Optimizing Mix Design for Alkali-Activated Concrete: A Comprehensive Review of Critical Selection Factors
Abstract
1. Introduction
2. Significance of Study
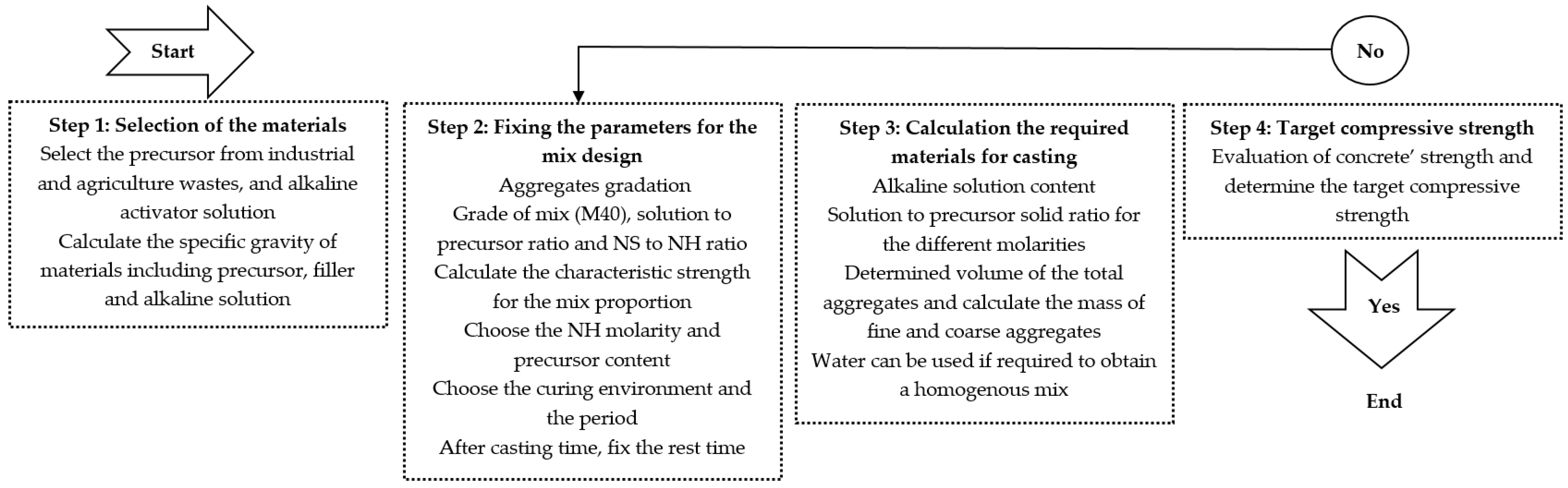
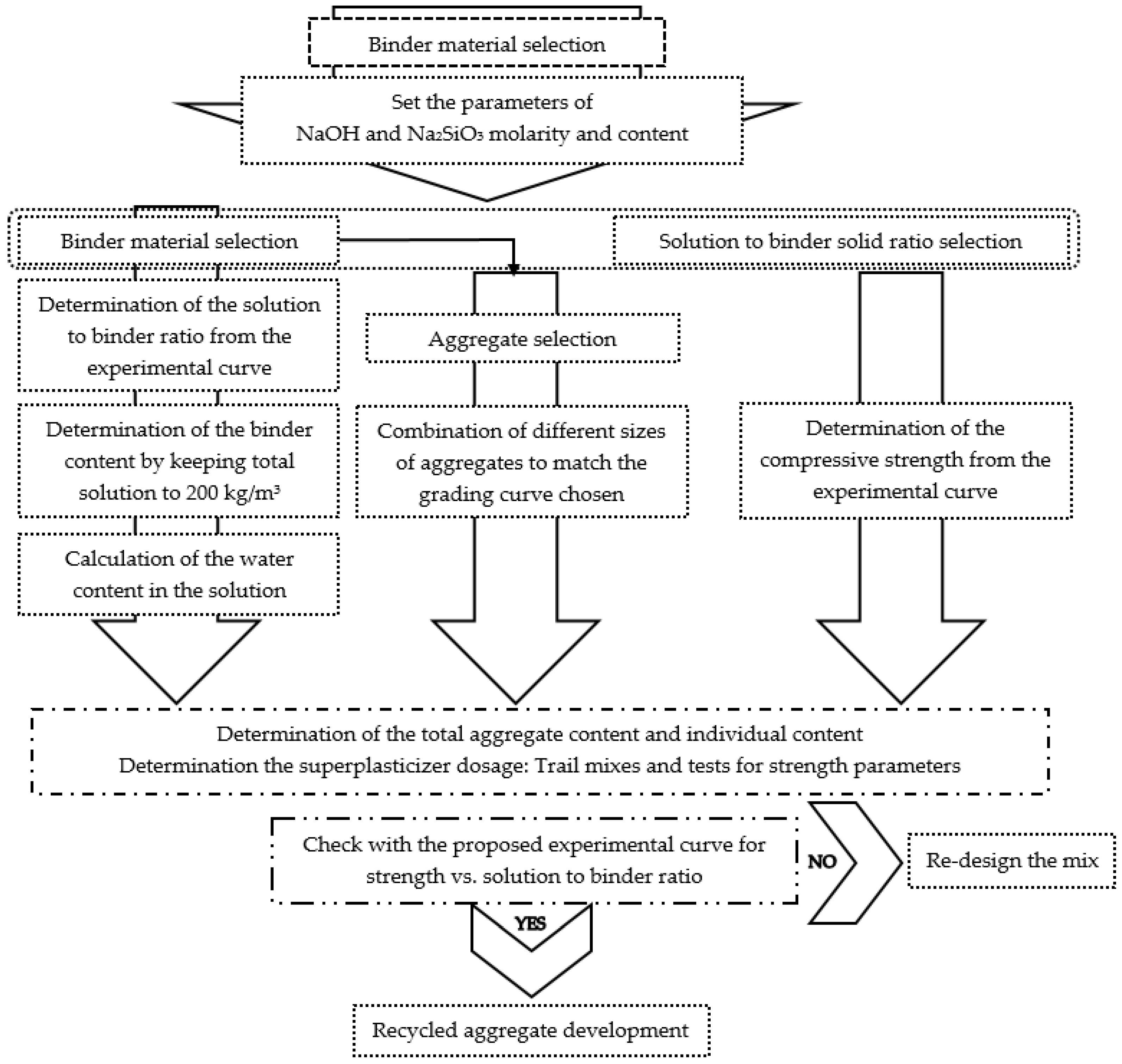
3. Alkali-Activated Mix Design Methods
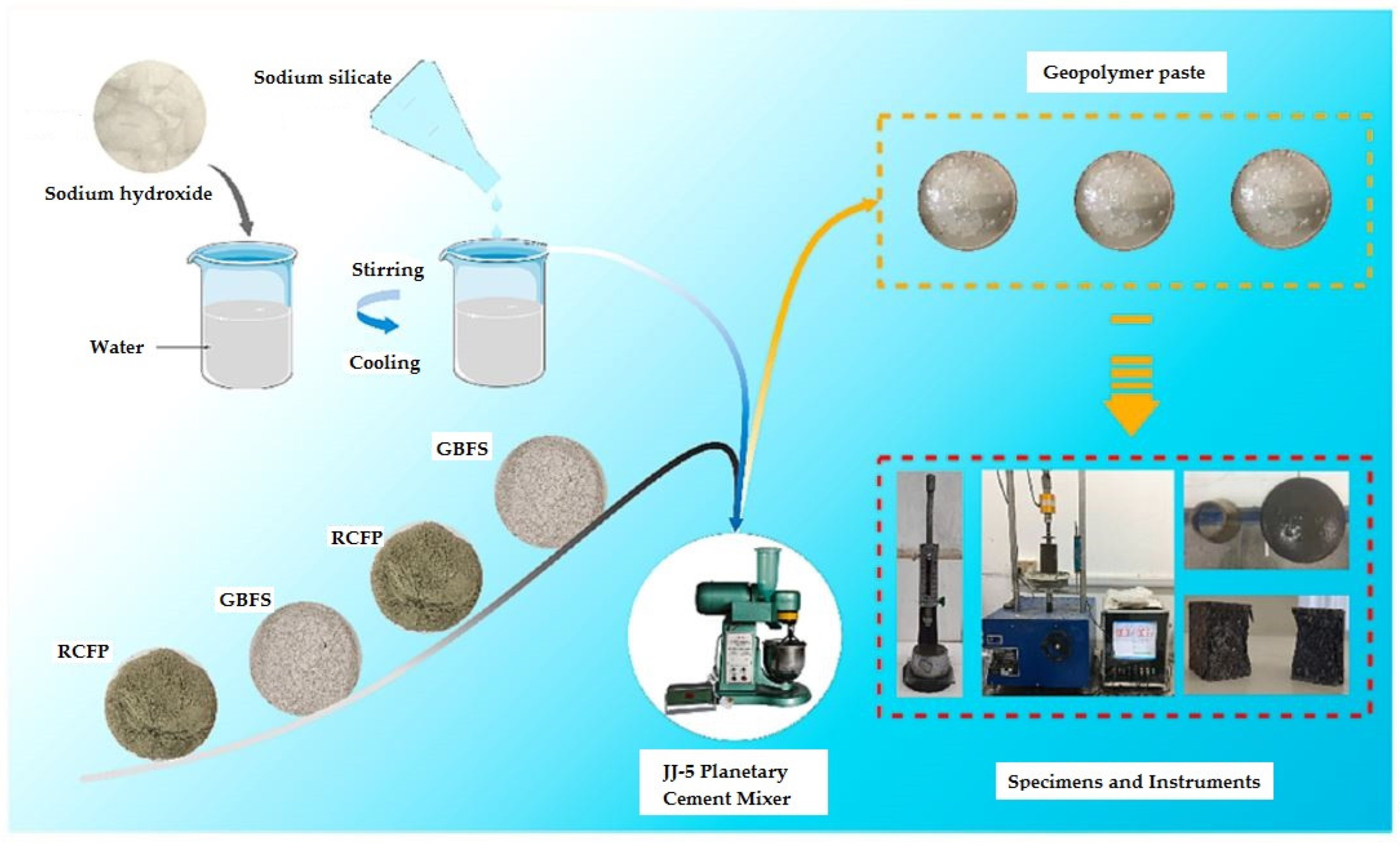
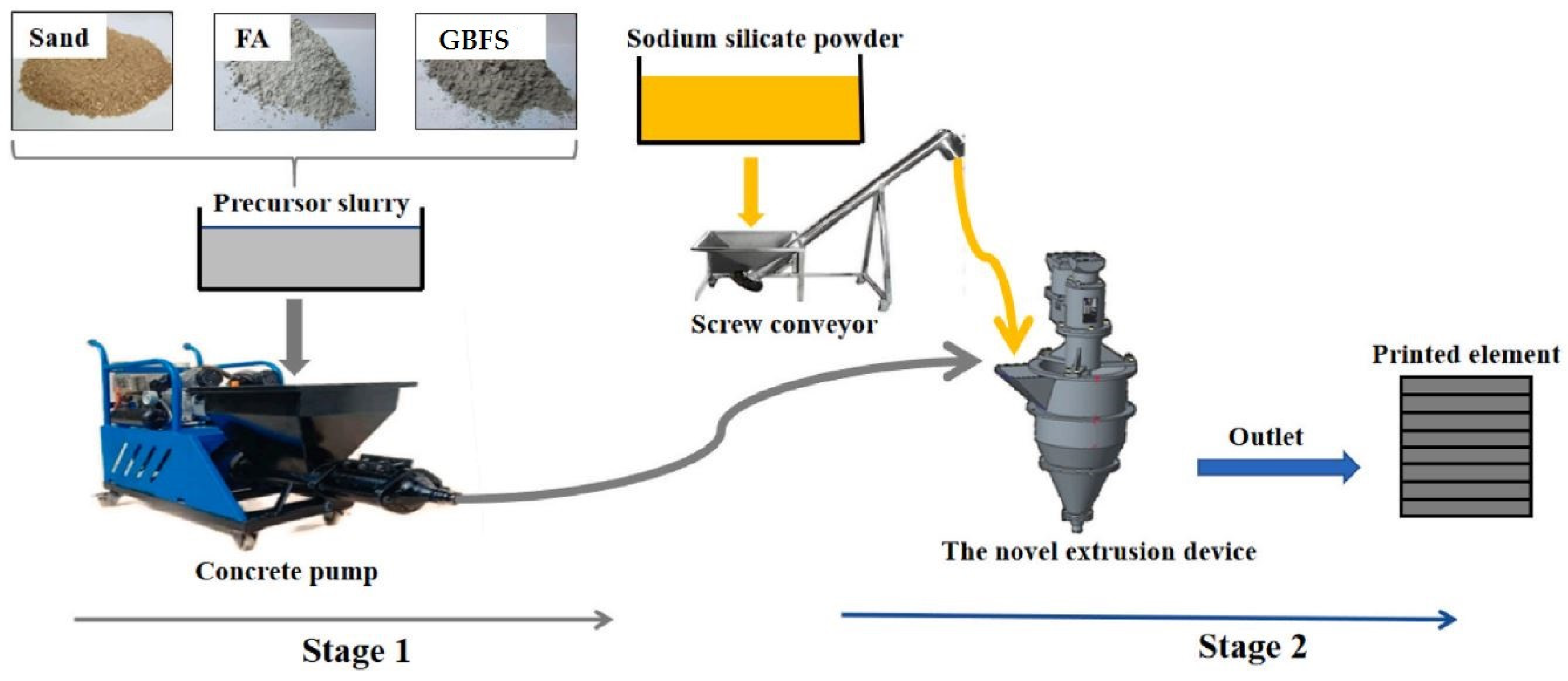
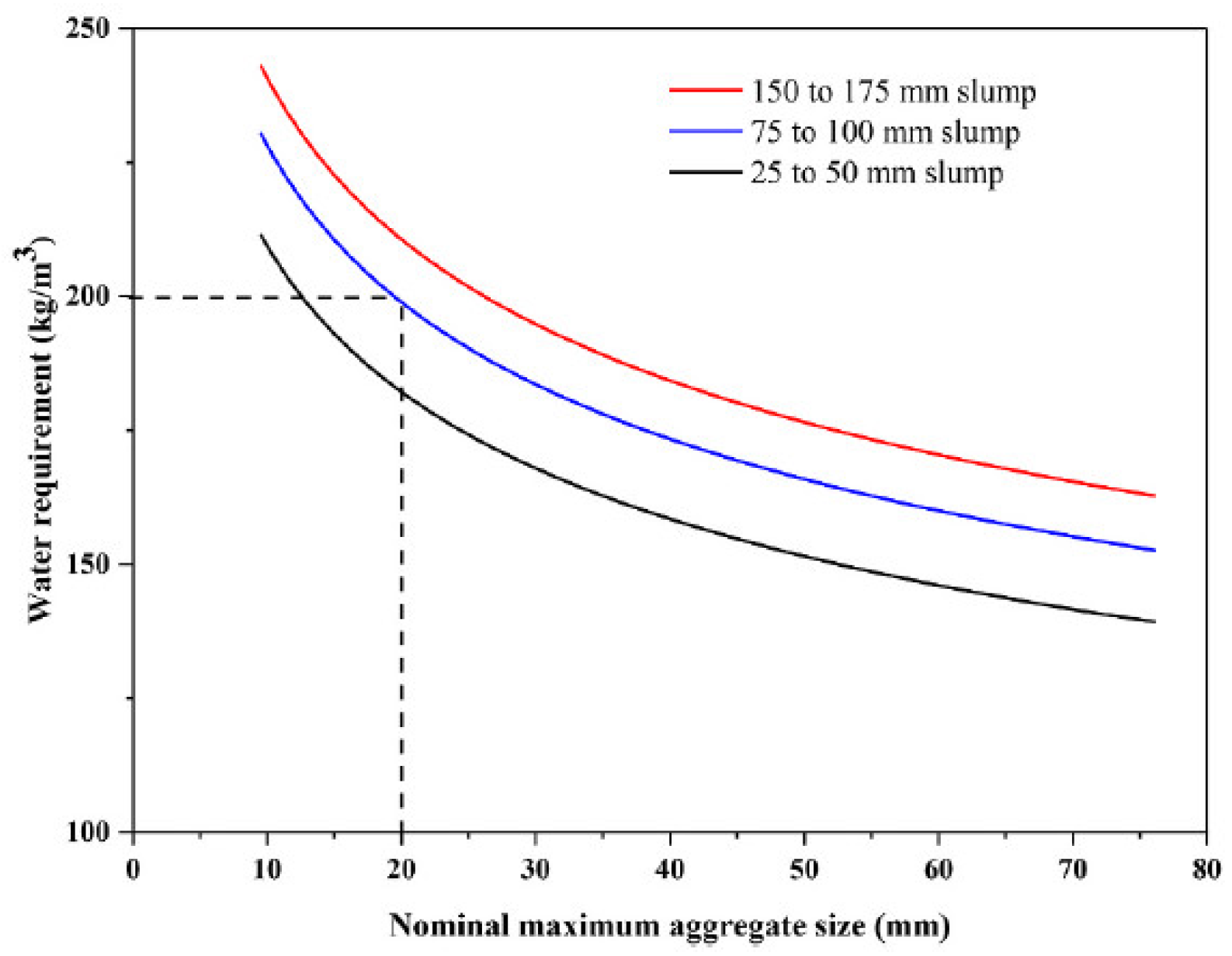
4. Preparation of Alkali-Activated Specimens
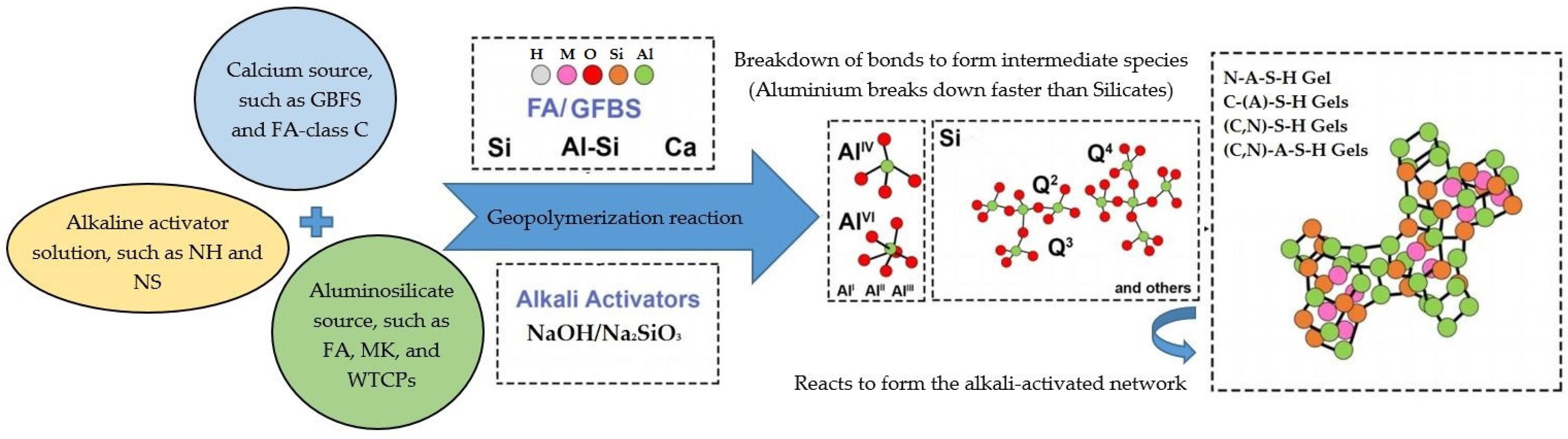
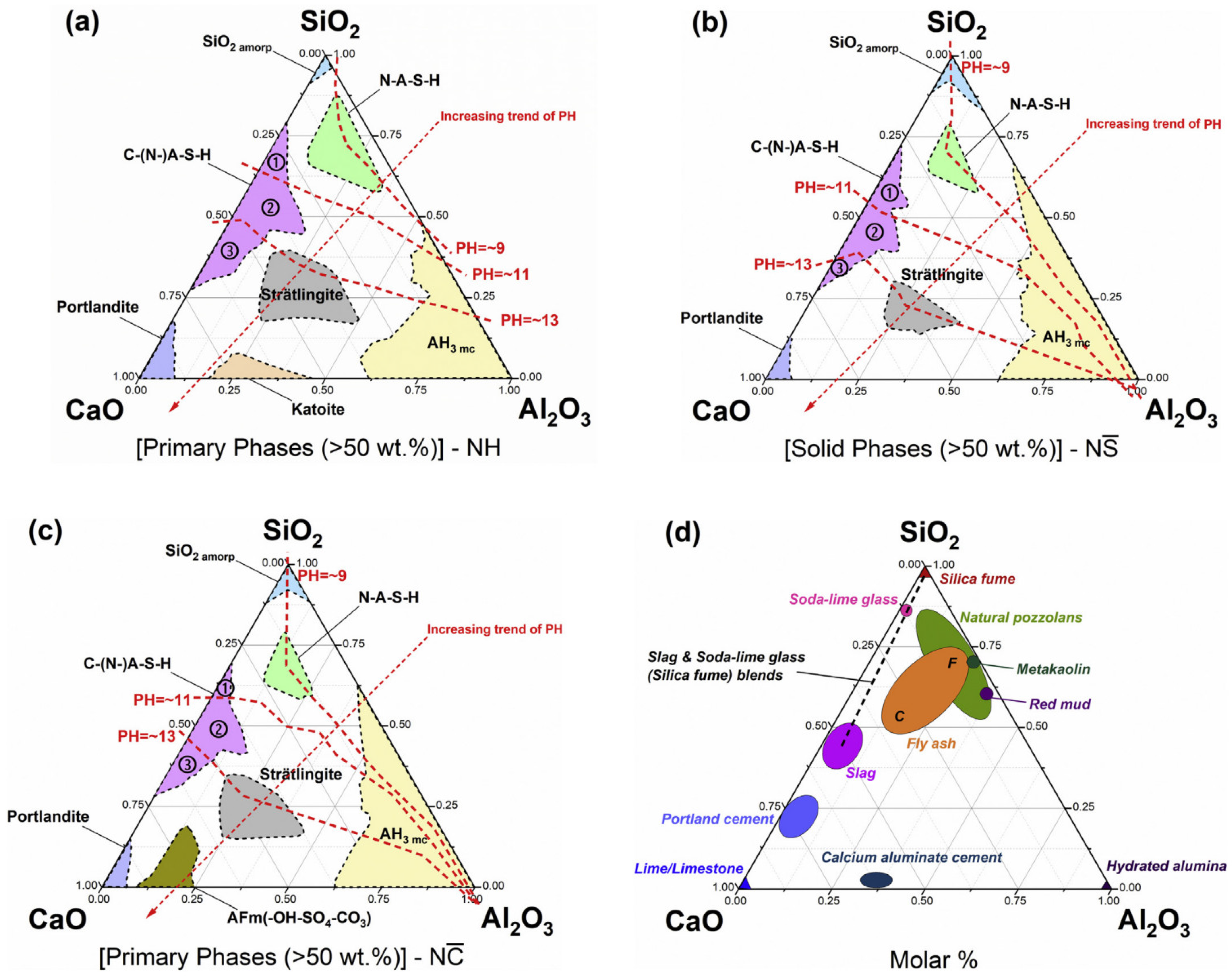
5. Geopolymerization Mechanism of AACs
6. Workability Performance
6.1. Effect of Binder Source, Chemical, and Physical Properties
6.2. Binder-Solution Effect
6.3. Solution Molarity Effect
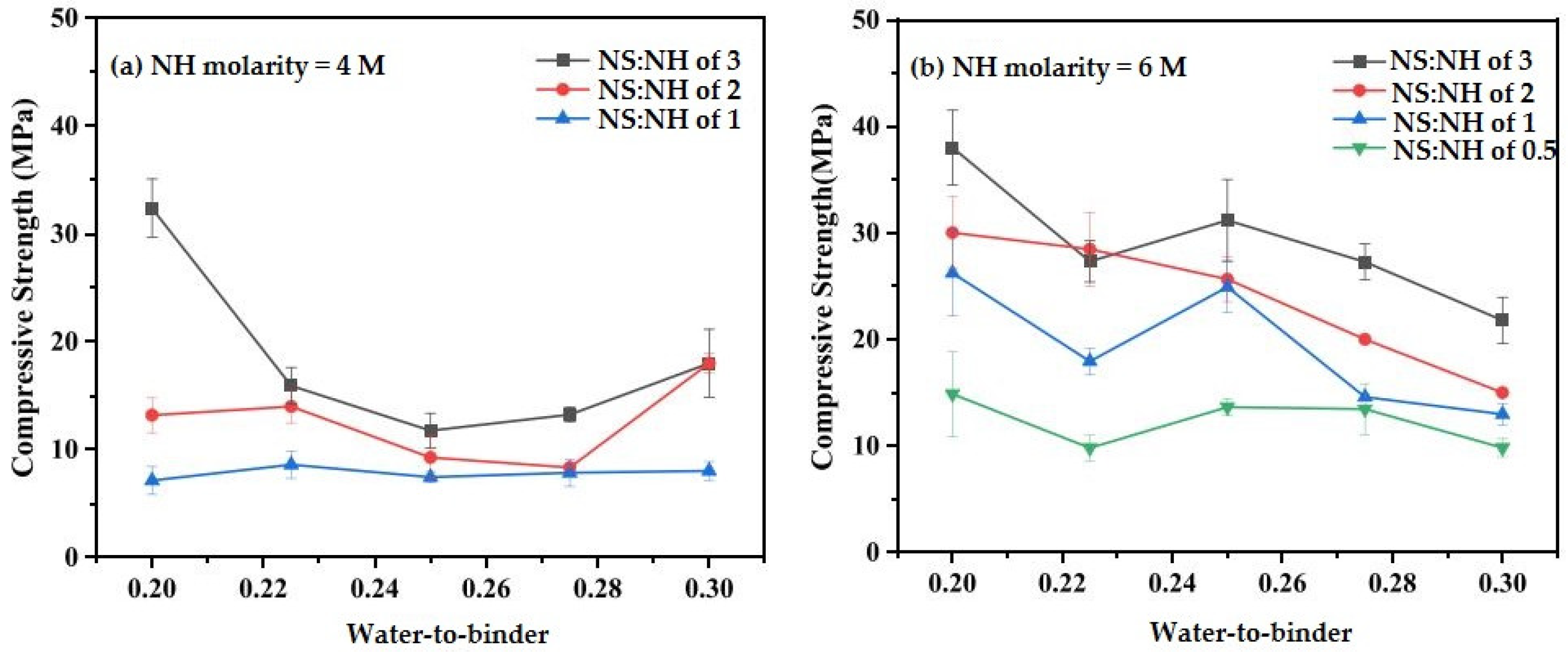
6.4. NS-to-NH Effect
6.5. Addition of Water, Superplasticizer, and Filler Content Effect
6.6. Correlation Matrix and Predictive Performance
7. Compressive Strength Performance
7.1. Effect of Binder Source and Chemical and Physical Properties
| Refs. | Reaction System | Molar Ratio | Workability and Strength Performance | |||
|---|---|---|---|---|---|---|
| Binder | AAS | Si/Al | Na/Al | Ca/Si | ||
| [139] | FA + GBFS | NS-NH | 1.67 | 0.40 | 0.78 | The authors reported that the CS was positively influenced by increasing the molar ratios of Si/Al, Na/Al, and Ca/Si, and with increasing these molar ratios to 1.67, 0.4, and 0.78, specimens achieved CS higher than 45 MPa. |
| [182] | MK + SF | NS-NH | 1.90 | 0.73 | - | The CS of prepared specimens was found to be significantly influenced by molar ratios of Si/Al and Na/Al. A positive effect was observed when the molar ratio of Si/Al increased from 1.62 to 1.9, and CS values increased from 8 MPa to 22 MPa; then dropped to 20 MPa with the molar ratio rising to 1.95. For the Na/Al molar ratio, the increase in the ratio from 0.43 to 0.73 led to an increase in the CS from 8 MPa to 32 MPa. However, a high loss (˃70%) in CS was observed when the molar ratio increased to 0.93, and the specimens showed lower performance. |
| [183] | RM + coal MK | NS-NH | - | 1 | - | From the reported results, CS values are significantly influenced by the Na/Al molar ratio. Increasing the molar ratio from 0.75 to 1.0 results in an excellent improvement in strength performance. However, increasing the molar ratio up to 1.0 (1.3) causes a drop in CS value, and the specimens lost more than 25% compared to the optimum molar ratio. |
| [140] | OPC kiln dust + RHA + SF | NS-NH | - | - | 0.52 | The flowability and both initial and final setting times are negatively influenced by increasing the molar ratio of Ca/Si, and the workability trend decreases with the increasing molar ratio. For strength performance, with the increase in the molar ratio of Ca/Si from 0.15 to 0.52, the CS was enhanced by more than 20%. However, increasing the molar concentration to 0.8 slightly leads to a drop in the CS value. A significant drop in CS was observed when the molar ratio reached 1.8, and specimens lost more than 40% of CS. |
| [55] | MK | NS-NH | 1.91 | - | - | The results show that increasing or reducing the Si/Al molar ratio to 2.5 or 1.25, respectively, results in a loss of CS of more than 6%. |
| [184] | MK + Meta-halloysite | NS-NH | 1.45 | 0.92 | - | The authors reported that increasing the molar ratios of Si/Al and Na/Al, from 1 and 0.60 to 1.45 and 0.92, respectively, positively enhanced the CS value from 30 MPa to more than 60 MPa. |
| [185] | FA + GBFS + Steel slag | NS-NH | 1.85 | 0.40 | 0.58 | The CS of prepared specimens was significantly influenced by the molar ratios of Si/Al, Na/Al, and Ca/Si. By reducing the molar ratio of Si/Al from 1.85 to 1.5, the CS dropped from 40 MPa to 28 MPa. Similarly, the reduction in Na/Al and Ca/Si molar values from 0.40 to 0.30 and 0.55 to 0.42 resulted in a significant loss in CS (˃22%). |
| [186] | Volcanic ash + MK | NS-NH | 1.90 | 0.61 | - | With the increase in the molar ratios of Si/Al and Na/Al to 2.30 and 0.75, respectively, more than 24% of CS was lost. |
| [187] | Fused volcanic ash + MK | NS-NH | 1.85 | 0.84 | - | Increasing the molar ratio of Na/Al from 0.84 to 1.15 results in a loss of CS of more than 20%. |
| [188] | Lithium slag + FA + SF | NS-NH | 1.31 | 0.29 | - | Significant loss of CS was found when the Na/Al molar ratio increased from 0.29 to 0.48. |
| [189] | FA + Steel slag | NS-NH | 1.62 | 0.31 | 0.42 | Increasing the molar ratio of Na/Al from 0.31 to more than 0.40 leads to a loss of more than 15% of CS. |
| [190] | Mine tailings slag + Calcium carbide residue | Soda residue + NH | 2.78 | 1.11 | 1.02 | The results obtained from this study show that the Si/Al molar ratio slightly influenced the CS of specimens. However, the most significant and largest drop in strength was observed when the molar ratios of Na/Al and Ca/Si increased to 1.26 and 1.7, respectively. |
| [191] | FA + Recycled fine powder | NS-NH | 3.53 | 0.71 | - | The results of CS slightly dropped when the Si/Al molar ratio increased up to 3.53. However, the specimens lost more than 20% of CS when the molar ratio of Na/Al increased from 0.71 to 0.80. |
| [192] | MK | NS-NH | 1.90 | - | - | Increasing the Si/Al molar content from 1.0 to 1.9 significantly enhanced the CS from 20 MPa to 79 MPa. However, an increase in silica content beyond that resulted in a drop in strength to 64 MPa. |
| [193] | Recycled concrete fine powder + Slag + Nano-SiO2 | NS-NH | 2.77 | - | 0.86 | It is observed that the CS is slightly influenced and drops with increasing silica content. |
| [194] | MK | NS-NH | 1.50 | - | - | A significant drop in CS (from 80 MPa to 42 MPa) occurred with increasing silica content up to the optimum level. |
| [195] | FA + OPC | NS-NH | 2.97 | 0.87 | 0.83 | CS was found to be enhanced with increasing the molar ratio of Ca/Si using GBFS as a source of calcium materials in the geopolymerization process. |
| [196] | FA + GBFS | NS-NH | - | - | 0.17 | The CS was slightly influenced by increasing the molar ratio of Ca/Si from 0.17 to 0.20. |
| [197] | MK + RHA + Chicken eggshell | NS-NH | - | - | 0.40 | Increasing the Ca/Si molar ratio to 0.40 resulted in a slight loss of CS, and a significant drop in strength was observed when the molar ratio reached 1.0. |
