Molecular Biophysics
Abstract
1. Introduction
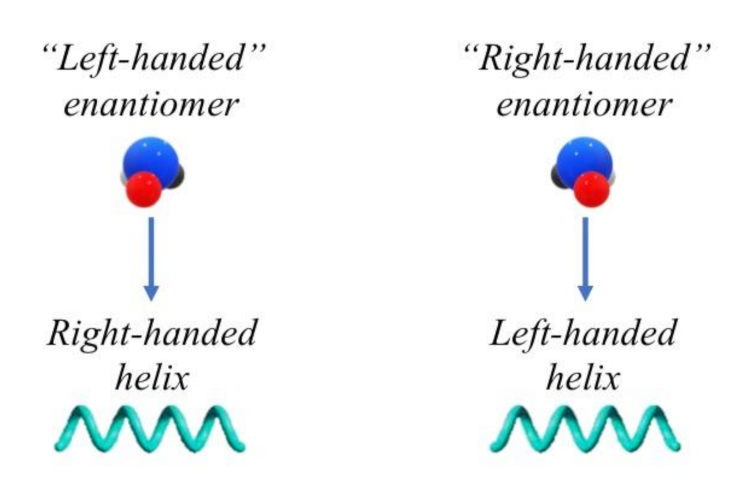
2. Chirality of Macromolecules
2.1. Proteins
2.1.1. Primary and Secondary Structure
2.1.2. Superhelices
2.1.3. Cytoskeleton
2.2. DNA
3. Chiral Hierarchy Establishment
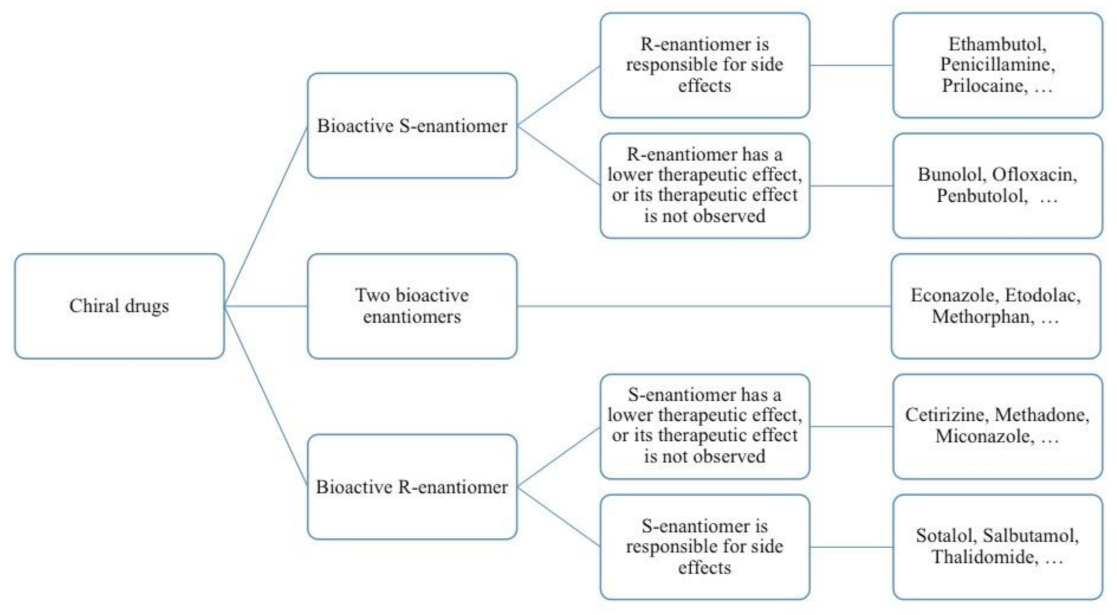
4. Chirality of Drugs
4.1. Drugs with a Bioactive “Left-Handed” S-Enantiomer
4.2. Drugs with A Bioactive “Right-Handed” R-Enantiomer
4.3. Drugs with Two Bioactive Enantiomers
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feller, G. Protein folding at extreme temperatures: Current issues. Semin. Cell Dev. Biol. 2018, 84, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, A.V. 50+ Years of Protein Folding. Biochemistry 2018, 83 (Suppl. 1), S3–S18. [Google Scholar] [CrossRef]
- Newberry, R.W.; Raines, R.T. Secondary Forces in Protein Folding. ACS Chem. Biol. 2019, 14, 1677–1686. [Google Scholar] [CrossRef]
- Cheung, M.S.; Gasic, A.G. Towards developing principles of protein folding and dynamics in the cell. Phys. Biol. 2018, 15, 063001. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, V.; Cerminara, M. When fast is better: Protein folding fundamentals and mechanisms from ultrafast approaches. Biochem. J. 2016, 473, 2545–2559. [Google Scholar] [CrossRef]
- Hong, N.S.; Petrović, D.; Lee, R.; Gryn’ova, G.; Purg, M.; Saunders, J.; Bauer, P.; Carr, P.D.; Lin, C.Y.; Mabbitt, P.D.; et al. The evolution of multiple active site configurations in a designed enzyme. Nat. Commun. 2018, 9, 3900. [Google Scholar] [CrossRef]
- Warelow, T.P.; Pushie, M.J.; Cotelesage, J.J.H.; Santini, J.M.; George, G.N. The active site structure and catalytic mechanism of arsenite oxidase. Sci. Rep. 2017, 7, 1757. [Google Scholar] [CrossRef] [PubMed]
- Kean, K.M.; Karplus, P.A. Structure and role for active site lid of lactate monooxygenase from Mycobacterium smegmatis. Protein Sci. 2019, 28, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Osuna, S.; Jiménez-Osés, G.; Noey, E.L.; Houk, K.N. Molecular dynamics explorations of active site structure in designed and evolved enzymes. Acc. Chem. Res. 2015, 48, 1080–1089. [Google Scholar] [CrossRef]
- Law, B.J.; Bennett, M.R.; Thompson, M.L.; Levy, C.; Shepherd, S.A.; Leys, D.; Micklefield, J. Effects of Active-Site Modification and Quaternary Structure on the Regioselectivity of Catechol-O-Methyltransferase. Angew. Chem. Int. Ed. Engl. 2016, 55, 2683–2687. [Google Scholar] [CrossRef]
- Jez, J.M. Revisiting protein structure, function, and evolution in the genomic era. J. Invertebr. Pathol. 2017, 142, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ikeya, T.; Güntert, P.; Ito, Y. Protein Structure Determination in Living Cells. Int. J. Mol. Sci. 2019, 20, 2442. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Protein intrinsic disorder and structure-function continuum. Prog. Mol. Biol. Transl. Sci. 2019, 166, 1–17. [Google Scholar] [CrossRef]
- Kuhlman, B.; Bradley, P. Advances in protein structure prediction and design. Nat. Rev. Mol. Cell Biol. 2019, 20, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.W. Origin of life. The origin of macromolecular chirality. Curr. Biol. 1994, 4, 758–760. [Google Scholar] [CrossRef]
- Kojić-Prodić, B.; Štefanić, Z. Symmetry versus Asymmetry in the Molecules of Life: Homomeric Protein Assemblies. Symmetry 2010, 2, 884–906. [Google Scholar] [CrossRef]
