Effect of the Rehydration Method on the Physical–Mechanical Properties of CO2-Cured Magnesium-Based Fiber Cement Boards
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Accelerated Carbonation Process
2.2.3. Board Characterization
3. Results
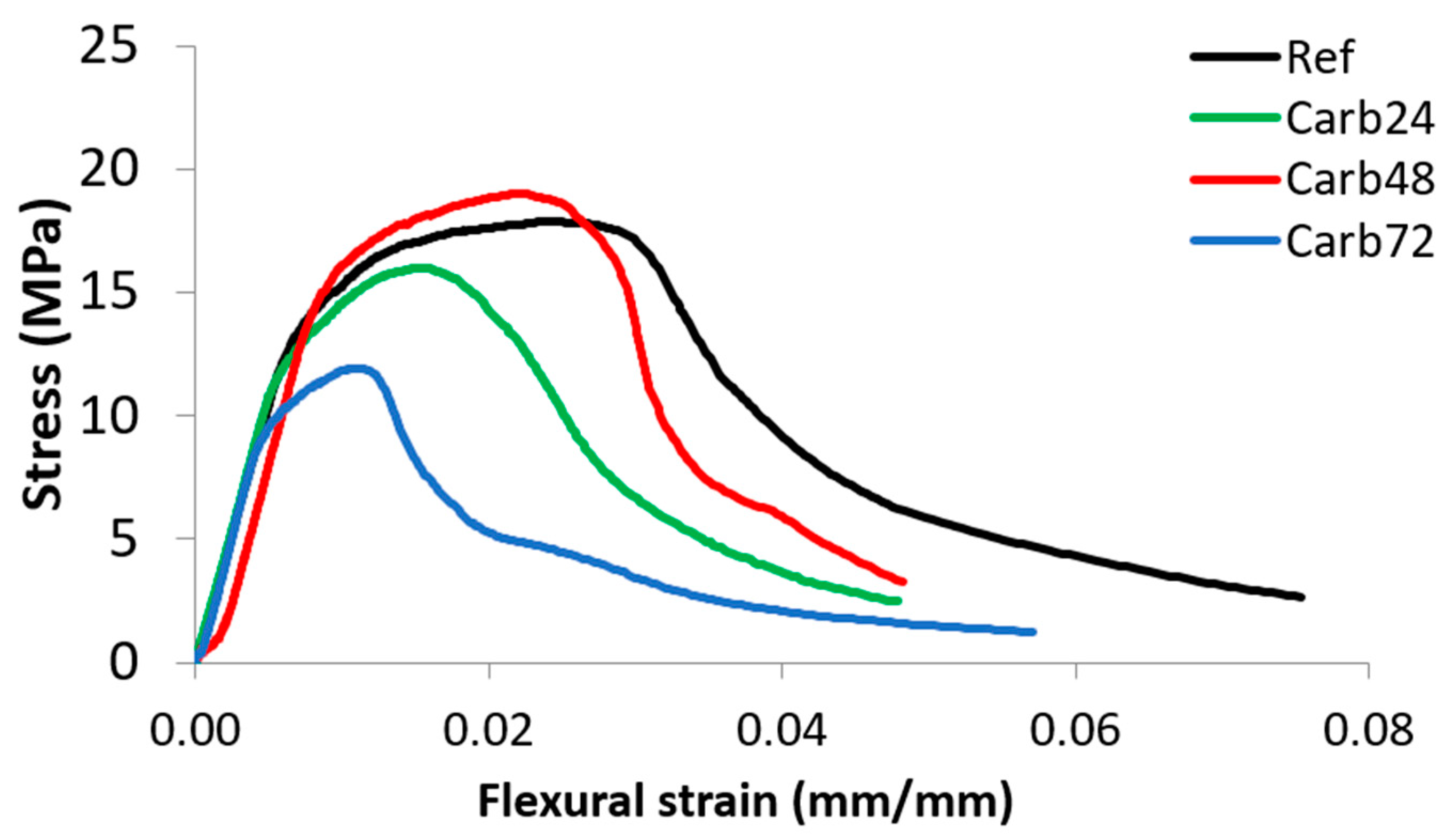