| [198] | FA + Calcium Aluminate Cement | NS-NH | - | - | 0.43 | CS significantly dropped with increasing the molar ratio of Ca/Si up to 0.43. |
| [199] | Iron ore tailings + lime + GBFS | NS | - | - | 0.35 | Increasing the Ca/Si ratio up to 0.35 resulted in a decrease in the strength values. |
| [103] | RCFP + GBFS | NS-NH | 2.68 | 0.89 | 0.89 | Flowability and initial and final setting times tend to decrease with an increasing Ca/Si molar ratio and with a reduction in the Na/Si and Si/Al molar ratios. CS tends to increase with the increasing molar ratio of Ca/Si. |
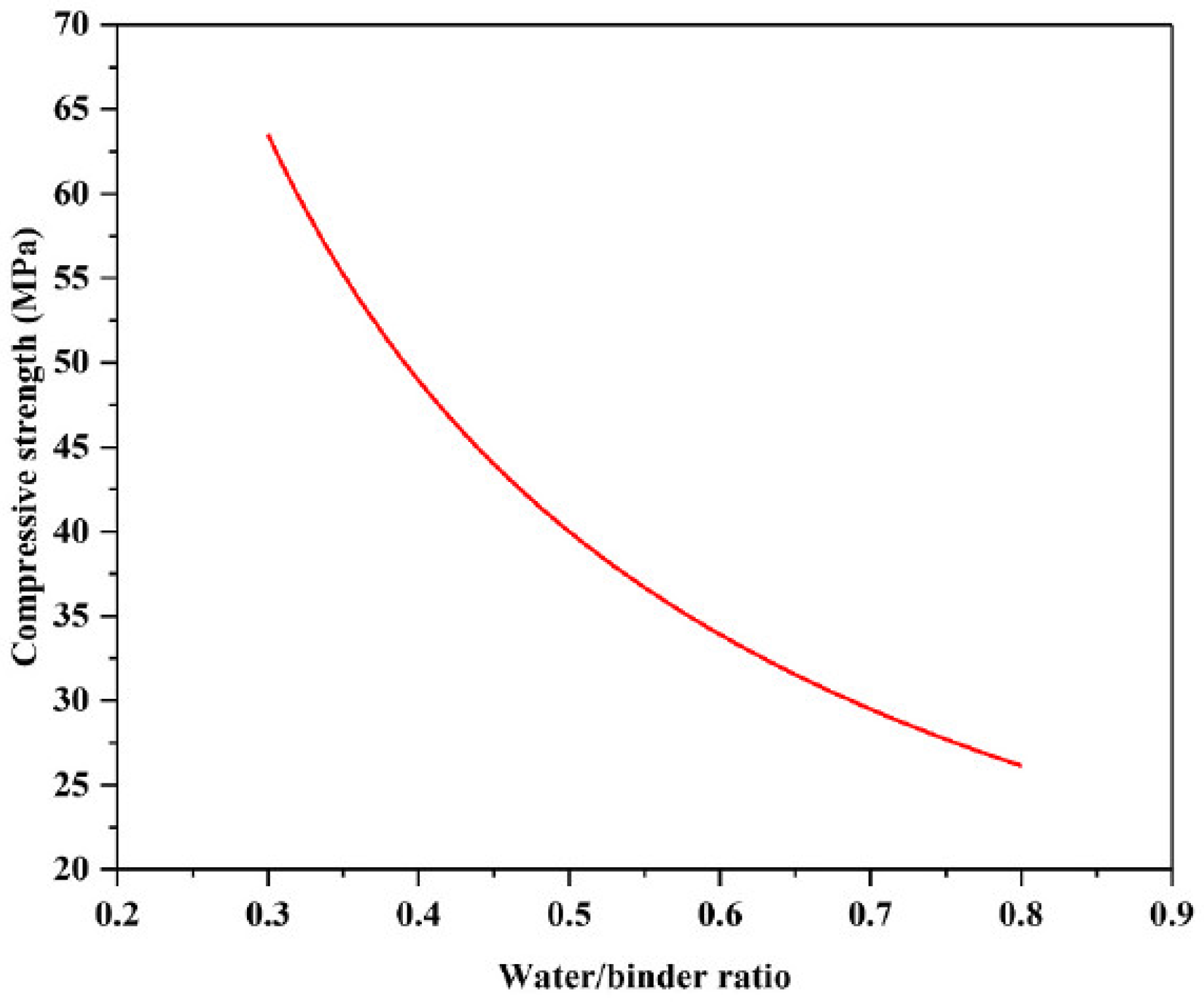
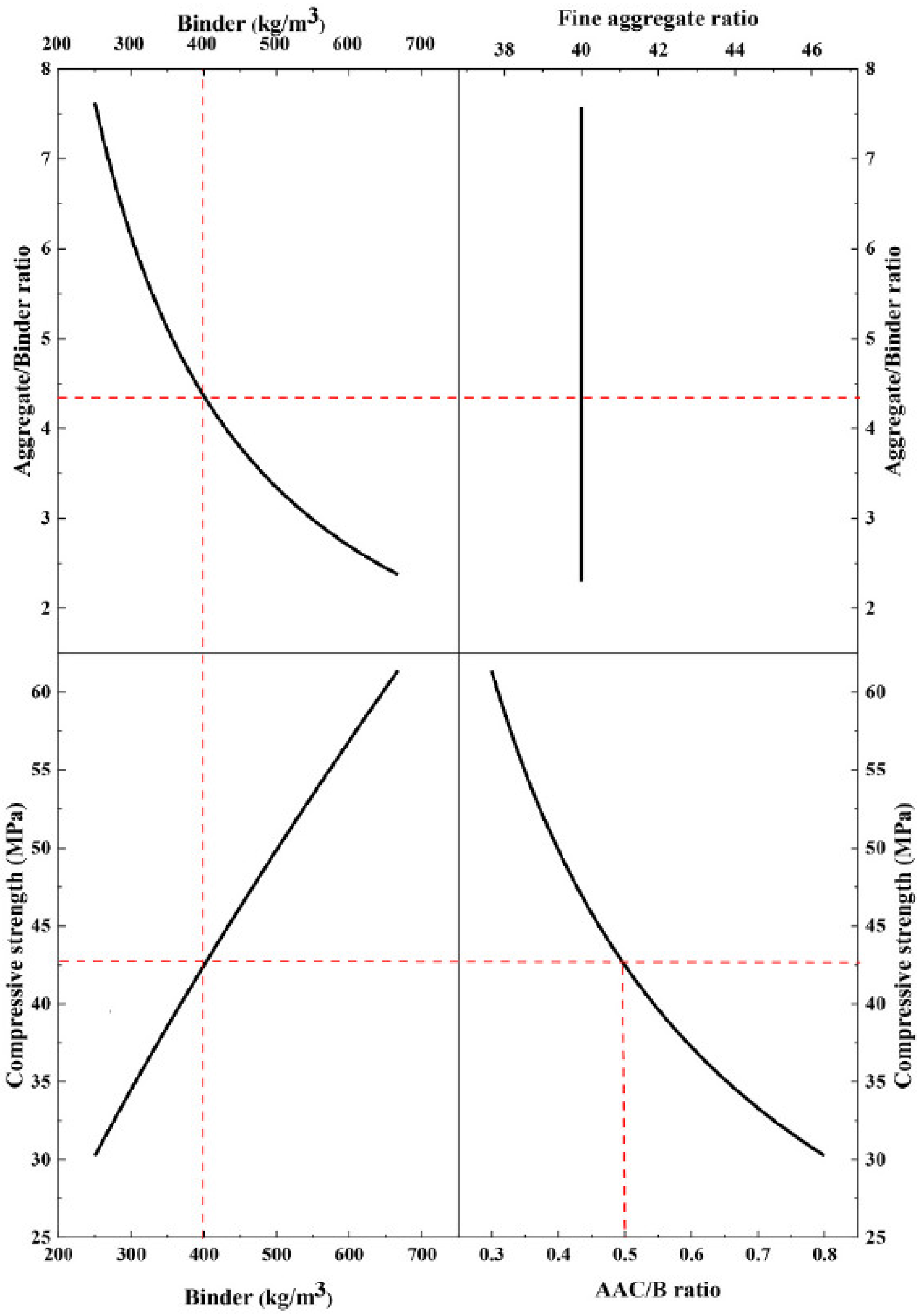
7.2. Effect of Solution on Binder Ratio
7.3. Effect of Activator Toxicity and Cost
7.4. Effect of Solution Type and Molarity
7.5. Effect of Solution Modulus (NS to NH) and Curing Methods
7.6. Effect of the Addition of Water, Superplasticizer, and Filler Content
7.7. Bond Zone and Aggregates Content
7.8. Effect of Curing Methods
7.9. Correlation Matrix and Predictive Performance
7.10. Relationship Between the Workability and Compressive Strength
8. Comparative Study
9. Conclusions
10. Recommendations and Future Vision
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AABs | Alkali-activated binders |
| AAC | Alkali-activated concrete |
| AAS | Alkaline activator solution |
| AS | Aluminosilicate |
| CA | Coarse aggregate |
| C-(A)-S-H | Calcium-alumino-silicate-hydrate |
| CH | Calcium hydroxide |
| CS | Compressive strength |
| FA | Fly ash |
| GBFS | Ground blast furnace slag |
| GPZ | Geopolymerization |
| ITZ | Interfacial transition zone |
| MK | Metakaolin |
| NH | Sodium hydroxide |
| NS | Sodium silicate |
| OPC | Ordinary Portland cement |
| RA | Recycled aggregate |
| RCFP | Recycled concrete fine powder |
| RHAs | Rice husk ash |
| RMs | Red mud |
| RS | River sand (Fine aggregate) |
| POFA | Palm oil fuel ash |
| PSO | Particle swarm optimization |
| SF | Silica fume |
| WGMs | Waste glass |
| WTCPs | Waste tile ceramics |
References
- Xu, J.-H.; Fleiter, T.; Eichhammer, W.; Fan, Y. Energy consumption and CO2 emissions in China’s cement industry: A perspective from LMDI decomposition analysis. Energy Policy 2012, 50, 821–832. [Google Scholar] [CrossRef]
- Du, G.; Sun, C.; Ouyang, X.; Zhang, C. A decomposition analysis of energy-related CO2 emissions in Chinese six high-energy intensive industries. J. Clean. Prod. 2018, 184, 1102–1112. [Google Scholar] [CrossRef]
- Talaei, A.; Pier, D.; Iyer, A.V.; Ahiduzzaman, M.; Kumar, A. Assessment of long-term energy efficiency improvement and greenhouse gas emissions mitigation options for the cement industry. Energy 2019, 170, 1051–1066. [Google Scholar] [CrossRef]
- Belaïd, F. How does concrete and cement industry transformation contribute to mitigating climate change challenges? Resour. Conserv. Recycl. Adv. 2022, 15, 200084. [Google Scholar] [CrossRef]
- Huseien, G.F.; Tang, W.; Yu, Y.; Wong, L.S.; Mirza, J.; Dong, K.; Gu, X. Evaluation of high-volume fly-ash cementitious binders incorporating nanosilica as eco-friendly sustainable concrete repair materials. Constr. Build. Mater. 2024, 447, 138022. [Google Scholar] [CrossRef]
- Joudah, Z.H.; Khalid, N.H.A.; Baghban, M.H.; Faridmehr, I.; Talip, A.R.A.; Huseien, G.F. Development sustainable concrete with high-volume wastes tile ceramic: Role of silica nanoparticles amalgamation. Case Stud. Constr. Mater. 2024, 21, e03733. [Google Scholar] [CrossRef]
- Huseien, G.F.; Joudah, Z.H.; Baghban, M.H.; A. Khalid, N.H.; Faridmehr, I.; Dong, K.; Li, Y.; Gu, X. Sustainability of Recycling Waste Ceramic Tiles in the Green Concrete Industry: A Comprehensive Review. Buildings 2025, 15, 2406. [Google Scholar] [CrossRef]
- Monteiro, P.J.; Miller, S.A.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698–699. [Google Scholar] [CrossRef]
- Mhaya, A.M.; Shahidan, S.; Algaifi, H.A.; Zuki, S.S.M.; Benjeddou, O.; Ibrahim, M.H.W.; Huseien, G.F. Thermal conductivity of coconut shell-incorporated concrete: A systematic assessment via theory and experiment. Sustainability 2022, 14, 16167. [Google Scholar] [CrossRef]
- Deraemaeker, A.; Dumoulin, C. Embedding ultrasonic transducers in concrete: A lifelong monitoring technology. Constr. Build. Mater. 2019, 194, 42–50. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.; Hussein, A.A. Geopolymer mortars as sustainable repair material: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ariffin, N.F.; Hussin, M.W. Synthesis and characterization of self-healing mortar with modified strength. J. Teknol. 2015, 76, 195–200. [Google Scholar] [CrossRef]
- Xu, D.; Cui, Y.; Li, H.; Yang, K.; Xu, W.; Chen, Y. On the future of Chinese cement industry. Cem. Concr. Res. 2015, 78, 2–13. [Google Scholar] [CrossRef]
- Zhang, W.; Li, K.; Zhou, D.; Zhang, W.; Gao, H. Decomposition of intensity of energy-related CO2 emission in Chinese provinces using the LMDI method. Energy Policy 2016, 92, 369–381. [Google Scholar] [CrossRef]
- Samadi, M.; Huseien, G.F.; Mohammadhosseini, H.; Lee, H.S.; Lim, N.H.A.S.; Tahir, M.M.; Alyousef, R. Waste ceramic as low cost and eco-friendly materials in the production of sustainable mortars. J. Clean. Prod. 2020, 266, 121825. [Google Scholar] [CrossRef]
- Xi, F.; Davis, S.J.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L. Substantial global carbon uptake by cement carbonation. Nat. Geosci. 2016, 9, 880–883. [Google Scholar] [CrossRef]
- Esmeray, E.; Atıs, M. Utilization of sewage sludge, oven slag and fly ash in clay brick production. Constr. Build. Mater. 2019, 194, 110–121. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.; Heikal, E.; El-Didamony, H.; Hashim, F.; Mohammed, A.H. Recycling of concrete waste to produce ready-mix alkali activated cement. Ceram. Int. 2018, 44, 7300–7304. [Google Scholar] [CrossRef]
- Huseien, G.F.; Ismail, M.; Tahir, M.M.; Mirza, J.; Khalid, N.H.A.; Asaad, M.A.; Husein, A.A.; Sarbini, N.N. Synergism between palm oil fuel ash and slag: Production of environmental-friendly alkali activated mortars with enhanced properties. Constr. Build. Mater. 2018, 170, 235–244. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Mirza, J.; Tahir, M.M.; Asaad, M.A.; Ismail, M.; Shah, K.W. Waste ceramic powder incorporated alkali activated mortars exposed to elevated Temperatures: Performance evaluation. Constr. Build. Mater. 2018, 187, 307–317. [Google Scholar] [CrossRef]