- Inaki, M.; Liu, J.; Matsuno, K. Cell chirality: Its origin and roles in left-right asymmetric development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150403. [Google Scholar] [CrossRef]
- Podlech, J. Origin of organic molecules and biomolecular homochirality. Cell Mol. Life Sci. 2001, 58, 44–60. [Google Scholar] [CrossRef]
- Hein, J.E.; Blackmond, D.G. On the origin of single chirality of amino acids and sugars in biogenesis. Acc. Chem. Res. 2012, 45, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Dorta-Urra, A.; Bargueño, P. Homochirality: A Perspective from Fundamental Physics. Symmetry 2019, 11, 661. [Google Scholar] [CrossRef]
- Blackmond, D.G. The Origin of Biological Homochirality. Cold Spring Harb. Perspect. Biol. 2019, 11, a032540. [Google Scholar] [CrossRef] [PubMed]
- Famiano, M.; Boyd, R.; Kajino, T.; Onaka, T.; Mo, Y. Astrophysical Sites that Can Produce Enantiomeric Amino Acids. Symmetry 2019, 11, 23. [Google Scholar] [CrossRef]
- Suzuki, N.; Itabashi, Y. Possible Roles of Amphiphilic Molecules in the Origin of Biological Homochirality. Symmetry 2019, 11, 966. [Google Scholar] [CrossRef]
- Takahashi, J.-I.; Kobayashi, K. Origin of Terrestrial Bioorganic Homochirality and Symmetry Breaking in the Universe. Symmetry 2019, 11, 919. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, M. Symmetry Breaking in Self-Assembled Nanoassemblies. Symmetry 2019, 11, 950. [Google Scholar] [CrossRef]
- Aav, R.; Mishra, K.A. The Breaking of Symmetry Leads to Chirality in Cucurbituril-Type Hosts. Symmetry 2018, 10, 98. [Google Scholar] [CrossRef]
- Tverdislov, V.A.; Yakovenko, L.V. Physical Aspects of the Emergence of Living Cell Precursors: The Ion and Chiral Asymmetries as Two Fundamental Asymmetry Types. Mosc. Univ. Phys. Bull. 2008, 63, 151–163. [Google Scholar] [CrossRef]
- Zlenko, D.; Zanin, A.; Skoblin, A.; Tverdislov, V.; Stovbun, S. Spontaneous resolution in racemic solutions of N-trifluoroacetylated α-aminoalcohols. J. Mol. Struct. 2019, 1183, 8–13. [Google Scholar] [CrossRef]
- Hirose, K.; Ukimi, M.; Ueda, S.; Onoda, C.; Kano, R.; Tsuda, K.; Hinohara, Y.; Tobe, Y. The Asymmetry is Derived from Mechanical Interlocking of Achiral Axle and Achiral Ring Components –Syntheses and Properties of Optically Pure [2]Rotaxanes–. Symmetry 2018, 10, 20. [Google Scholar] [CrossRef]
- Ustrnul, L.; Kaabel, S.; Burankova, T.; Martõnova, J.; Adamson, J.; Konrad, N.; Burk, P.; Borovkov, V.; Aav, R. Supramolecular chirogenesis in zinc porphyrins by enantiopure hemicucurbit[n]urils (n = 6, 8). Chem. Commun. 2019, 55, 14434–14437. [Google Scholar] [CrossRef] [PubMed]
- Rickhaus, M.; Mayor, M.; Juríček, M. Chirality in curved polyaromatic systems. Chem. Soc. Rev. 2017, 46, 1643–1660. [Google Scholar] [CrossRef]
- Chen, Z.; Choi, C.K.K.; Wang, Q. Origin of the Plasmonic Chirality of Gold Nanorod Trimers Templated by DNA Origami. ACS Appl. Mater. Interfaces 2018, 10, 26835–26840. [Google Scholar] [CrossRef]