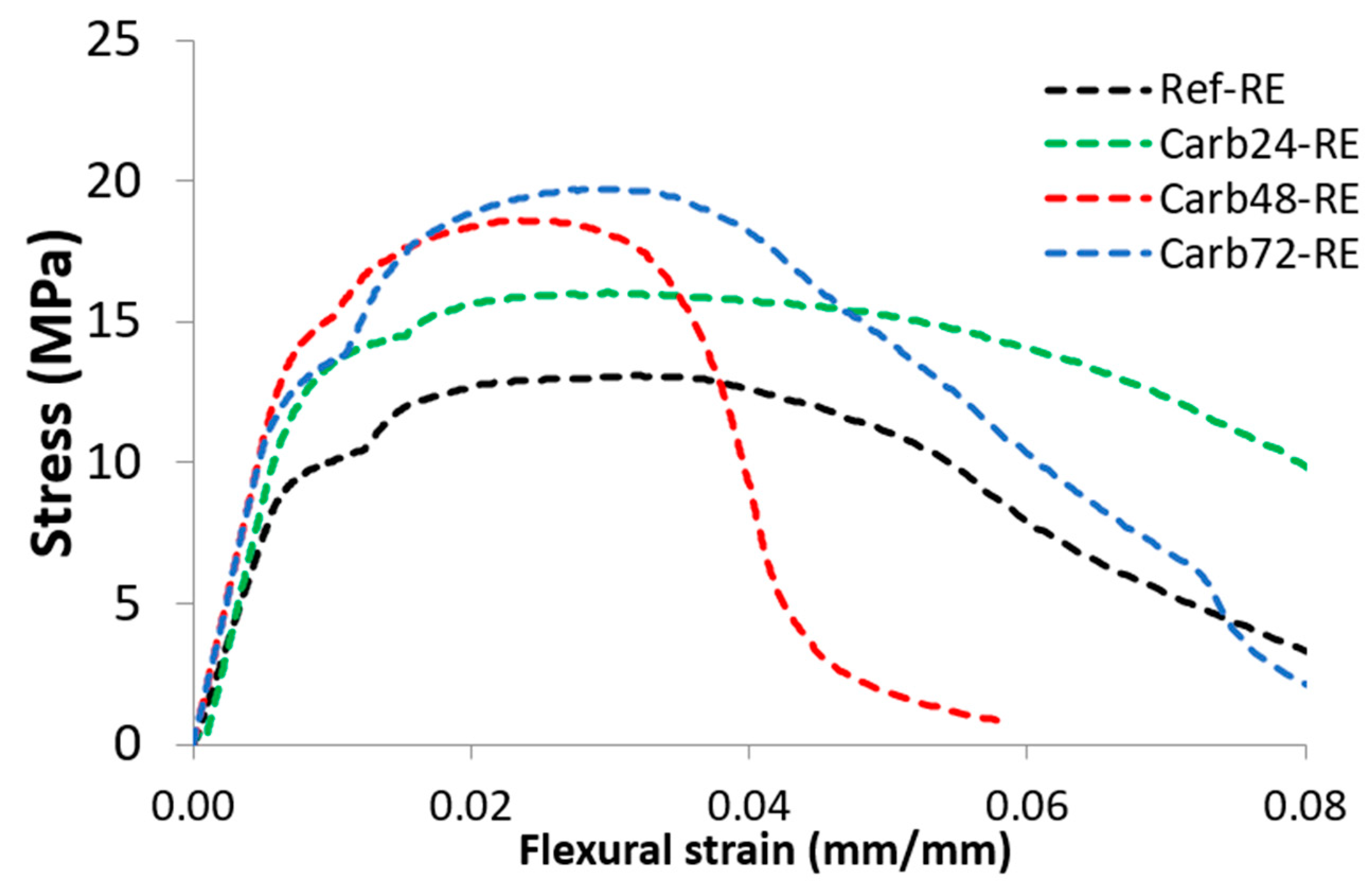
3.1. Mechanical Properties
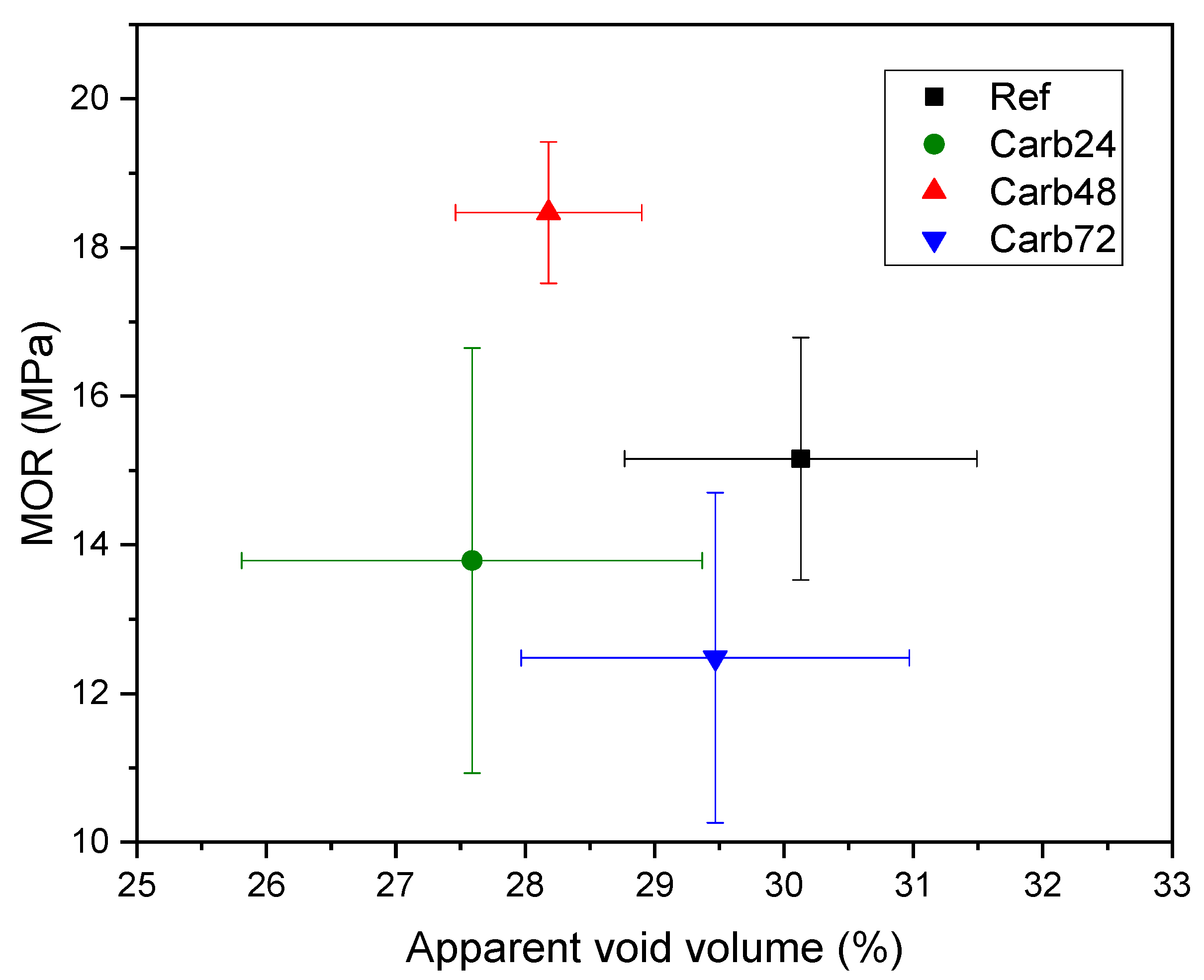
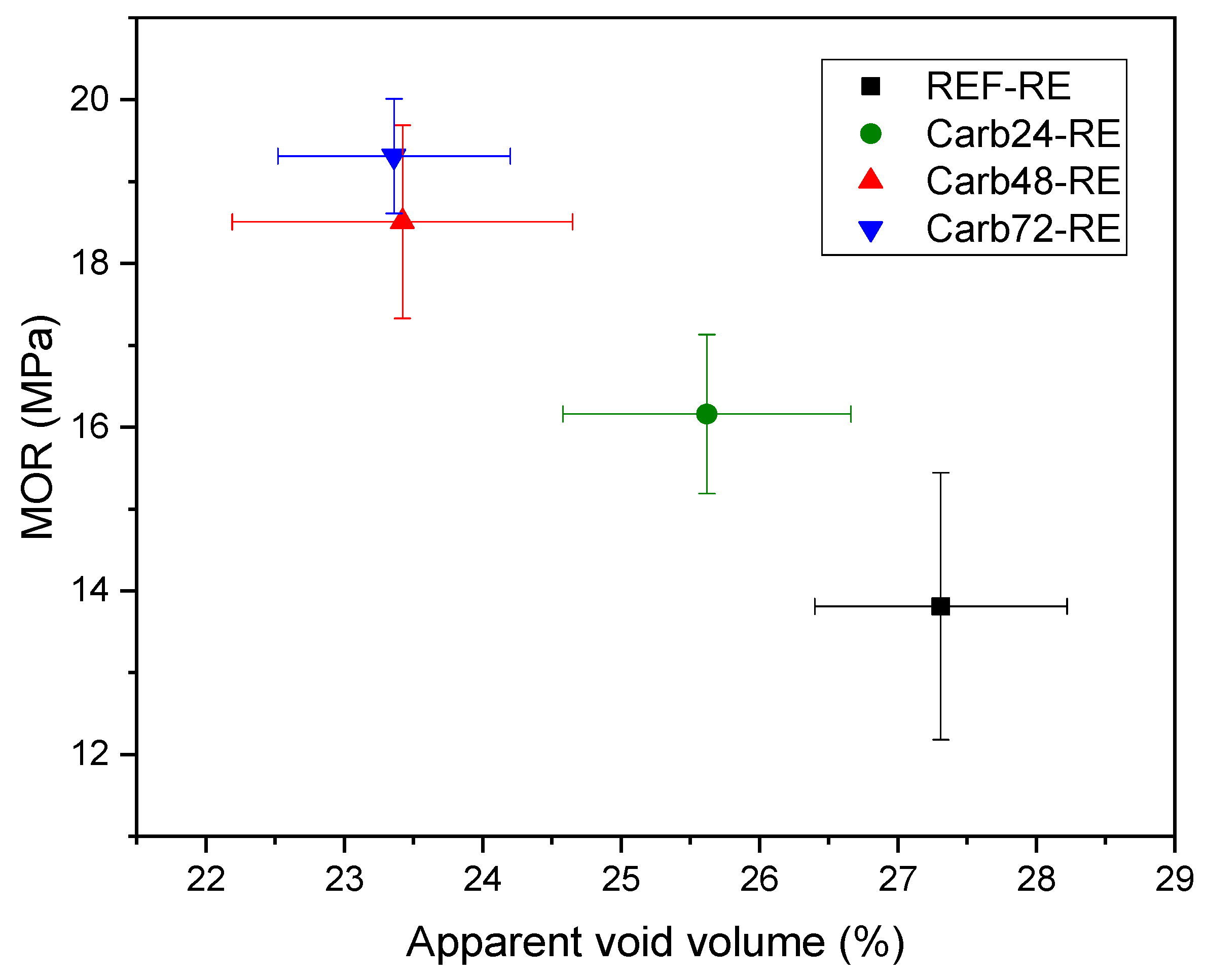
3.2. Physical Properties
3.3. Thermogravimetric Analysis (TGA/DTG)
3.4. Phase Analysis
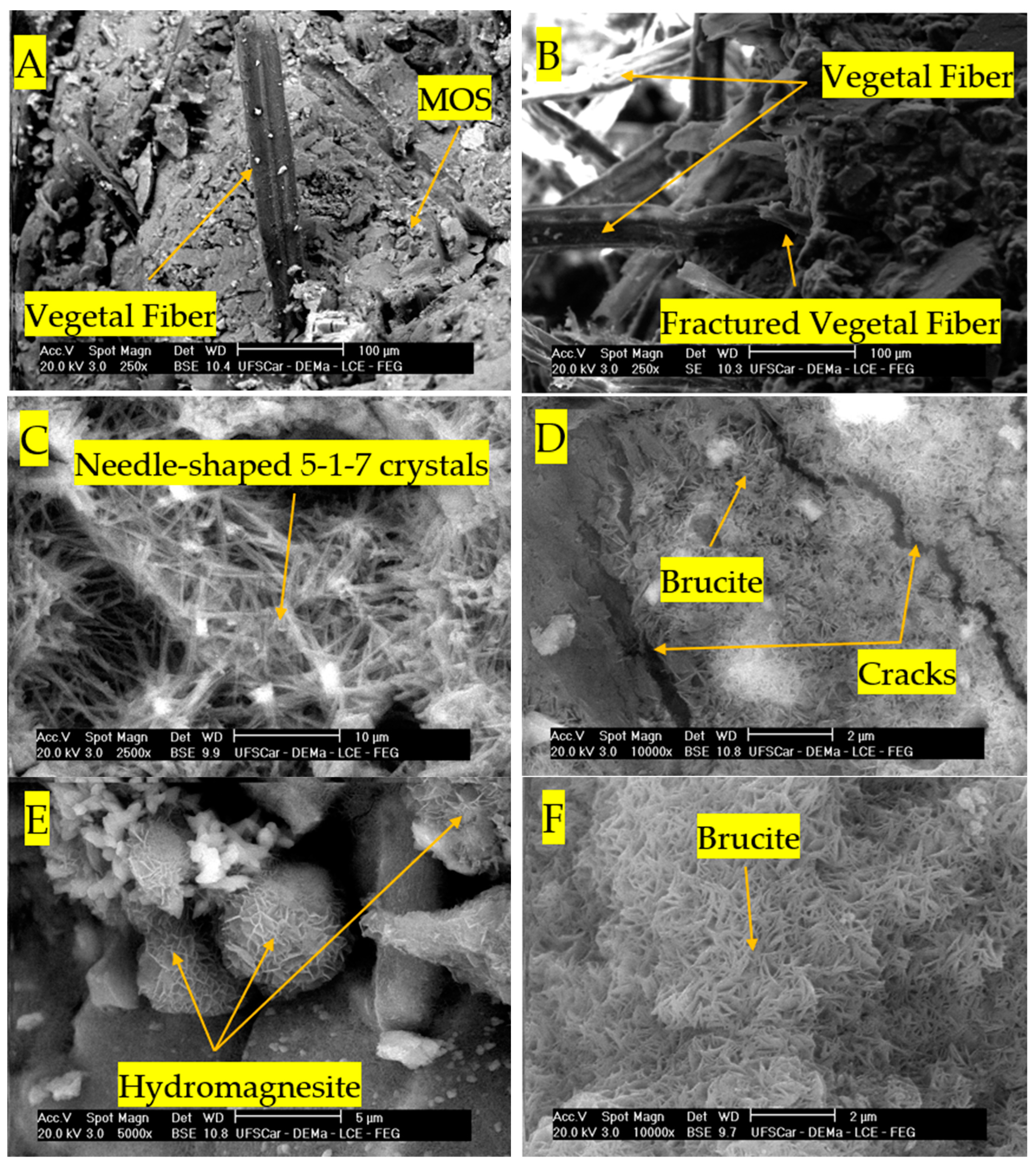
3.5. SEM Analysis
4. Discussion
5. Conclusions
- The production of boards using the Hatschek process simulation was successful, and the composites presented satisfactory mechanical behavior after different types of curing were applied.
- The accelerated carbonation process of the boards resulted in more significant improvements in the rehydrated composites with the formation of hydromagnesite after curing in a CO2-rich environment.
- The improvement of the mechanical performance of the boards was associated with the formation of new carbonation products, preferentially in the void space of the matrix. These new carbonation product formations were confirmed using TGA and SEM analysis.
- The pre-curing applied before carbonation proved to be a crucial step to control to obtain boards without microcracks and enhanced mechanical properties.