- Huseien, G.F.; Faridmehr, I.; Nehdi, M.L.; Abadel, A.A.; Aiken, T.A.; Ghoshal, S. Structure, morphology and compressive strength of Alkali-activated mortars containing waste bottle glass nanoparticles. Constr. Build. Mater. 2022, 342, 128005. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Mirza, J.; Tahir, M.M. Evaluation of alkali-activated mortars containing high volume waste ceramic powder and fly ash replacing GBFS. Constr. Build. Mater. 2019, 210, 78–92. [Google Scholar] [CrossRef]
- Chithambaram, S.J.; Kumar, S.; Prasad, M.M.; Adak, D. Effect of parameters on the compressive strength of fly ash based geopolymer concrete. Struct. Concr. 2018, 19, 1202–1209. [Google Scholar] [CrossRef]
- Eryılmaz, K.; Polat, R. Sustainable concrete production: Mechanical and durability behaviour of slag-based geopolymer containing recycled geopolymer aggregate. J. Build. Eng. 2024, 96, 110512. [Google Scholar] [CrossRef]
- Faridmehr, I.; Nehdi, M.L.; Huseien, G.F.; Baghban, M.H.; Sam, A.R.M.; Algaifi, H.A. Experimental and informational modeling study of sustainable self-compacting geopolymer concrete. Sustainability 2021, 13, 7444. [Google Scholar] [CrossRef]
- Assi, L.N.; Carter, K.; Deaver, E.; Ziehl, P. Review of availability of source materials for geopolymer/sustainable concrete. J. Clean. Prod. 2020, 263, 121477. [Google Scholar] [CrossRef]
- Algaifi, H.A.; Huseien, G.F.; Syamsir, A.; Qaid, A.; Baharom, S.; Mhaya, A.M. Optimizing durability and performance in high-volume fly ash-based alkali-activated mortar with palm oil fuel ash and slag: A response surface methodology approach. Dev. Built Environ. 2024, 18, 100427. [Google Scholar] [CrossRef]
- Hamzah, H.K.; Georgescu, D.P.; Khalid, N.H.A.; Hussein, G.F. Strength performance of free cement mortars incorporating fly ash and slag: Effects of alkaline activator solution dosage. Open J. Sci. Technol. 2020, 3, 87–98. [Google Scholar] [CrossRef]
- Yanting, M.; Bo, D.; Xiaotong, Y.; Da, C.; Yingdi, L. Study on mechanical properties and durability of geopolymer concrete with oyster shell aggregate. Constr. Build. Mater. 2025, 472, 140926. [Google Scholar] [CrossRef]
- Faridmehr, I.; Nehdi, M.L.; Nikoo, M.; Huseien, G.F.; Ozbakkaloglu, T. Life-Cycle Assessment of Alkali-Activated Materials Incorporating Industrial Byproducts. Materials 2021, 14, 2401. [Google Scholar] [CrossRef]
- Chand, G.; Ram, S.; Kumar, S.; Gupta, U. Microstructural and engineering properties investigation of sustainable hybrid concrete produced from industrial wastes. Clean. Eng. Technol. 2021, 2, 100052. [Google Scholar] [CrossRef]
- Feng, B.; Lu, Z.; Zhang, Y.; Zhang, X.; He, L.; Li, Y. Physical, mechanical properties and microstructure of waste GBFS-based geopolymer incorporated with metakaolin and SiC whiskers (SiCw). J. Build. Eng. 2024, 91, 109653. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Ma, T.; Gu, G.; Chen, C.; Hu, J. Understanding the changes in engineering behaviors and microstructure of FA-GBFS based geopolymer paste with addition of silica fume. J. Build. Eng. 2023, 70, 106450. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Tahir, M.M.; Mirza, J. Properties of ceramic tile waste based alkali-activated mortars incorporating GBFS and fly ash. Constr. Build. Mater. 2019, 214, 355–368. [Google Scholar] [CrossRef]
- Bai, F.; Bai, B.; Chen, J.; Nie, Q.; Liu, J. Development of an ambient cured high-strength red mud-based geopolymer through calcination activation. Sustain. Chem. Pharm. 2024, 42, 101866. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.; Ariffin, M.A.M. Effect of metakaolin replaced granulated blast furnace slag on fresh and early strength properties of geopolymer mortar. Ain Shams Eng. J. 2018, 9, 1557–1566. [Google Scholar] [CrossRef]
- Mhaya, A.M.; Shahidan, S.; Goel, A.; Huseien, G.F. Effect of metakaolin content and shape design on strength performance of lightweight rubberized geopolymer mortars incorporated slag-waste glass powders. Constr. Build. Mater. 2024, 432, 136500. [Google Scholar] [CrossRef]
- Poojalakshmi, E.; Patel, J.; Sunantha, B.; Thomas, B.S.; Ramaswamy, K.; Khan, R.A. Effect of mechanical activation on the properties of rice husk ash-based one part geopolymer. Mater. Today Proc. 2023, 1–7. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Cheng, Y.; Huseien, G.F.; Shah, K.W. Shrinkage mechanisms and shrinkage-mitigating strategies of alkali-activated slag composites: A critical review. Constr. Build. Mater. 2022, 318, 125993. [Google Scholar] [CrossRef]
- Kubba, Z.; Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Ismail, M.; Tahir, M.M.; Mirza, J. Impact of curing temperatures and alkaline activators on compressive strength and porosity of ternary blended geopolymer mortars. Case Stud. Constr. Mater. 2018, 9, e00205. [Google Scholar] [CrossRef]
- Huseien, G.F.; Hamzah, H.K.; Sam, A.R.M.; Khalid, N.H.A.; Shah, K.W.; Deogrescu, D.P.; Mirza, J. Alkali-activated mortars blended with glass bottle waste nano powder: Environmental benefit and sustainability. J. Clean. Prod. 2020, 243, 118636. [Google Scholar] [CrossRef]
- Huseien, G.F.; Shah, K.W. Durability and life cycle evaluation of self-compacting concrete containing fly ash as GBFS replacement with alkali activation. Constr. Build. Mater. 2020, 235, 117458. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Luo, W. Fresh properties, mechanical strength and microstructure of fly ash geopolymer paste reinforced with sawdust. Constr. Build. Mater. 2016, 111, 600–610. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Pacheco-Torgal, F.; Félix, T.; Tahri, W.; Aguiar, J.B. Mix design, properties and cost analysis of fly ash-based geopolymer foam. Constr. Build. Mater. 2015, 80, 18–30. [Google Scholar] [CrossRef]
- İlkentapar, S.; Atiş, C.; Karahan, O.; Avşaroğlu, E.G. Influence of duration of heat curing and extra rest period after heat curing on the strength and transport characteristic of alkali activated class F fly ash geopolymer mortar. Constr. Build. Mater. 2017, 151, 363–369. [Google Scholar] [CrossRef]
- Rihan, M.A.M.; Onchiri, R.O.; Gathimba, N.; Sabuni, B. Assessing the Durability Performance of Geopolymer Concrete Utilizing Fly Ash and Sugarcane Bagasse Ash as Sustainable Binders. Open Ceram. 2024, 20, 100687. [Google Scholar] [CrossRef]