- Lahav, M. Question 4: Basic Questions about the Origin of Life: On Chirobiogenesis. Orig. Life Evol. Biosph. 2007, 37, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Weissbuch, I.; Lahav, M. Crystalline Architectures as Templates of Relevance to the Origins of Homochirality. Chem. Rev. 2011, 111, 3236–3267. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Tanaka, M.; Suzuki, T.; Sekine, A.; Kawasaki, T.; Soai, K.; Shiro, M.; Lahav, M.; Asahi, T. Absolute chirality of the γ-polymorph of glycine: Correlation of the absolute structure with the optical rotation. Chem. Commun. 2012, 48, 6031–6033. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Ozaki, H.; Tsuchiya, S.; Asahi, T.; Lahav, M.; Kawasaki, T.; Soai, K. Achiral amino acid glycine acts as an origin of homochirality in asymmetric autocatalysis. Org. Biomol. Chem. 2019, 17, 4200–4203. [Google Scholar] [CrossRef]
- Weissbuch, I.; Illos, R.A.; Bolbach, G.; Lahav, M. Racemic beta-sheets as templates of relevance to the origin of homochirality of peptides: Lessons from crystal chemistry. Acc. Chem. Res. 2009, 42, 1128–1140. [Google Scholar] [CrossRef]
- Illos, R.A.; Bisogno, F.R.; Clodic, G.; Bolbach, G.; Weissbuch, I.; Lahav, M. Oligopeptides and copeptides of homochiral sequence, via beta-sheets, from mixtures of racemic alpha-amino acids, in a one-pot reaction in water; relevance to biochirogenesis. J. Am. Chem. Soc. 2008, 130, 8651–8659. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2003. [Google Scholar]
- Flügel, R.M. Chirality and Life: A Short Introduction to the Early Phases of Chemical Evolution; Springer: Berlin, Germany, 2011. [Google Scholar]
- De Zotti, M.; Formaggio, F.; Crisma, M.; Peggion, C.; Moretto, A.; Toniolo, C. Handedness preference and switching of peptide helices. Part I: Helices based on protein amino acids. J. Pept. Sci. 2014, 20, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Chothia, C. Conformation of twisted beta-pleated sheets in proteins. J. Mol. Biol. 1973, 75, 295–302. [Google Scholar] [CrossRef]
- Joseph, A.P.; Srinivasan, N.; de Brevern, A.G. Cis-trans peptide variations in structurally similar proteins. Amino Acids 2012, 43, 1369–1381. [Google Scholar] [CrossRef]
- Finkelstein, A.V.; Ptitsyn, O.B. Protein Physics: A Course of Lectures, 2nd ed.; Academic Press, An Imprint of Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Pauling, L.; Corey, R.B. Compound helical configurations of polypeptide chains: Structure of proteins of the α-keratin type. Nature 1953, 171, 59–61. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R.B.; Branson, H.R. The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl. Acad. Sci. USA 1951, 37, 205–211. [Google Scholar] [CrossRef]
- Crick, F.H. Is alpha-keratin a coiled coil? Nature 1952, 170, 882–883. [Google Scholar] [CrossRef]
- Rose, A.; Schraegle, S.J.; Stahlberg, E.A.; Meier, I. Coiled-coil protein composition of 22 proteomes differences and common themes in subcellular infrastructure and traffic control. BMC Evol. Biol. 2005, 5, 66. [Google Scholar] [CrossRef]
- Rackham, O.J.; Madera, M.; Armstrong, C.T.; Vincent, T.L.; Woolfson, D.N.; Gough, J. The evolution and structure prediction of coiled coils across all genomes. J. Mol. Biol. 2010, 403, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Lupas, A.N.; Gruber, M. The structure of alpha-helical coiled coils. Adv. Protein Chem. 2005, 70, 37–78, Erratum in: Proteins 1993, 17, 219. [Google Scholar] [CrossRef]
- Yu, Y.B. Coiled-coils: Stability, specificity, and drug delivery potential. Adv. Drug Deliv. Rev. 2002, 54, 1113–1129. [Google Scholar] [CrossRef]
- Lobyshev, V.I.; Solovei, A.B. Structure of bound water and topological rearrangement waves. Biophysics 2011, 56, 848–856. [Google Scholar] [CrossRef]