- Gopalakrishna, B.; Dinakar, P. An innovative approach to fly ash-based geopolymer concrete mix design: Utilizing 100% recycled aggregates. Structures 2024, 66, 106819. [Google Scholar] [CrossRef]
- Ahıskalı, A.; Ahıskalı, M.; Bayraktar, O.Y.; Kaplan, G.; Assaad, J. Mechanical and durability properties of polymer fiber reinforced one-part foam geopolymer concrete: A sustainable strategy for the recycling of waste steel slag aggregate and fly ash. Constr. Build. Mater. 2024, 440, 137492. [Google Scholar] [CrossRef]
- Li, F.; Fan, S.; Xiao, S.; Huo, J.; Yuan, Y.; Chen, Z. Flexural performance and damage of reinforced fly ash and slag-based geopolymer concrete after coupling effect of freeze-thaw cycles and sustained loading. Structures 2024, 64, 106537. [Google Scholar] [CrossRef]
- Bagheri, A.; Nazari, A.; Sanjayan, J.G.; Rajeev, P. Alkali activated materials vs geopolymers: Role of boron as an eco-friendly replacement. Constr. Build. Mater. 2017, 146, 297–302. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of geopolymer mortar under ambient temperature for in situ applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef]
- Huseien, G.F.; Ismail, M.; Khalid, N.H.A.; Hussin, M.W.; Mirza, J. Compressive strength and microstructure of assorted wastes incorporated geopolymer mortars: Effect of solution molarity. Alex. Eng. J. 2018, 57, 3375–3386. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; Mejía de Gutiérrez, R.; Gordillo, M.; Provis, J.L. Mechanical and thermal characterisation of geopolymers based on silicate-activated metakaolin/slag blends. J. Mater. Sci. 2011, 46, 5477–5486. [Google Scholar] [CrossRef]
- De Silva, P.; Sagoe-Crenstil, K. Medium-term phase stability of Na2O–Al2O3–SiO2–H2O geopolymer systems. Cem. Concr. Res. 2008, 38, 870–876. [Google Scholar] [CrossRef]
- De Silva, P.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Hatanaka, S.; Cao, T. High-strength geopolymer using fine high-calcium fly ash. J. Mater. Civ. Eng. 2011, 23, 264–270. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Ohno, M.; Li, V.C. An integrated design method of Engineered Geopolymer Composite. Cem. Concr. Compos. 2018, 88, 73–85. [Google Scholar] [CrossRef]
- Aleem, M.A.; Arumairaj, P. Optimum mix for the geopolymer concrete. Indian J. Sci. Technol. 2012, 5, 2299–2301. [Google Scholar] [CrossRef]
- Kupaei, R.H.; Alengaram, U.J.; Jumaat, M.Z.B.; Nikraz, H. Mix design for fly ash based oil palm shell geopolymer lightweight concrete. Constr. Build. Mater. 2013, 43, 490–496. [Google Scholar] [CrossRef]
- Hadi, M.N.; Farhan, N.A.; Sheikh, M.N. Design of geopolymer concrete with GGBFS at ambient curing condition using Taguchi method. Constr. Build. Mater. 2017, 140, 424–431. [Google Scholar] [CrossRef]
- Kantro, D.L. Influence of water-reducing admixtures on properties of cement paste—A miniature slump test. Cem. Concr. Aggreg. 1980, 2, 95–102. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J. Early age strength and workability of slag pastes activated by sodium silicates. Mag. Concr. Res. 2001, 53, 321–326. [Google Scholar] [CrossRef]
- Collins, F.; Lambert, J.; Duan, W.H. The influences of admixtures on the dispersion, workability, and strength of carbon nanotube–OPC paste mixtures. Cem. Concr. Compos. 2012, 34, 201–207. [Google Scholar] [CrossRef]
- Lloyd, N.; Rangan, V. Geopolymer concrete with fly ash. In Proceedings of the Second International Conference on sustainable construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; pp. 1493–1504. [Google Scholar]
- Anuradha, R.; Sreevidya, V.; Venkatasubramani, R.; Rangan, B.V. Modified guidelines for geopolymer concrete mix design using Indian standard. Asian J. Civ. Eng. 2012, 13, 353–364. [Google Scholar]
- Karthik, S.; Mohan, K.S.R. A taguchi approach for optimizing design mixture of geopolymer concrete incorporating fly ash, ground granulated blast furnace slag and silica fume. Crystals 2021, 11, 1279. [Google Scholar] [CrossRef]
- Bondar, D.; Nanukuttan, S.; Provis, J.L.; Soutsos, M. Efficient mix design of alkali activated slag concretes based on packing fraction of ingredients and paste thickness. J. Clean. Prod. 2019, 218, 438–449. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Zhang, Z.; Zhu, D.; Hwang, H.-J.; Zhu, Y.; Sun, T. A mixture proportioning method for the development of performance-based alkali-activated slag-based concrete. Cem. Concr. Compos. 2018, 93, 163–174. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Haruna, S.; Liew, M. Optimization and characterization of cast in-situ alkali-activated pastes by response surface methodology. Constr. Build. Mater. 2019, 225, 776–787. [Google Scholar] [CrossRef]
- Mermerdaş, K.; Algın, Z.; Oleiwi, S.M.; Nassani, D.E. Optimization of lightweight GGBFS and FA geopolymer mortars by response surface method. Constr. Build. Mater. 2017, 139, 159–171. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Molino, B.; De Vincenzo, A.; Ferone, C.; Messina, F.; Colangelo, F.; Cioffi, R. Recycling of clay sediments for geopolymer binder production. A new perspective for reservoir management in the framework of Italian legislation: The Occhito reservoir case study. Materials 2014, 7, 5603–5616. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, B.; Dinakar, P. Mix design development of fly ash-GGBS based recycled aggregate geopolymer concrete. J. Build. Eng. 2023, 63, 105551. [Google Scholar] [CrossRef]
- Pattanayak, N.; Behera, H.K.; Das, S.S. Mix design strategy and optimization considering characteristic evaluation of geopolymer concrete. J. Build. Eng. 2024, 91, 109557. [Google Scholar] [CrossRef]
- Nataraja, M.; Shivakumara, M.; Dalawai, V.N. Effect of design parameters on the proportioning of mass concrete using fly ash and ground granulated blast furnace slag. Mater. Today Proc. 2022, 62, 5329–5335. [Google Scholar] [CrossRef]
- Huseien, G.F.; Kubba, Z.; Ghoshal, S.K. Engineering attributes of ternary geopolymer mortars containing high volumes of palm oil fuel ash: Impact of elevated temperature exposure. Fire. 2023, 6, 340. [Google Scholar] [CrossRef]
- IS 10262: 2019; Concrete Mix Proportioning-Guidelines (Second Revision). Bureau of Indian Standards (BIS): New Delhi, India, 2019.
- Pavithra, P.; Reddy, M.S.; Dinakar, P.; Rao, B.H.; Satpathy, B.; Mohanty, A. A mix design procedure for geopolymer concrete with fly ash. J. Clean. Prod. 2016, 133, 117–125. [Google Scholar] [CrossRef]
- Ji, H.; Lyu, Y.; Ying, W.; Liu, J.-C.; Ye, H. Machine learning guided iterative mix design of geopolymer concrete. J. Build. Eng. 2024, 91, 109710. [Google Scholar] [CrossRef]
- Rafeet, A.; Vinai, R.; Soutsos, M.; Sha, W. Guidelines for mix proportioning of fly ash/GGBS based alkali activated concretes. Constr. Build. Mater. 2017, 147, 130–142. [Google Scholar] [CrossRef]
- Vora, P.R.; Dave, U.V. Parametric studies on compressive strength of geopolymer concrete. Procedia Eng. 2013, 51, 210–219. [Google Scholar] [CrossRef]
- Lloyd, N.; Rangan, B.V. Geopolymer concrete: A review of development and opportunities. In Proceedings of the 35th Conference on Our World in Concrete & Structures, Singapore, 25–27 August 2010; pp. 25–27. [Google Scholar]
- Nguyen, T.T.; Goodier, C.I.; Austin, S.A. Factors affecting the slump and strength development of geopolymer concrete. Constr. Build. Mater. 2020, 261, 119945. [Google Scholar] [CrossRef]
- Ghafoor, M.T.; Khan, Q.S.; Qazi, A.U.; Sheikh, M.N.; Hadi, M. Influence of alkaline activators on the mechanical properties of fly ash based geopolymer concrete cured at ambient temperature. Constr. Build. Mater. 2021, 273, 121752. [Google Scholar] [CrossRef]
- Luan, C.; Shi, X.; Zhang, K.; Utashev, N.; Yang, F.; Dai, J.; Wang, Q. A mix design method of fly ash geopolymer concrete based on factors analysis. Constr. Build. Mater. 2021, 272, 121612. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, M.; Salem, H.A. Effect of water addition, plasticizer and alkaline solution constitution on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 121, 694–703. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, M.; Salem, H.A. Effect of cement addition, solution resting time and curing characteristics on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 123, 581–593. [Google Scholar] [CrossRef]
- Bartos, P. Fresh Concrete: Properties and Tests; Elsevier: Amsterdam, The Netherlands, 2013; Volume 38. [Google Scholar]
- Varaprasad, B.; Reddy, K.N.K.; Reddy, K.N.K. Strength and workability of low lime fly-ash based geopolymer concrete. Indian J. Sci. Technol. 2010, 3, 1188–1189. [Google Scholar] [CrossRef]
- Jindal, B.B.; Singhal, D.; Sharma, S.K.; Ashish, D.K. Improving compressive strength of low calcium fly ash geopolymer concrete with alccofine. Adv. Concr. Constr. 2017, 5, 17–29. [Google Scholar] [CrossRef]
- Hadi, M.N.; Zhang, H.; Parkinson, S. Optimum mix design of geopolymer pastes and concretes cured in ambient condition based on compressive strength, setting time and workability. J. Build. Eng. 2019, 23, 301–313. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Morsy, M.; Alsayed, S.; Al-Salloum, Y.; Almusallam, T. Effect of sodium silicate to sodium hydroxide ratios on strength and microstructure of fly ash geopolymer binder. Arab. J. Sci. Eng. 2014, 39, 4333–4339. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Sirivivatnanon, V. Workability and strength of coarse high calcium fly ash geopolymer. Cem. Concr. Compos. 2007, 29, 224–229. [Google Scholar] [CrossRef]
- Wardhono, A.; Law, D.W.; Strano, A. The strength of alkali-activated slag/fly ash mortar blends at ambient temperature. Procedia Eng. 2015, 125, 650–656. [Google Scholar] [CrossRef]
- Mehdi, N.; Ghazanfarah, H.; Iman, F.; Huseien, G.F.; Bedon, C. Investigating the fresh and mechanical properties of wood sawdust-modified lightweight geopolymer concrete. Adva. Struct. Eng. 2023, 26, 1287–1306. [Google Scholar] [CrossRef]
- Reddy, M.S.; Dinakar, P.; Rao, B.H. Mix design development of fly ash and ground granulated blast furnace slag based geopolymer concrete. J. Build. Eng. 2018, 20, 712–722. [Google Scholar] [CrossRef]
- Jansen, M.S. Effect of Water-Solids Ratio on the Compressive Strength, Degree of Reaction and Microstructural Characterization of Fly Ash-Waste Glass-Based Geopolymers. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2017. [Google Scholar]
- Phoo-ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA–GBFS geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Gordon, S.I.; Guilfoos, B. Introduction to Modeling and Simulation with MATLAB® and Python; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Arpitha, B.; Parthasarathy, P. Enhanced approach for geopolymer mix-design and performance evaluation: Integrating hybrid Taguchi-GRA-PCA for improved properties and behavioral insights. Constr. Build. Mater. 2024, 433, 136701. [Google Scholar]
- Huo, W.; Zhu, Z.; Sun, H.; Yang, L.; Zhang, C. Estimating the relationships between initial constituent molar ratios and physical–mechanical properties of RCFP-GBFS based geopolymers. Constr. Build. Mater. 2023, 406, 133409. [Google Scholar] [CrossRef]
- Sheng, Z.; Zhu, B.; Cai, J.; Li, X.; Meng, L.; Zhang, Y.; Pan, J. Development of a novel extrusion device to improve the printability of 3D printable geopolymer concrete. J. Build. Eng. 2024, 88, 109079. [Google Scholar] [CrossRef]
- Abed, M.H.; Abbas, I.S.; Hamed, M.; Canakci, H. Rheological, fresh, and mechanical properties of mechanochemically activated geopolymer grout: A comparative study with conventionally activated geopolymer grout. Constr. Build. Mater. 2022, 322, 126338. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Wong, V.; Jervis, W.; Fishburn, B.; Numata, T.; Joe, W.; Rawal, A.; Sorrell, C.C.; Koshy, P. Long-term strength evolution in ambient-cured solid-activator geopolymer compositions. Minerals 2021, 11, 143. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Hussin, M.W. Influence of different curing temperatures and alkali activators on properties of GBFS geopolymer mortars containing fly ash and palm-oil fuel ash. Constr. Build. Mater. 2016, 125, 1229–1240. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Budiea, A.; Mirza, J. Utilizing spend garnets as sand replacement in alkali-activated mortars containing fly ash and GBFS. Constr. Build. Mater. 2019, 225, 132–145. [Google Scholar] [CrossRef]
- Mirza, J.; Huseien, G.F.; Shah, K.W.; Kirgiz, M.S. Reduction in ecology, environment, economy and energy in concrete industry using waste materials. J. Adv. Compos. Mater. Constr. Environ. Nano Technol. 2022, 1–6. [Google Scholar]
- Huseien, G.F.; Tahir, M.M.; Mirza, J.; Ismail, M.; Shah, K.W.; Asaad, M.A. Effects of POFA replaced with FA on durability properties of GBFS included alkali activated mortars. Constr. Build. Mater. 2018, 175, 174–186. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S.P. Fresh and hardened properties of fly ash–slag blended geopolymer paste and mortar. Int. J. Concr. Struct. Mater. 2019, 13, 47. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Mehrotra, S. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Marjanović, N.; Komljenović, M.; Baščarević, Z.; Nikolić, V.; Petrović, R. Physical–mechanical and microstructural properties of alkali-activated fly ash–blast furnace slag blends. Ceram. Int. 2015, 41, 1421–1435. [Google Scholar] [CrossRef]
- Palacios, M.; Gismera, S.; Alonso, M.d.M.; de Lacaillerie, J.d.E.; Lothenbach, B.; Favier, A.; Brumaud, C.; Puertas, F. Early reactivity of sodium silicate-activated slag pastes and its impact on rheological properties. Cem. Concr. Res. 2021, 140, 106302. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Shi, C.; Li, N.; Jiao, D.; Yuan, Q. Rheology of alkali-activated materials: A review. Cem. Concr. Compos. 2021, 121, 104061. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mitigation strategies in alkali-activated slag. Cem. Concr. Res. 2017, 101, 131–143. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mechanisms of alkali-activated slag. Cem. Concr. Res. 2016, 88, 126–135. [Google Scholar] [CrossRef]
- Hojati, M.; Radlińska, A. Shrinkage and strength development of alkali-activated fly ash-slag binary cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, N.; Lee, H.-K. Fresh and hardened properties of alkali-activated fly ash/slag pastes with superplasticizers. Constr. Build. Mater. 2014, 50, 169–176. [Google Scholar] [CrossRef]
- Tan, H.; Zou, F.; Ma, B.; Guo, Y.; Li, X.; Mei, J. Effect of competitive adsorption between sodium gluconate and polycarboxylate superplasticizer on rheology of cement paste. Constr. Build. Mater. 2017, 144, 338–346. [Google Scholar] [CrossRef]
- Alghamdi, H.; Nair, S.A.; Neithalath, N. Insights into material design, extrusion rheology, and properties of 3D-printable alkali-activated fly ash-based binders. Mater. Des. 2019, 167, 107634. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhou, D.; Khayat, K.H.; Feys, D.; Shi, C. On the measurement of evolution of structural build-up of cement paste with time by static yield stress test vs. small amplitude oscillatory shear test. Cem. Concr. Res. 2017, 99, 183–189. [Google Scholar] [CrossRef]
- Si, R.; Guo, S.; Dai, Q.; Wang, J. Atomic-structure, microstructure and mechanical properties of glass powder modified metakaolin-based geopolymer. Constr. Build. Mater. 2020, 254, 119303. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Optimization of brick waste-based geopolymer binders at ambient temperature and pre-targeted chemical parameters. J. Clean. Prod. 2020, 268, 122285. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Development of normal and very high strength geopolymer binders based on concrete waste at ambient environment. J. Clean. Prod. 2021, 279, 123436. [Google Scholar] [CrossRef]
- Dehghani, A.; Aslani, F.; Panah, N.G. Effects of initial SiO2/Al2O3 molar ratio and slag on fly ash-based ambient cured geopolymer properties. Constr. Build. Mater. 2021, 293, 123527. [Google Scholar] [CrossRef]
- Punurai, W.; Kroehong, W.; Saptamongkol, A.; Chindaprasirt, P. Mechanical properties, microstructure and drying shrinkage of hybrid fly ash-basalt fiber geopolymer paste. Constr. Build. Mater. 2018, 186, 62–70. [Google Scholar] [CrossRef]
- Gao, K.; Lin, K.-L.; Wang, D.; Hwang, C.-L.; Shiu, H.-S.; Chang, Y.-M.; Cheng, T.-W. Effects SiO2/Na2O molar ratio on mechanical properties and the microstructure of nano-SiO2 metakaolin-based geopolymers. Constr. Build. Mater. 2014, 53, 503–510. [Google Scholar] [CrossRef]
- Huseien, G.; Ismail, M.; Tahir, M.; Mirza, J.; Hussein, A.; Khalid, N.; Sarbini, N. Performance of sustainable alkali activated mortars containing solid waste ceramic powder. Chem. Eng. Trans. 2018, 63, 673–678. [Google Scholar]
- Chindaprasirt, P.; De Silva, P.; Sagoe-Crentsil, K.; Hanjitsuwan, S. Effect of SiO2 and Al2O3 on the setting and hardening of high calcium fly ash-based geopolymer systems. J. Mater. Sci. 2012, 47, 4876–4883. [Google Scholar] [CrossRef]
- van Jaarsveld, J.; Van Deventer, J. Effect of the alkali metal activator on the properties of fly ash-based geopolymers. Ind. Eng. Chem. Res. 1999, 38, 3932–3941. [Google Scholar] [CrossRef]