- Lupas, A.N.; Bassler, J.; Dunin-Horkawicz, S. The Structure and Topology of α-Helical Coiled Coils. In Fibrous Proteins: Structures and Mechanisms. Subcellular Biochemistry; Parry, D., Squire, J., Eds.; Springer: Cham, Switzerland, 2017; Volume 82, pp. 95–129. [Google Scholar]
- Moutevelis, E.; Woolfson, D. A periodic table of coiled-coil protein structures. J. Mol. Biol. 2009, 385, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Cohen, C. Pitch diversity in alpha-helical coiled coils. Proteins 1993, 15, 223–234. [Google Scholar] [CrossRef]
- Stetefeld, J.; Jenny, M.; Schulthess, T.; Landwehr, R.; Engel, J.; Kammerer, R.A. Crystal structure of a naturally occurring parallel right-handed coiled coil tetramer. Nat. Struct. Mol. Biol. 2000, 7, 772–776. [Google Scholar] [CrossRef]
- Harbury, P.B.; Plecs, J.J.; Tidor, B.; Alber, T.; Kim, P.S. High-resolution protein design with backbone freedom. Science 1998, 282, 1462–1467. [Google Scholar] [CrossRef]
- Peters, J.; Baumeister, W.; Lupas, A. Hyperthermostable surface layer protein tetrabrachion from the archaebacterium Staphylothermus marinus: Evidence for the presence of a right-handed coiled coil derived from the primary structure. J. Mol. Biol. 1996, 257, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Treutlein, H.R.; Lemmon, M.A.; Engelman, D.M.; Brunger, A.T. The glycophorin A transmembrane domain dimer: Sequence-specific propensity for a right-handed supercoil of helices. Biochemistry 1992, 31, 12726–12733. [Google Scholar] [CrossRef] [PubMed]
- Dure, L., 3rd. A repeating 11-mer amino acid motif and plant desiccation. Plant J. 1993, 3, 363–369. [Google Scholar] [CrossRef]
- Parry, D.A.D.; Fraser, R.D.B.; Squire, J.M. Fifty years of coiled-coils and a-helical bundles: A close relationship between sequence and structure. J. Struct. Biol. 2008, 163, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Korn, E.D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol. Rev. 1982, 62, 672–737. [Google Scholar] [CrossRef] [PubMed]
- Milligan, R.A.; Whittaker, M.; Safer, D. Molecular structure of F-actin and location of surface binding sites. Nature 1990, 348, 217–221. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin Structure and Function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Aebi, U. Intermediate Filaments: Molecular Structure, Assembly Mechanism, and Integration into Functionally Distinct Intracellular Scaffolds. Annu. Rev. Biochem. 2004, 73, 749–789. [Google Scholar] [CrossRef]
- Murthy, N.S.; Wang, W.; Kamath, Y. Structure of intermediate filament assembly in hair deduced from hydration studies using small-angle neutron scattering. J. Struct. Biol. 2019, 206, 295–304. [Google Scholar] [CrossRef]
- Crick, F.H.C. The packing of α-helices: Simple coiled-coils. Acta Crystallogr. 1953, 6, 689. [Google Scholar] [CrossRef]
- Bray, D.J.; Walsh, T.R.; Noro, M.G.; Notman, R. Complete Structure of an Epithelial Keratin Dimer: Implications for Intermediate Filament Assembly. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Weber, K. Intermediate filaments: Structure, dynamics, function, and disease. Annu. Rev. Biochem. 1994, 63, 345–382. [Google Scholar] [CrossRef] [PubMed]