- Yaseri, S.; Hajiaghaei, G.; Mohammadi, F.; Mahdikhani, M.; Farokhzad, R. The role of synthesis parameters on the workability, setting and strength properties of binary binder based geopolymer paste. Constr. Build. Mater. 2017, 157, 534–545. [Google Scholar] [CrossRef]
- Kaze, C.R.; Djobo, J.N.Y.; Nana, A.; Tchakoute, H.K.; Kamseu, E.; Melo, U.C.; Leonelli, C.; Rahier, H. Effect of silicate modulus on the setting, mechanical strength and microstructure of iron-rich aluminosilicate (laterite) based-geopolymer cured at room temperature. Ceram. Int. 2018, 44, 21442–21450. [Google Scholar] [CrossRef]
- Karakoç, M.B.; Türkmen, I.; Maraş, M.M.; Kantarci, F.; Demirboğa, R.; Toprak, M.U. Mechanical properties and setting time of ferrochrome slag based geopolymer paste and mortar. Constr. Build. Mater. 2014, 72, 283–292. [Google Scholar] [CrossRef]
- Malkawi, A.B.; Nuruddin, M.F.; Fauzi, A.; Almattarneh, H.; Mohammed, B.S. Effects of alkaline solution on properties of the HCFA geopolymer mortars. Procedia Eng. 2016, 148, 710–717. [Google Scholar] [CrossRef]
- Chen, X.; Sutrisno, A.; Struble, L.J. Effects of calcium on setting mechanism of metakaolin-based geopolymer. J. Am. Ceram. Soc. 2018, 101, 957–968. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Alrefaei, Y.; Dai, J.-G. Influence of coal fly ash on the early performance enhancement and formation mechanisms of silico-aluminophosphate geopolymer. Cem. Concr. Res. 2020, 127, 105932. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Pu, S.; Wan, Y.; Xu, X.; Song, S.; Zhang, J.; Yao, K.; Hu, L. Multi-technical characterization and correlations between properties of standard cured alkali-activated high-calcium FA binders with GGBS as additive. Constr. Build. Mater. 2020, 241, 117996. [Google Scholar] [CrossRef]
- Yaseri, S.; Verki, V.M.; Mahdikhani, M. Utilization of high volume cement kiln dust and rice husk ash in the production of sustainable geopolymer. J. Clean. Prod. 2019, 230, 592–602. [Google Scholar] [CrossRef]
- Wang, W.; Fan, C.; Wang, B.; Zhang, X.; Liu, Z. Workability, rheology, and geopolymerization of fly ash geopolymer: Role of alkali content, modulus, and water–binder ratio. Constr. Build. Mater. 2023, 367, 130357. [Google Scholar] [CrossRef]
- Wang, C.; Kayali, O.; Liow, J.-L.; Troitzsch, U. Participation and disturbance of superplasticisers in early-stage reaction of class F fly ash-based geopolymer. Constr. Build. Mater. 2023, 403, 133176. [Google Scholar] [CrossRef]
- Jiao, Z.; Li, X.; Yu, Q.; Yao, Q.; Guan, X.; Hu, P. Chloride resistance of class C/class F fly ash-based geopolymer mortars with different strength grades. Case Stud. Constr. Mater. 2023, 18, e01811. [Google Scholar] [CrossRef]
- Addis, L.B.; Sendekie, Z.B.; Habtu, N.G.; de Ligny, D.; Roether, J.A.; Boccaccini, A.R. Optimization of process parameters for the synthesis of class F fly ash-based geopolymer binders. J. Clean. Prod. 2023, 415, 137849. [Google Scholar] [CrossRef]
- Yavuz, E.; Gul, N.I.K.; Kockal, N.U. Characterization of class C and F fly ashes based geopolymers incorporating silica fume. Ceram. Int. 2022, 48, 32213–32225. [Google Scholar] [CrossRef]
- Fan, H.; Liu, S.; Zhou, W.; Wang, Z.; Liao, K.; Wu, T.; Li, X.; Luo, Z.; Xu, F. Composite control of workability and strength of FA-GBFS based geopolymer. Mater. Lett. 2025, 385, 138138. [Google Scholar] [CrossRef]
- Aygun, B.F.; Uysal, M.; Bilir, T.; Çoşgun, T.; Dilbas, H. An investigation on physical, mechanical and microstructural properties of electricity-based cured GBFS-FA geopolymer. Constr. Build. Mater. 2025, 458, 139526. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, J.; Wang, Y.; Yi, C.; Su, T. Characterizing the interfacial bonding behaviour and influential mechanism of metakaolin-based geopolymer-concrete composites under shearing load: Experimental and modelling study. Compos. Struct. 2025, 369, 119336. [Google Scholar] [CrossRef]
- Altawil, H.; Olgun, M. Optimization of mechanical properties of geopolymer mortar based on Class C fly ash and silica fume: A Taguchi method approach. Case Stud. Constr. Mater. 2025, 22, e04332. [Google Scholar] [CrossRef]
- Huseien, G.F.; Shah, K.W. Performance evaluation of alkali-activated mortars containing industrial wastes as surface repair materials. J. Build. Eng. 2020, 30, 101234. [Google Scholar] [CrossRef]
- Asaad, M.A.; Huseien, G.F.; Memon, R.P.; Ghoshal, S.K.; Mohammadhosseini, H.; Alyousef, R. Enduring performance of alkali-activated mortars with metakaolin as granulated blast furnace slag replacement. Case Stud. Constr. Mater. 2022, 16, p.e00845. [Google Scholar] [CrossRef]
- Choi, H.; Pour-Ghaz, M.; Park, S. Compressive strength degradation of metakaolin-based geopolymer with an excessively high S/A ratio: Insights from nanoindentation on NASH Gel structure. J. Build. Eng. 2025, 108, 112870. [Google Scholar] [CrossRef]
- Gao, R.; Mao, J.; Ruan, S.; Qiu, Y.; Ye, S.; Chen, S.; Liu, Y.; Yan, D. A comparative study of metakaolin-based geopolymers with immobilised organic liquids: Vegetable oil, mineral oil, and organosilicon liquids. J. Build. Eng. 2025, 108, 112860. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, M.; Fu, D.; He, C.; Wang, S.; Zhai, X. Synthesis of red mud/metakaolin based geopolymer for adsorption of methylene blue and tetracycline hydrochloride from water. Colloids Surf. A Physicochem. Eng. Asp. 2025, 722, 137155. [Google Scholar] [CrossRef]
- Sanalkumar, K.U.A.; Yang, E.-H. Effect of mix design factors on the self-stress-sensing behavior of metakaolin-based geopolymer. Cem. Concr. Compos. 2025, 161, 106093. [Google Scholar] [CrossRef]
- Costa, L.M.; de Souza, T.d.C.C.; de Oliveira, R.K.F.G.; Almeida, N.G.S.; Houmard, M. Effects of silicas and aluminosilicate synthesized by sol-gel process on the structural properties of metakaolin-based geopolymers. Appl. Clay Sci. 2025, 267, 107743. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Mirza, J. Effects of ceramic tile powder waste on properties of self-compacted alkali-activated concrete. Constr. Build. Mater. 2020, 236, 117574. [Google Scholar] [CrossRef]
- Abdelmonem, A.; Azam, A.; Alruwaili, A.; Ouda, A.S.; Abd Elrahman, M. Effect of ceramic tile waste addition on the performance of slag-based geopolymer upon exposure to marine conditions: Physico-mechanical characteristics, and shielding proficiency against ionizing radiation. Sustain. Chem. Pharm. 2025, 43, 101886. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Development and characterization of binary recycled ceramic tile and brick wastes-based geopolymers at ambient and high temperatures. Constr. Build. Mater. 2021, 301, 124138. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Development of ceramic tile waste geopolymer binders based on pre-targeted chemical ratios and ambient curing. Constr. Build. Mater. 2020, 258, 120297. [Google Scholar] [CrossRef]
- Chub-Uppakarn, T.; Chompoorat, T.; Thepumong, T.; Sae-Long, W.; Khamplod, A.; Chaiprapat, S. Influence of partial substitution of metakaolin by palm oil fuel ash and alumina waste ash on compressive strength and microstructure in metakaolin-based geopolymer mortar. Case Stud. Constr. Mater. 2023, 19, e02519. [Google Scholar] [CrossRef]
- Salih, M.A.; Farzadnia, N.; Demirboga, R.; Ali, A.A.A. Effect of elevated temperatures on mechanical and microstructural properties of alkali-activated mortar made up of POFA and GGBS. Constr. Build. Mater. 2022, 328, 127041. [Google Scholar] [CrossRef]
- Bayrak, B.; Alcan, H.G.; Tanyıldızı, M.; Kaplan, G.; İpek, S.; Aydın, A.C.; Güneyisi, E. Effects of silica fume and rice husk ash contents on engineering properties and high-temperature resistance of slag-based prepacked geopolymers. J. Build. Eng. 2024, 92, 109746. [Google Scholar] [CrossRef]
- Abbasi, M.; Hosseinpour, I.; Salimi, M.; Astaneh, A.G.; Payan, M. A comparative study on stabilization efficiency of kaolinite and montmorillonite clays with fly ash (FA) and rice husk ash (RHA)-based geopolymers. J. Mater. Res. Technol. 2025, 36, 2332–2347. [Google Scholar] [CrossRef]
- Hao, H.; Liu, X.; Dong, X.; Li, J.; Li, J.; Xu, X.; Chang, S. Study on the optimal ratios and strength formation mechanism of mechanical activation red mud based geopolymer. J. Build. Eng. 2025, 104, 112401. [Google Scholar] [CrossRef]
- Yang, D.; Wang, P.; Chen, W.; Liu, L.; Huang, Y.; Xiang, X.; Wang, G.; Wu, J. Effects of red mud, desert sand, and ground granulated blast furnace slag on the mechanical properties and microstructure of fly ash-based geopolymer. Constr. Build. Mater. 2025, 468, 140471. [Google Scholar] [CrossRef]
- El Harouachi, H.; Ahoudi, D.; Moukannaa, S.; Aaddouz, M.; Elgettafi, M.; Mansori, M.; Loutou, M. Effect of glass waste and sodium hydroxide concentration on fly ash-pyrophyllite based geopolymer composites: Microstructural investigations and performance optimization. Case Stud. Constr. Mater. 2025, 22, e04392. [Google Scholar] [CrossRef]
- Dinh, H.L.; Liu, J.; Doh, J.-H.; Ong, D.E. Influence of Si/Al molar ratio and ca content on the performance of fly ash-based geopolymer incorporating waste glass and GGBFS. Constr. Build. Mater. 2024, 411, 134741. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Skibsted, J.; Fernández-Jiménez, A.; Palomo, A. Alkaline solution/binder ratio as a determining factor in the alkaline activation of aluminosilicates. Cem. Concr. Res. 2012, 42, 1242–1251. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Pham, T.M. Effective utilisation of ultrafine slag to improve mechanical and durability properties of recycled aggregates geopolymer concrete. Clean. Eng. Technol. 2021, 5, 100330. [Google Scholar] [CrossRef]
- Jithendra, C.; Elavenil, S. Effects of silica fume on workability and compressive strength properties of aluminosilicate based flowable geopolymer mortar under ambient curing. Silicon 2020, 12, 1965–1974. [Google Scholar] [CrossRef]
- Arun, B.; Nagaraja, P.; Mahalingasharma, S. Combined effect of flyash & GGBS on workability and mechanical properties of self compacting geopolymer concrete. Int. J. Pure Appl. Math. 2018, 119, 1369–1380. [Google Scholar]
- Kumar, N.K.; Reddy, I.R. A study on the effect of NaOH molarity on flyash based self compacting geopolymer concrete. Mater. Today Proc. 2023, 1–5. [Google Scholar] [CrossRef]
- Babu, D.V. Assessing the performance of molarity and alkaline activator ratio on engineering properties of self-compacting alkaline activated concrete at ambient temperature. J. Build. Eng. 2018, 20, 137–155. [Google Scholar] [CrossRef]
- Al Bakri, A.; Abdulkareem, O.A.; Rafiza, A.; Zarina, Y.; Norazian, M.; Kamarudin, H. Review on Processing of low calcium fly ash geopolymer concrete. Aust. J. Basic Appl. Sci. 2013, 7, 342–349. [Google Scholar]
- Fang, G.; Ho, W.K.; Tu, W.; Zhang, M. Workability and mechanical properties of alkali-activated fly ash-slag concrete cured at ambient temperature. Constr. Build. Mater. 2018, 172, 476–487. [Google Scholar] [CrossRef]
- AS 3972-2010; General Purpose and Blended Cements. Standards Australia: Sydney, Australia, 2010.
- ACI 211.1-91; Standard Practice for Selecting Proportions for Normal, Heavyweight, and Mass Concrete. American Concrete Institute: Farmington Hills, MI, USA, 1996; pp. 1–38.
- Huseien, G.F.; Hussein, Z.J.; Kubba, Z.; Mikhail Nikolaevich, B.; Mirza, J. Improved bond strength performance of geopolymer mortars: Role of high volume ground blast furnace slag, fly ash, and palm oil fuel ash incorporation. Minerals 2023, 13, 1096. [Google Scholar] [CrossRef]
- Huseien, G.F.; Khamehchi, M.; Kubba, Z.; Benjeddou, O.; Mahmoodi, M.J. Freeze-thaw cycle and abrasion resistance of alkali-activated FA and POFA-based mortars: Role of high volume GBFS incorporation. Heliyon 2023, 9, e17672. [Google Scholar] [CrossRef]
- Huseien, G.F.; Kubba, Z.; Mhaya, A.M.; Malik, N.H.; Mirza, J. Impact resistance enhancement of sustainable geopolymer composites using high volume tile ceramic wastes. J. Compos. Sci. 2023, 7, 73. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H.; Xing, Z.; Wang, R.; Dai, S. The effect of various Si/Al, Na/Al molar ratios and free water on micromorphology and macro-strength of metakaolin-based geopolymer. Materials 2021, 14, 3845. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Lu, Y.; Bai, X. Effects of Na/Al ratio on mechanical properties and microstructure of red mud-coal metakaolin geopolymer. Constr. Build. Mater. 2020, 263, 120653. [Google Scholar] [CrossRef]
- Kaze, C.R.; Jiofack, S.B.K.; Cengiz, Ö.; Alomayri, T.S.; Adesina, A.; Rahier, H. Reactivity and mechanical performance of geopolymer binders from metakaolin/meta-halloysite blends. Constr. Build. Mater. 2022, 336, 127546. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Pu, S.; Wan, Y.; Huo, W.; Song, S.; Zhang, J.; Yao, K.; Hu, L. Efficient use of steel slag in alkali-activated fly ash-steel slag-ground granulated blast furnace slag ternary blends. Constr. Build. Mater. 2020, 259, 119814. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, Z.; Zhang, R.; Li, F. Preparation, characterization and rheological analysis of eco-friendly road geopolymer grouting materials based on volcanic ash and metakaolin. J. Clean. Prod. 2021, 312, 127822. [Google Scholar] [CrossRef]
- Kouamo, H.T.; Mbey, J.; Elimbi, A.; Diffo, B.K.; Njopwouo, D. Synthesis of volcanic ash-based geopolymer mortars by fusion method: Effects of adding metakaolin to fused volcanic ash. Ceram. Int. 2013, 39, 1613–1621. [Google Scholar] [CrossRef]
- Javed, U.; Shaikh, F.U.A.; Sarker, P.K. Microstructural investigation of lithium slag geopolymer pastes containing silica fume and fly ash as additive chemical modifiers. Cem. Concr. Compos. 2022, 134, 104736. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Peng, Y.; Wan, Y.; Xu, X.; Pu, S.; Song, S.; Wei, Y. Effect of steel slag on fresh, hardened and microstructural properties of high-calcium fly ash based geopolymers at standard curing condition. Constr. Build. Mater. 2019, 229, 116933. [Google Scholar] [CrossRef]
- Qing, L.; Xiaochang, L.; Chuanming, L.; Xianjun, L. Synthesis and optimization of green one-part geopolymer from mine tailings and slag: Calcium carbide residue and soda residue as supplementary alkali sources. Constr. Build. Mater. 2022, 353, 129013. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, P.; Kapoor, K. Utilization of recycled fine powder as an activator in fly ash based geopolymer mortar. Constr. Build. Mater. 2022, 323, 126581. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, Z.; Sun, H.; Gao, Q.; Zhang, J.; Wan, Y.; Zhang, C. Reaction kinetics, mechanical properties, and microstructure of nano-modified recycled concrete fine powder/slag based geopolymers. J. Clean. Prod. 2022, 372, 133715. [Google Scholar] [CrossRef]
- Subaer, S.; Haris, A.; Nurhayati, N.; Andi, I.; Ekaputri, J.J. The influence of Si: Al and Na: Al on the physical and microstructure characters of geopolymers based on metakaolin. Mater. Sci. Forum 2016, 841, 170–177. [Google Scholar]
- Phoo-Ngernkham, T.; Sata, V.; Hanjitsuwan, S.; Ridtirud, C.; Hatanaka, S.; Chindaprasirt, P. High calcium fly ash geopolymer mortar containing Portland cement for use as repair material. Constr. Build. Mater. 2015, 98, 482–488. [Google Scholar] [CrossRef]
- Chithambaram, S.J.; Kumar, S.; Prasad, M. Thermo-mechanical characteristics of geopolymer mortar. Constr. Build. Mater. 2019, 213, 100–108. [Google Scholar] [CrossRef]
- Mabah, D.; Tchakouté, H.; Fotio, D.; Rüscher, C.; Kamseu, E.; Bignozzi, M.; Leonelli, C. Influence of the molar ratios CaO/SiO2 contained in the sustainable microcomposites on the mechanical and microstructural properties of (Ca, Na)-poly (sialate-siloxo) networks. Mater. Chem. Phys. 2019, 238, 121928. [Google Scholar] [CrossRef]
- Kim, H.-J.; Khalid, H.R. The effects of calcium aluminate cement substitution on physicochemical properties of geopolymer–zeolite composites. Arab. J. Sci. Eng. 2022, 47, 5073–5078. [Google Scholar] [CrossRef]
- Das, P.; Beulah, M.; Hossiney, N.; Dunna, U.; Kavitha, S. A probable mathematical relationship between (Si/Al) ratio and (Ca/Si) ratio on the compressive strength of an iron ore tailings sample arising out of geopolymeric reactions. J. Min. Metall. A Min. 2019, 55, 27–36. [Google Scholar] [CrossRef]
- Shah, K.W.; Huseien, G.F. Bond strength performance of ceramic, fly ash and GBFS ternary wastes combined alkali-activated mortars exposed to aggressive environments. Constr. Build. Mater. 2020, 251, 119088. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Reig, L.; Tashima, M.M.; Borrachero, M.; Monzó, J.; Cheeseman, C.; Payá, J. Properties and microstructure of alkali-activated red clay brick waste. Constr. Build. Mater. 2013, 43, 98–106. [Google Scholar] [CrossRef]
- Ye, T.; Xiao, J.; Duan, Z.; Li, S. Geopolymers made of recycled brick and concrete powder–A critical review. Constr. Build. Mater. 2022, 330, 127232. [Google Scholar] [CrossRef]
- Görhan, G.; Aslaner, R.; Şinik, O. The effect of curing on the properties of metakaolin and fly ash-based geopolymer paste. Compos. Part B Eng. 2016, 97, 329–335. [Google Scholar] [CrossRef]
- El-Gamal, S.; Selim, F. Utilization of some industrial wastes for eco-friendly cement production. Sustain. Mater. Technol. 2017, 12, 9–17. [Google Scholar] [CrossRef]
- Şahin, O.; İlcan, H.; Ateşli, A.T.; Kul, A.; Yıldırım, G.; Şahmaran, M. Construction and demolition waste-based geopolymers suited for use in 3-dimensional additive manufacturing. Cem. Concr. Compos. 2021, 121, 104088. [Google Scholar] [CrossRef]
- Panias, D.; Giannopoulou, I.P.; Perraki, T. Effect of synthesis parameters on the mechanical properties of fly ash-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 246–254. [Google Scholar] [CrossRef]
- Ahmari, S.; Ren, X.; Toufigh, V.; Zhang, L. Production of geopolymeric binder from blended waste concrete powder and fly ash. Constr. Build. Mater. 2012, 35, 718–729. [Google Scholar] [CrossRef]
- Temuujin, J.; Van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-ngernkham, T.; Li, L.-y.; Damrongwiriyanupap, N.; Chindaprasirt, P. Strength development and durability of alkali-activated fly ash mortar with calcium carbide residue as additive. Constr. Build. Mater. 2018, 162, 714–723. [Google Scholar] [CrossRef]
- Yadollahi, M.M.; Benli, A.; Demirboğa, R. The effects of silica modulus and aging on compressive strength of pumice-based geopolymer composites. Constr. Build. Mater. 2015, 94, 767–774. [Google Scholar] [CrossRef]
- Turkoglu, M.; Bayraktar, O.Y.; Benli, A.; Kaplan, G. Effect of cement clinker type, curing regime and activator dosage on the performance of one-part alkali-activated hybrid slag/clinker composites. J. Build. Eng. 2023, 68, 106164. [Google Scholar] [CrossRef]
- Yadollahi, M.M.; Benli, A.; Demirboga, R. Application of adaptive neuro-fuzzy technique and regression models to predict the compressive strength of geopolymer composites. Neural Comput. Appl. 2017, 28, 1453–1461. [Google Scholar] [CrossRef]
- Rathod, N.; Chippagiri, R.; Ralegaonkar, R.V. Cleaner production of geopolymer materials: A critical review of waste-derived activators. Mater. Today Proc. 2023, 1–7. [Google Scholar] [CrossRef]
- Hamzah, H.K.; Huseien, G.F.; Asaad, M.A.; Georgescu, D.P.; Ghoshal, S.; Alrshoudi, F. Effect of waste glass bottles-derived nanopowder as slag replacement on mortars with alkali activation: Durability characteristics. Case Stud. Constr. Mater. 2021, 15, e00775. [Google Scholar] [CrossRef]
- Shah, S.F.A.; Zhang, S.; Xuan, D.; Poon, C.S. Development of a novel mixing strategy for set-on-demand printing of one-part geopolymer using municipal solid waste incineration bottom ash and blast furnace slag. J. Build. Eng. 2025, 106, 112632. [Google Scholar] [CrossRef]
- Ouellet-Plamondon, C.; Habert, G. Life cycle assessment (LCA) of alkali-activated cements and concretes. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 663–686. [Google Scholar]
- Faridmehr, I.; Bedon, C.; Huseien, G.F.; Nikoo, M.; Baghban, M.H. Assessment of mechanical properties and structural morphology of alkali-activated mortars with industrial waste materials. Sustainability 2021, 13, 2062. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer cements to minimize carbon dioxide greenhouse warming. Ceram. Trans. 1993, 37, 165–182. [Google Scholar]
- Fawer, M.; Concannon, M.; Rieber, W. Life cycle inventories for the production of sodium silicates. Int. J. Life Cycle Assess. 1999, 4, 207–212. [Google Scholar] [CrossRef]
- Habert, G.; De Lacaillerie, J.D.E.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Mellado, A.; Catalán, C.; Bouzón, N.; Borrachero, M.; Monzó, J.; Payá, J. Carbon footprint of geopolymeric mortar: Study of the contribution of the alkaline activating solution and assessment of an alternative route. RSC Adv. 2014, 4, 23846–23852. [Google Scholar] [CrossRef]
- Bianco, I.; Tomos, B.A.D.; Vinai, R. Analysis of the environmental impacts of alkali-activated concrete produced with waste glass-derived silicate activator–A LCA study. J. Clean. Prod. 2021, 316, 128383. [Google Scholar] [CrossRef]
- Das, S.K.; Mishra, J.; Singh, S.K.; Mustakim, S.M.; Patel, A.; Das, S.K.; Behera, U. Characterization and utilization of rice husk ash (RHA) in fly ash–Blast furnace slag based geopolymer concrete for sustainable future. Mater. Today Proc. 2020, 33, 5162–5167. [Google Scholar] [CrossRef]
- Bajpai, R.; Choudhary, K.; Srivastava, A.; Sangwan, K.S.; Singh, M. Environmental impact assessment of fly ash and silica fume based geopolymer concrete. J. Clean. Prod. 2020, 254, 120147. [Google Scholar] [CrossRef]
- Font, A.; Soriano, L.; Reig, L.; Tashima, M.; Borrachero, M.; Monzó, J.; Payá, J. Use of residual diatomaceous earth as a silica source in geopolymer production. Mater. Lett. 2018, 223, 10–13. [Google Scholar] [CrossRef]
- Wu, X.; Shen, Y.; Hu, L. Performance of geopolymer concrete activated by sodium silicate and silica fume activator. Case Stud. Constr. Mater. 2022, 17, e01513. [Google Scholar] [CrossRef]
- Dadsetan, S.; Siad, H.; Lachemi, M.; Mahmoodi, O.; Sahmaran, M. Sodium glass liquid from glass waste as a user-friendly hardener in structural geopolymer systems. Cem. Concr. Compos. 2022, 130, 104525. [Google Scholar] [CrossRef]
- Humur, G.; Cevik, A. Effects of hybrid fibers and nanosilica on mechanical and durability properties of lightweight engineered geopolymer composites subjected to cyclic loading and heating–cooling cycles. Constr. Build. Mater. 2022, 326, 126846. [Google Scholar] [CrossRef]
- Autef, A.; Joussein, E.; Gasgnier, G.; Rossignol, S. Role of the silica source on the geopolymerization rate. J. Non-Cryst. Solids 2012, 358, 2886–2893. [Google Scholar] [CrossRef]
- Rathod, N.; Chippagiri, R.; Gavali, H.R.; Ralegaonkar, R.V. Development of sustainable masonry blocks using industrial rejects and alkali activation. In Proceedings of the 3rd International Conference on Innovative Technologies for Clean and Sustainable Development: ITCSD 2020; Springer: Cham, Switzerland, 2021; pp. 357–368. [Google Scholar]
- Cheng, Y.; Cong, P.; Zhao, Q.; Hao, H.; Mei, L.; Zhang, A.; Han, Z.; Hu, M. Study on the effectiveness of silica fume-derived activator as a substitute for water glass in fly ash-based geopolymer. J. Build. Eng. 2022, 51, 104228. [Google Scholar] [CrossRef]
- Al Bakri, A.; Kamarudin, H.; Bnhussain, M.; Nizar, I.K.; Rafiza, A.; Zarina, Y. The processing, characterization, and properties of fly ash based geopolymer concrete. Rev. Adv. Mater. Sci 2012, 30, 90–97. [Google Scholar]
- Self-Compacting Concrete European Project Group. The European Guidelines for Self-Compacting Concrete; International Bureau for Precast Concrete (BIBM): Brussels, Belgium, 2005; Volume 22, p. 563. [Google Scholar]
- Kumar, M.; Kumar, A.; Solanki, D.; Mungule, M. Low molarity geopolymer concrete: Effects on compressive strength, elastic modulus, sorptivity and chloride migration. Constr. Build. Mater. 2023, 409, 134065. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mahmood, L.J.; Muhammad, M.A.; Faraj, R.H.; Qaidi, S.M.; Sor, N.H.; Mohammed, A.S.; Mohammed, A.A. Geopolymer concrete as a cleaner construction material: An overview on materials and structural performances. Clean. Mater. 2022, 5, 100111. [Google Scholar] [CrossRef]
- Bayrak, B.; Benli, A.; Alcan, H.G.; Çelebi, O.; Kaplan, G.; Aydın, A.C. Recycling of waste marble powder and waste colemanite in ternary-blended green geopolymer composites: Mechanical, durability and microstructural properties. J. Build. Eng. 2023, 73, 106661. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.Y.; Khadar, S.D.A. Molarity activity effect on mechanical and microstructure properties of geopolymer concrete: A review. Case Stud. Constr. Mater. 2022, 16, e01014. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Farooq, F.; Jin, X.; Javed, M.F.; Akbar, A.; Shah, M.I.; Aslam, F.; Alyousef, R. Geopolymer concrete as sustainable material: A state of the art review. Constr. Build. Mater. 2021, 306, 124762. [Google Scholar] [CrossRef]
- Hasnaoui, A.; Ghorbel, E.; Wardeh, G. Effect of curing conditions on the performance of geopolymer concrete based on granulated blast furnace slag and metakaolin. J. Mater. Civ. Eng. 2021, 33, 04020501. [Google Scholar] [CrossRef]
- Koksal, F.; Bayraktar, O.Y.; Bodur, B.; Benli, A.; Kaplan, G. Insulating and fire-resistant performance of slag and brick powder based one-part alkali-activated lightweight mortars. Struct. Concr. 2023, 24, 3128–3146. [Google Scholar] [CrossRef]
- Part, W.K.; Ramli, M.; Cheah, C.B. An overview on the influence of various factors on the properties of geopolymer concrete derived from industrial by-products. Constr. Build. Mater. 2015, 77, 370–395. [Google Scholar] [CrossRef]
- Rifaai, Y.; Yahia, A.; Mostafa, A.; Aggoun, S.; Kadri, E.-H. Rheology of fly ash-based geopolymer: Effect of NaOH concentration. Constr. Build. Mater. 2019, 223, 583–594. [Google Scholar] [CrossRef]
- Junaid, M.T.; Kayali, O.; Khennane, A.; Black, J. A mix design procedure for low calcium alkali activated fly ash-based concretes. Constr. Build. Mater. 2015, 79, 301–310. [Google Scholar] [CrossRef]