- Kapinos, L.E.; Burkhard, P.; Herrmann, H.; Aebi, U.; Strelkov, S.V. Simultaneous formation of right- and left-handed anti-parallel coiled-coil interfaces by a coil2 fragment of human lamin A. J. Mol. Biol. 2011, 408, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Dechat, T.; Adam, S.A.; Taimen, P.; Shimi, T.; Goldman, R.D. Nuclear Lamins. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef]
- Amos, L.; Klug, A. Arrangement of subunits in flagellar microtubules. J. Cell Sci. 1974, 14, 523–549. [Google Scholar]
- Chrétien, D.; Kenney, J.M.; Fuller, S.D.; Wade, R.H. Determination of microtubule polarity by cryo-electron microscopy. Structure 1996, 4, 1031–1040. [Google Scholar] [CrossRef]
- Malyshko, E.V.; Murtazina, A.R.; Tverdislov, V.A. Chirality as a physical basis of hierarchical periodization of biomacromolecular structures. Biophysics 2020, 65, 181–185. [Google Scholar] [CrossRef]
- Nevzorov, I.A.; Levitsky, D.I. Tropomyosin: Double helix from the protein world. Biochemistry 2011, 76, 1507–1527. [Google Scholar] [CrossRef]
- Samatey, F.A.; Imada, K.; Nagashima, S.; Vonderviszt, F.; Kumasaka, T.; Yamamoto, M.; Namba, K. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature 2001, 410, 331–337. [Google Scholar] [CrossRef]
- Raos, G. Degrees of chirality in helical structures. Macromol. Theory Simul. 2002, 11, 739–750. [Google Scholar] [CrossRef]
- Petitjean, M. Chirality and Symmetry Measures: A Transdisciplinary Review. Entropy 2003, 5, 271–312. [Google Scholar] [CrossRef]
- Randić, M.; Razinger, M. Molecular shapes and chirality. J. Chem. Inf. Comput. Sci. 1996, 36, 429–441. [Google Scholar] [CrossRef]
- Dryzun, C.; Zait, A.; Avnir, D. Quantitative symmetry and chirality--a fast computational algorithm for large structures: Proteins, macromolecules, nanotubes, and unit cells. J. Comput. Chem. 2011, 32, 2526–2538. [Google Scholar] [CrossRef]
- Yewande, E.O.; Neal, M.P.; Low, R. The Hausdorff chirality measure and a proposed Hausdorff structure measure. Mol. Phys. 2009, 107, 281–291. [Google Scholar] [CrossRef]
- Ramachandran, G.N.; Ramakrishnan, C.; Sasisekharan, V. Stereochemistry of polypeptide chain configurations. J. Mol. Biol. 1963, 7, 95–99. [Google Scholar] [CrossRef]
- Sidorova, A.E.; Malyshko, E.V.; Kotov, A.R.; Tverdislov, V.A.; Ustinin, M.N. Quantitative criteria of chirality in hierarchical protein structures. Biophysics 2019, 64, 155–166. [Google Scholar] [CrossRef]
- Fogg, J.M.; Catanese, D.J.; Randall, G.L.; Swick, M.C.; Zechiedrich, L. Differences between Positively and Negatively Supercoiled DNA that Topoisomerases May Distinguish. IMA Vol. Math. Its Appl. 2009, 73–121. [Google Scholar] [CrossRef]
- Widom, J. Toward a unified model of chromatin folding. Annu. Rev. Biophys. Biophys. Chem. 1989, 18, 365–395. [Google Scholar] [CrossRef] [PubMed]
- Hamiche, A.; Carot, V.; Alilat, M.; Lucia, F.D.; O’donohue, M.-F.; Revet, B.; Prunell, A. Interaction of histone (H3-H4)2 tetramer of the nucleosome with positively supercoiled DNA minicircles: Potential flipping of the protein from a left- to a right- handed superhelical form. Proc. Natl. Acad. USA 1996, 93, 7588–7593. [Google Scholar] [CrossRef] [PubMed]
- Arents, G.; Burlingame, R.W.; Wang, B.C.; Love, W.E.; Moudrianakis, E.N. The nucleosomal core histone octamer at 3.1 A resolution: A tripartite protein assembly and a left-handed superhelix. Proc. Natl. Acad. Sci. USA 1991, 88, 10148–10152. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dou, S.-X.; Wang, P.-Y. The histone octamer influences the wrapping direction of DNA on it: Brownian dynamics simulation of the nucleosome chirality. J. Theor. Biol. 2005, 235, 365–372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tverdislov, V.A. Chirality as a primary switch of hierarchical levels in molecular biological systems. Biophysics 2013, 58, 128–132. [Google Scholar] [CrossRef]
- Tverdislov, V.A.; Malyshko, E.V. On regularities of spontaneous formation of structural hierarchies in chiral systems of non-living and living nature. Phys. Uspekhi 2019, 189, 375–385. [Google Scholar] [CrossRef]
- Tverdislov, V.A.; Malyshko, E.V. Chiral Dualism as an Instrument of Hierarchical Structure Formation in Molecular Biology. Symmetry 2020, 12, 587. [Google Scholar] [CrossRef]
- Xue, S.; Xing, P.; Zhang, J.; Zeng, Y.; Zhao, Y. Diverse Role of Solvents in Controlling Supramolecular Chirality. Chemistry 2019, 25, 7426–7437. [Google Scholar] [CrossRef]
- Kousar, A.; Feng, C. Controlled mechanical properties and supramolecular chirality of hydrogels via pH change. MethodsX 2019, 6, 417–423. [Google Scholar] [CrossRef]
- Cobos, K.; Quiñoá, E.; Riguera, R.; Freire, F. Chiral-to-Chiral Communication in Polymers: A Unique Approach To Control Both Helical Sense and Chirality at the Periphery. J. Am. Chem. Soc. 2018, 140, 12239–12246. [Google Scholar] [CrossRef]
- Wang, F.; Feng, C.L. Stoichiometry-Controlled Inversion of Supramolecular Chirality in Nanostructures Co-assembled with Bipyridines. Chemistry 2018, 24, 1509–1513. [Google Scholar] [CrossRef]
- Zhao, D.; van Leeuwen, T.; Cheng, J.; Feringa, B.L. Dynamic control of chirality and self-assembly of double-stranded helicates with light. Nat. Chem. 2017, 9, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Ferrand, Y.; Chandramouli, N.; Kauffmann, B.; Aube, C.; Dubreuil, D.; Huc, I. Identification of a Foldaxane Kinetic Byproduct during Guest-Induced Single to Double Helix Conversion. J. Am. Chem. Soc. 2012, 134, 15656–15659. [Google Scholar] [CrossRef]
- Danila, I.; Riobé, F.; Piron, F.; Puigmartí-Luis, J.; Wallis, J.D.; Linares, M.; Ågren, H.; Beljonne, D.; Amabilino, D.B.; Avarvari, N. Hierarchical chiral expression from the nano- to mesoscale in synthetic supramolecular helical fibers of a nonamphiphilic C3-Symmetrical π-functional molecule. J. Am. Chem. Soc. 2011, 133, 8344–8353. [Google Scholar] [CrossRef] [PubMed]
- Berthier, D.; Buffeteau, T.; Léger, J.-M.; Oda, R.; Huc, I. From Chiral Counterions to Twisted Membranes. J. Am. Chem. Soc. 2002, 124, 13486–13494. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Fukushima, T.; Niki, M.; Kosaka, A.; Ishii, N.; Aida, T. Self-assembled graphitic nanotubes with one-handed helical arrays of a chiral amphiphilic molecular graphene. Proc. Natl. Acad. Sci. USA 2005, 102, 10801–10806. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, L.; Chen, J.; Liu, M. Hierarchical Self-Assembly of a Porphyrin into Chiral Macroscopic Flowers with Superhydrophobic and Enantioselective Property. ACS Nano 2017, 11, 12453–12460. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, E. What Is Life? The Physical Aspect of the Living Cell; University Press: Cambridge, UK, 1944. [Google Scholar]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef]