- Yadollahi, M.M.; Benli, A. Stress-strain behavior of geopolymer under uniaxial compression. Comput. Concr. 2017, 20, 381–389. [Google Scholar]
- Xu, S.; Yuan, P.; Liu, J.; Pan, Z.; Liu, Z.; Su, Y.; Li, J.; Wu, C. Development and preliminary mix design of ultra-high-performance concrete based on geopolymer. Constr. Build. Mater. 2021, 308, 125110. [Google Scholar] [CrossRef]
- Kathirvel, P.; Sreekumaran, S. Sustainable development of ultra high performance concrete using geopolymer technology. J. Build. Eng. 2021, 39, 102267. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Shi, C.; Zhu, D.; Li, N.; Deng, Y. Development of ultra-high performance geopolymer concrete (UHPGC): Influence of steel fiber on mechanical properties. Cem. Concr. Compos. 2020, 112, 103670. [Google Scholar] [CrossRef]
- Yadollahi, M.; Benli, A.; Demirboğa, R. Effects of elevated temperature on pumice based geopolymer composites. Plast. Rubber Compos. 2015, 44, 226–237. [Google Scholar] [CrossRef]
- Reddy, M.S.; Dinakar, P.; Rao, B.H. A review of the influence of source material’s oxide composition on the compressive strength of geopolymer concrete. Microporous Mesoporous Mater. 2016, 234, 12–23. [Google Scholar] [CrossRef]
- Bignozzi, M.C.; Manzi, S.; Natali, M.E.; Rickard, W.D.; Van Riessen, A. Room temperature alkali activation of fly ash: The effect of Na2O/SiO2 ratio. Constr. Build. Mater. 2014, 69, 262–270. [Google Scholar] [CrossRef]
- Cheng, T.-W.; Chiu, J. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Škvára, F.; Jílek, T.; Kopecký, L. Geopolymer materials based on fly ash. Ceram.-Silik 2005, 49, 195–204. [Google Scholar]
- Xu, H.; van Deventer, J.S. The effect of alkali metals on the formation of geopolymeric gels from alkali-feldspars. Colloids Surf. A Physicochem. Eng. Asp. 2003, 216, 27–44. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.; Rangan, B.V. Fly ash-based geopolymer concrete. Aust. J. Struct. Eng. 2005, 6, 77–86. [Google Scholar] [CrossRef]
- Lee, W.; Van Deventer, J. Structural reorganisation of class F fly ash in alkaline silicate solutions. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 49–66. [Google Scholar] [CrossRef]
- Dave, S.V.; Bhogayata, A. The strength oriented mix design for geopolymer concrete using Taguchi method and Indian concrete mix design code. Constr. Build. Mater. 2020, 262, 120853. [Google Scholar] [CrossRef]
- Tsujino, M.; Noguchi, T.; Tamura, M.; Kanematsu, M.; Maruyama, I. Application of conventionally recycled coarse aggregate to concrete structure by surface modification treatment. J. Adv. Concr. Technol. 2007, 5, 13–25. [Google Scholar] [CrossRef]
- Kou, S.-c.; Poon, C.S. Enhancing the durability properties of concrete prepared with coarse recycled aggregate. Constr. Build. Mater. 2012, 35, 69–76. [Google Scholar] [CrossRef]
- Spaeth, V.; Tegguer, A.D. Improvement of recycled concrete aggregate properties by polymer treatments. Int. J. Sustain. Built Environ. 2013, 2, 143–152. [Google Scholar] [CrossRef]
- Qiu, J.; Tng, D.Q.S.; Yang, E.-H. Surface treatment of recycled concrete aggregates through microbial carbonate precipitation. Constr. Build. Mater. 2014, 57, 144–150. [Google Scholar] [CrossRef]
- Kim, Y.; Hanif, A.; Kazmi, S.M.; Munir, M.J.; Park, C. Properties enhancement of recycled aggregate concrete through pretreatment of coarse aggregates–Comparative assessment of assorted techniques. J. Clean. Prod. 2018, 191, 339–349. [Google Scholar] [CrossRef]
- Nuaklong, P.; Sata, V.; Chindaprasirt, P. Properties of metakaolin-high calcium fly ash geopolymer concrete containing recycled aggregate from crushed concrete specimens. Constr. Build. Mater. 2018, 161, 365–373. [Google Scholar] [CrossRef]
- Shaikh, F.U.A. Mechanical and durability properties of fly ash geopolymer concrete containing recycled coarse aggregates. Int. J. Sustain. Built Environ. 2016, 5, 277–287. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R. Mechanical properties and durability of mortar containing fine fraction of demolition wastes produced by selective demolition in South Italy. Compos. Part B Eng. 2017, 115, 43–50. [Google Scholar] [CrossRef]
- Sáez, P.V.; Osmani, M. A diagnosis of construction and demolition waste generation and recovery practice in the European Union. J. Clean. Prod. 2019, 241, 118400. [Google Scholar] [CrossRef]
- Posi, P.; Thongjapo, P.; Thamultree, N.; Boontee, P.; Kasemsiri, P.; Chindaprasirt, P. Pressed lightweight fly ash-OPC geopolymer concrete containing recycled lightweight concrete aggregate. Constr. Build. Mater. 2016, 127, 450–456. [Google Scholar] [CrossRef]
- Singh, R.P.; Vanapalli, K.R.; Cheela, V.R.S.; Peddireddy, S.R.; Sharma, H.B.; Mohanty, B. Fly ash, GGBS, and silica fume based geopolymer concrete with recycled aggregates: Properties and environmental impacts. Constr. Build. Mater. 2023, 378, 131168. [Google Scholar] [CrossRef]
- Qaidi, S.; Najm, H.M.; Abed, S.M.; Ahmed, H.U.; Al Dughaishi, H.; Al Lawati, J.; Sabri, M.M.; Alkhatib, F.; Milad, A. Fly ash-based geopolymer composites: A review of the compressive strength and microstructure analysis. Materials 2022, 15, 7098. [Google Scholar] [CrossRef] [PubMed]
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Bernal, S.A.; van Deventer, J.S.; Provis, J.L. Structural evolution of synthetic alkali-activated CaO-MgO-Na2O-Al2O3-SiO2 materials is influenced by Mg content. Cem. Concr. Res. 2017, 99, 155–171. [Google Scholar] [CrossRef]
- Xiao, R.; Jiang, X.; Zhang, M.; Polaczyk, P.; Huang, B. Analytical investigation of phase assemblages of alkali-activated materials in CaO-SiO2-Al2O3 systems: The management of reaction products and designing of precursors. Mater. Des. 2020, 194, 108975. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of fly ashes. Potential reactivity as alkaline cements☆. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Lee, W.; Van Deventer, J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Riahi, S.; Nazari, A.; Zaarei, D.; Khalaj, G.; Bohlooli, H.; Kaykha, M.M. Compressive strength of ash-based geopolymers at early ages designed by Taguchi method. Mater. Des. 2012, 37, 443–449. [Google Scholar] [CrossRef]
- Mijarsh, M.; Johari, M.M.; Ahmad, Z. Synthesis of geopolymer from large amounts of treated palm oil fuel ash: Application of the Taguchi method in investigating the main parameters affecting compressive strength. Constr. Build. Mater. 2014, 52, 473–481. [Google Scholar] [CrossRef]
- Prusty, J.K.; Pradhan, B. Multi-response optimization using Taguchi-Grey relational analysis for composition of fly ash-ground granulated blast furnace slag based geopolymer concrete. Constr. Build. Mater. 2020, 241, 118049. [Google Scholar] [CrossRef]
- Shoaei, P.; Musaeei, H.R.; Mirlohi, F.; Ameri, F.; Bahrami, N. Waste ceramic powder-based geopolymer mortars: Effect of curing temperature and alkaline solution-to-binder ratio. Constr. Build. Mater. 2019, 227, 116686. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Xing, J.; Sun, X. Fly Ash/Blast Furnace Slag-Based Geopolymer as a Potential Binder for Mine Backfilling: Effect of Binder Type and Activator Concentration. Adv. Mater. Sci. Eng. 2019, 2019, 2028109. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Development of optimized binary ceramic tile and concrete wastes geopolymer binders for in-situ applications. J. Build. Eng. 2021, 43, 102906. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. (1980–2015) 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Hunpratub, S.; Thongbai, P.; Maensiri, S.; Sata, V.; Chindaprasirt, P. Effects of NaOH concentrations on physical and electrical properties of high calcium fly ash geopolymer paste. Cem. Concr. Compos. 2014, 45, 9–14. [Google Scholar] [CrossRef]
- Elyamany, H.E.; Abd Elmoaty, M.; Elshaboury, A.M. Setting time and 7-day strength of geopolymer mortar with various binders. Constr. Build. Mater. 2018, 187, 974–983. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Rao, R.; Wang, C.; Fang, C. Effects of combined usage of GGBS and fly ash on workability and mechanical properties of alkali activated geopolymer concrete with recycled aggregate. Compos. Part B Eng. 2019, 164, 179–190. [Google Scholar] [CrossRef]
- Lee, W.; Van Deventer, J. The effect of ionic contaminants on the early-age properties of alkali-activated fly ash-based cements. Cem. Concr. Res. 2002, 32, 577–584. [Google Scholar] [CrossRef]
- Mallikarjuna Rao, G.; Gunneswara Rao, T. Final setting time and compressive strength of fly ash and GGBS-based geopolymer paste and mortar. Arab. J. Sci. Eng. 2015, 40, 3067–3074. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Some factors affecting early hydration of alkali-slag cements. Cem. Concr. Res. 1996, 26, 439–447. [Google Scholar] [CrossRef]
- Weng, L.; Sagoe-Crentsil, K. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part I—Low Si/Al ratio systems. J. Mater. Sci. 2007, 42, 2997–3006. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio: Part I: FTIR study. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]















| Refs | Source | Elements, Weight % | Workability Performance | ||
|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | |||
| [108,142,143,144,145,146,147] | FA class F | 1.46–7.94 | 49.55–60.5 | 21.5–29.8 | Longer initial and final setting times with higher workability. |
| [143,145,148,149] | FA class C | 14.1–74.9 | 12.6–51.80 | 8.47–20.5 | Significantly reduce the setting times and workability. |
| [40,42,108,146,147,150] | GBFS | 35.6–51.8 | 26.87–40.60 | 10.90–13.3 | Very fast setting times, high viscosity, and lower flowability. |
| [36,148,151,152,153,154,155,156] | MK | 0.02– 0.61 | 49.6–59.5 | 35.1–44.1 | Enhance the setting time and workability. |
| [34,157,158,159,160] | WTCPs | 0.02–6.01 | 60.6–72.60 | 10.3–26.90 | Higher setting time and excellent workability performance. |
| [19,108,111,161,162] | POFA | 10.2–11.8 | 45.9–64.20 | 0.64–4.25 | Delay the setting time and improve the workability. |
| [156,163,164] | RHAs | 0.50–0.99 | 86.2–96.5 | 0.10–0.60 | Accelerated setting, increasing viscosity, and reducing workability. |
| [154,165,166] | RMs | 0.96–9.14 | 21.6–51.93 | 17.39–30.1 | Accelerated or delayed setting, depending on other factors, such as the activator. |
| [37,41,167,168] | WGMs | 1.75–11.2 | 69.14–70.65 | 1.49–13.06 | A high dosage of waste glass reduces workability. |
| [145,149,152,155,156] | SF | 0.1–0.14 | 78.02–97.30 | 0.06–10.6 | The high surface area and reactivity of SF particles lead to an accelerated geopolymerization process, increased the viscosity, and reduced the setting time and workability. |
| Parameter | CS, Day | Molarity | % GBFS | A/B | NS:NH | Total | Error | |
|---|---|---|---|---|---|---|---|---|
| Level 1 | 7 | 33.32 | 28.99 | 33.09 | 32.69 | - | - | |
| 28 | 33.38 | 29.12 | 33.33 | 32.92 | - | - | ||
| Level 2 | 7 | 32.14 | 32.83 | 32.47 | 32.45 | - | - | |
| 28 | 32.36 | 32.91 | 32.90 | 32.74 | - | - | ||
| Level 3 | 7 | 32.32 | 35.96 | 32.22 | 32.63 | - | - | |
| 28 | 32.68 | 36.39 | 32.19 | 32.75 | - | - | ||
| Delta | 7 | 1.18 | 6.97 | 0.88 | 0.24 | - | - | |
| 28 | 1.02 | 7.27 | 1.14 | 0.18 | - | - | ||
| Rank | 7 | 2 | 1 | 3 | 4 | - | - | |
| 28 | 3 | 1 | 2 | 4 | - | - | ||
| DOF | 7 | 2 | 2 | 2 | 2 | 8 | 0 | |
| 28 | 2 | 2 | 2 | 2 | 8 | 0 | ||
| Sum of squares | SSm | 7 | 2.43 | 73.02 | 1.22 | 0.09 | 76.77 | - |
| 28 | 1.63 | 79.29 | 1.98 | 0.06 | 82.97 | - | ||
| SSt | 7 | 76.77 | - | - | - | - | - | |
| 28 | 0.81 | 39.64 | 0.99 | 0.03 | - | - | ||
| Mean sum of squares | 7 | 1.22 | 36.51 | 0.61 | 0.05 | - | - | |
| 28 | ||||||||
| % Contribution | 7 | 3.17 | 95.12 | 1.59 | 0.12 | 100 | - | |
| 28 | 1.96 | 95.57 | 2.40 | 0.07 | 100 | - | ||
| Factors | Workability Performance | Strength Development |
|---|---|---|
| High-calcium materials, such as slag and Class C fly ash. | An increased content of high-calcium materials in the alkali-activated matrix accelerates the hydration process and raises the viscosity, which notably decreases flowability and shortens both the initial and final setting times. | Materials with high calcium content positively influenced the development of compressive strength, with strength values increasing as the calcium content rose under ambient curing temperatures. |
| Aluminosilicate materials, such as fly ash (Class F), metakaolin, waste glass, and ceramic. | Compared to high-calcium materials, aluminosilicate materials exhibited greater flowability as well as longer initial and final setting times. | Specimens of alkali-activated concrete containing high-aluminosilicate materials exhibited slower early strength development and required elevated curing temperatures. |
| Binder-to-solution ratio | The optimal binder-to-solution ratio depends on the type and the chemical and physical properties of the binder. However, the workability of concrete generally improves as the solution content increases. | Increasing the solution content in the matrix beyond the optimal level results in a reduction in strength performance. |
| Sodium hydroxide molarity | A high concentration of sodium hydroxide led to a significant reduction in both flowability and setting times. | Specimens prepared with molarities of 10, 12, and 14 demonstrated the highest strength performance compared to specimens prepared at other concentration levels. |
| Ratio of sodium silicate to sodium hydroxide | A high sodium silicate-to-sodium hydroxide ratio increases the solution’s viscosity, resulting in reduced flowability and shorter setting times. | The ratio of 2.5 between sodium silicate and sodium hydroxide is considered optimal for achieving maximum strength performance. |
| Effect of the addition of water and superplasticizer | The addition of water and superplasticizer significantly improved the workability performance. | Excess water adversely affects strength performance. However, the use of superplasticizers is strongly recommended to improve both workability and strength, rather than adding more water. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huseien, G.F.; Baghban, M.H.; Faridmehr, I.; Dong, K. Optimizing Mix Design for Alkali-Activated Concrete: A Comprehensive Review of Critical Selection Factors. CivilEng 2025, 6, 43. https://doi.org/10.3390/civileng6030043
Huseien GF, Baghban MH, Faridmehr I, Dong K. Optimizing Mix Design for Alkali-Activated Concrete: A Comprehensive Review of Critical Selection Factors. CivilEng. 2025; 6(3):43. https://doi.org/10.3390/civileng6030043
Chicago/Turabian StyleHuseien, Ghasan Fahim, Mohammad Hajmohammadian Baghban, Iman Faridmehr, and Kaijun Dong. 2025. "Optimizing Mix Design for Alkali-Activated Concrete: A Comprehensive Review of Critical Selection Factors" CivilEng 6, no. 3: 43. https://doi.org/10.3390/civileng6030043
APA StyleHuseien, G. F., Baghban, M. H., Faridmehr, I., & Dong, K. (2025). Optimizing Mix Design for Alkali-Activated Concrete: A Comprehensive Review of Critical Selection Factors. CivilEng, 6(3), 43. https://doi.org/10.3390/civileng6030043