- Mane, S. Racemic drug resolution: A comprehensive guide. Anal. Methods 2016, 8, 7567–7586. [Google Scholar] [CrossRef]
- Nation, R.L. Chirality in New Drug Development. Clin. Pharm. 1994, 27, 249–255. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. IJBS 2006, 2, 85–100. [Google Scholar]
- Smith, S.W. Chiral toxicology: It’s the same thing...only different. Toxicol. Sci. Off. J. Soc. Toxicol. 2009, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Budău, M.; Hancu, G.; Rusu, A.; Cârcu-Dobrin, M.; Muntean, D.L. Chirality of Modern Antidepressants: An Overview. Adv. Pharm. Bull. 2017, 7, 495–500. [Google Scholar] [CrossRef]
- Raikar, P.; Gurupadayya, B.; Koganti, V.S. Recent Advances in Chiral Separation of Antihistamine Drugs: Analytical and Bioanalytical Methods. Curr. Drug Deliv. 2018, 15, 1393–1410. [Google Scholar] [CrossRef] [PubMed]
- Čižmáriková, R.; Habala, L.; Valentová, J.; Markuliak, M. Survey of Pharmacological Activity and Pharmacokinetics of Selected β-Adrenergic Blockers in Regard to Their Stereochemistry. Appl. Sci. 2019, 9, 625. [Google Scholar] [CrossRef]
- Van Wart, S.A.; Mager, D.E. Clinical pharmacokinetics and pharmacodynamics of stereoisomeric drugs. In Drug Stereochemistry: Analytical Methods and Pharmacology, 3rd ed.; Jozwiak, K., Lough, W.J., Wainer, I.W., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 206–240. [Google Scholar]
- Wang, Y.; Zhou, J.; Han, Q.; Chen, Q.; Guo, L.; Fu, Y. Chiral recognition of penicillamine enantiomers based on DNA-MWNT complex modified electrode. Electroanalysis 2012, 24, 1561–1566. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Clissold, S.P. Ocular levobunolol. Drugs 1987, 34, 648–661. [Google Scholar] [CrossRef]
- Shrivastav, P.S.; Buha, S.M.; Sanyal, M. Detection and quantitation of β-blockers in plasma and urine. Bioanalysis 2010, 2, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, J.; Jungck, D.; Koch, A. The Molecular Mechanisms of Thalidomide Teratogenicity and Implications for Modern Medicine. Curr. Mol. Med. 2017, 17, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Lenz, W. A short history of thalidomide embryopathy. Teratology 1988, 38, 203–215. [Google Scholar] [CrossRef]
- Franks, M.E.; Macpherson, G.R.; Figg, W.D. Thalidomide. Lancet 2004, 363, 1802–1811. [Google Scholar] [CrossRef]
- Mori, T.; Ito, T.; Liu, S.; Ando, H.; Sakamoto, S.; Yamaguchi, Y.; Tokunaga, E.; Shibata, N.; Handa, H.; Hakoshima, T. Structural basis of thalidomide enantiomer binding to cereblon. Sci. Rep. 2018, 8, 1294. [Google Scholar] [CrossRef]
- Rentsch, K.M. The importance of stereoselective determination of drugs in the clinical laboratory. J. Biochem. Biophys. Methods 2002, 54, 1–9. [Google Scholar] [CrossRef]
- Tedesco, D.; Pietra, A.; Rossi, F.; Garagnani, M.; Borrello, E.; Bertucci, C.; Andrisano, V. Determination of dextromethorphan and levomethorphan in seized heroin samples by enantioselective HPLC and electronic CD. J. Pharm. Biomed. Anal. 2013, 81–82, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Mangas-Sánchez, J.; Busto, E.; Gotor-Fernández, V.; Malpartida, F.; Gotor, V. Asymmetric Chemoenzymatic Synthesis of Miconazole and Econazole Enantiomers. The Importance of Chirality in Their Biological Evaluation. J. Org. Chem. 2011, 76, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Foley, A.R.; Warner, C.; Zhang, X.; Rolandi, M.; Abrams, B.; Raskatov, J.A. Suppression of Oligomer Formation and Formation of Non-Toxic Fibrils upon Addition of Mirror-Image Aβ42 to the Natural l-Enantiomer. Angew. Chem. Int. Ed. Engl. 2017, 56, 11506–11510. [Google Scholar] [CrossRef]
- Raskatov, J.A.; Teplow, D.B. Using chirality to probe the conformational dynamics and assembly of intrinsically disordered amyloid proteins. Sci. Rep. 2017, 7, 12433. [Google Scholar] [CrossRef] [PubMed]
- Foley, A.R.; Finn, T.S.; Kung, T.; Hatami, A.; Lee, H.W.; Jia, M.; Rolandi, M.; Raskatov, J.A. Trapping and Characterization of Nontoxic Aβ42 Aggregation Intermediates. ACS Chem. Neurosci. 2019, 10, 3880–3887. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Finn, T.S.; Kuhn, A.J.; Abrams, B.; Raskatov, J.A. Chirality Dependence of Amyloid β Cellular Uptake and a New Mechanistic Perspective. ChemBioChem A Eur. J. Chem. Biol. 2019, 20, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Foley, A.R.; Lee, H.W.; Raskatov, J.A. A Focused Chiral Mutant Library of the Amyloid β 42 Central Electrostatic Cluster as a Tool to Stabilize Aggregation Intermediates. J. Org. Chem. 2020, 85, 1385–1391. [Google Scholar] [CrossRef]
- Rubin, N.; Perugia, E.; Goldschmidt, M.; Fridkin, M.; Addadi, L. Chirality of amyloid suprastructures. J. Am. Chem. Soc. 2008, 130, 4602–4603. [Google Scholar] [CrossRef]
- Rubin, N.; Perugia, E.; Wolf, S.G.; Klein, E.; Fridkin, M.; Addadi, L. Relation between serum amyloid A truncated peptides and their suprastructure chirality. J. Am. Chem. Soc. 2010, 132, 4242–4248. [Google Scholar] [CrossRef]
- Usov, I.; Adamcik, J.; Mezzenga, R. Polymorphism complexity and handedness inversion in serum albumin amyloid fibrils. ACS Nano 2013, 7, 10465–10474. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L.; Corey, R.B. Configurations of Polypeptide Chains with Favored Orientations around Single Bonds: Two New Pleated Sheets. Proc. Natl. Acad. Sci. USA 1951, 37, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L.; Corey, R.B. Two Rippled-Sheet Configurations of Polypeptide Chains, and a Note about the Pleated Sheets. Proc. Natl. Acad. Sci. USA 1953, 39, 253–256. [Google Scholar] [CrossRef]
- Urban, J.M.; Ho, J.; Piester, G.; Fu, R.; Nilsson, B.L. Rippled β-Sheet Formation by an Amyloid-β Fragment Indicates Expanded Scope of Sequence Space for Enantiomeric β-Sheet Peptide Coassembly. Molecules 2019, 24, 1983. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malyshko, E.V.; Semenova, E.V.; Bagrova, O.E.; Murtazina, A.R.; Tverdislov, V.A. Chiral Dualism as a Unifying Principle in Molecular Biophysics. Biophysica 2021, 1, 22-37. https://doi.org/10.3390/biophysica1010003
Malyshko EV, Semenova EV, Bagrova OE, Murtazina AR, Tverdislov VA. Chiral Dualism as a Unifying Principle in Molecular Biophysics. Biophysica. 2021; 1(1):22-37. https://doi.org/10.3390/biophysica1010003
Chicago/Turabian StyleMalyshko, Ekaterina V., Ekaterina V. Semenova, Olga E. Bagrova, Alina R. Murtazina, and Vsevolod A. Tverdislov. 2021. "Chiral Dualism as a Unifying Principle in Molecular Biophysics" Biophysica 1, no. 1: 22-37. https://doi.org/10.3390/biophysica1010003
APA StyleMalyshko, E. V., Semenova, E. V., Bagrova, O. E., Murtazina, A. R., & Tverdislov, V. A. (2021). Chiral Dualism as a Unifying Principle in Molecular Biophysics. Biophysica, 1(1), 22-37. https://doi.org/10.3390/biophysica1010003