- The use of MOS cement in the production of fiber cement boards can be an alternative to the traditionally employed inorganic binders since they exhibit superior mechanical properties that can be further enhanced by subjecting the materials to accelerated carbonation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- He, Z.; Zhu, X.; Wang, J.; Mu, M.; Wang, Y. Comparison of CO2 emissions from OPC and recycled cement production. Constr. Build. Mater. 2019, 211, 965–973. [Google Scholar] [CrossRef]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental impact of cement production: Detail of the different processes and cement plant variability evaluation. J. Clean. Prod. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Maddalena, R.; Roberts, J.J.; Hamilton, A. Can Portland cement be replaced by low-carbon alternative materials? A study on the thermal properties and carbon emissions of innovative cements. J. Clean. Prod. 2018, 186, 933–942. [Google Scholar] [CrossRef]
- Meng, D.; Unluer, C.; Yang, E.H.; Qian, S. Recent advances in magnesium-based materials: CO2 sequestration and utilization, mechanical properties and environmental impact. Cem. Concr. Compos. 2023, 138, 104983. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, L.; Yu, J.; Yu, K. Mechanical properties of high ductile magnesium oxychloride cement-based composites after water soaking. Cem. Concr. Compos. 2019, 97, 248–258. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Comparative life cycle assessment of reactive MgO and Portland cement production. J. Clean. Prod. 2016, 137, 258–273. [Google Scholar] [CrossRef]
- Liska, M.; Al-Tabbaa, A.; Carter, K.; Fifield, J. Scaled-up commercial production of reactive magnesium cement pressed masonry units. Part I: Production. Proc. Inst. Civ. Eng. Constr. Mater. 2012, 165, 211–223. [Google Scholar] [CrossRef]
- Shand, M.A. The Chemistry and Technology of Magnesia; Wiley-Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- Nobre, J.; Ahmed, H.; Bravo, M.; Evangelista, L.; De Brito, J. Magnesia (Mgo) production and characterization, and its influence on the performance of cementitious materials: A review. Materials 2020, 13, 4752. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef] [PubMed]
- Schorcht, F.; Kourti, I.; Scalet, B.M.; Roudier, S.; Sancho, L.D. European Commission, Joint Research Centre ([EC JRC]), European Integrated Pollution Prevention and Control Bureau (EIPPCB). Best Available Techniques (BAT) Reference Document for the Production of Cement, Lime and Magnesium Oxide; Publications Office of the European Union: Luxembourg, 2013. [Google Scholar]
- Shand, M.A. Magnesium oxysulfate cement. Magnes. Cem. 2020, 1, 75–83. [Google Scholar] [CrossRef]
- Chen, F. Study on preparation and properties of modified magnesium oxysulfate cements. Chem. Eng. Trans. 2017, 62, 973–978. [Google Scholar] [CrossRef]
- Ba, M.; Xue, T.; He, Z.; Wang, H.; Liu, J. Carbonation of magnesium oxysulfate cement and its influence on mechanical performance. Constr. Build. Mater. 2019, 223, 1030–1037. [Google Scholar] [CrossRef]
- Gu, K.; Chen, B.; Yu, H.; Zhang, N.; Bi, W.; Guan, Y. Characterization of magnesium-calcium oxysulfate cement prepared by replacing MgSO4 in magnesium oxysulfate cement with untreated desulfurization gypsum. Cem. Concr. Compos. 2021, 121, 104091. [Google Scholar] [CrossRef]
- Hao, Y.; Li, C.; Zhao, F. Study on water resistance modification of magnesium oxysulfate cement. IOP Conf. Ser. Mater. Sci. Eng. 2019, 493, 012079. [Google Scholar] [CrossRef]
- Demediuk, T.; Cole, W.F. A study on magnesium oxysulphates. Aust. J. Chem. 1957, 10, 287–294. [Google Scholar] [CrossRef]
- Grünhäuser Soares, E.; Castro-Gomes, J. Carbonation curing influencing factors of Carbonated Reactive Magnesia Cements (CRMC)—A review. J. Clean. Prod. 2021, 305, 127210. [Google Scholar] [CrossRef]
- Mármol, G.; Savastano, H. Study of the degradation of non-conventional MgO-SiO2 cement reinforced with lignocellulosic fibers. Cem. Concr. Compos. 2017, 80, 258–267. [Google Scholar] [CrossRef]
- Dung, N.T.; Lesimple, A.; Hay, R.; Celik, K.; Unluer, C. Formation of carbonate phases and their effect on the performance of reactive MgO cement formulations. Cem. Concr. Res. 2019, 125, 105894. [Google Scholar] [CrossRef]
- Sun, Q.; Ba, M.; Zhang, D.; Zhou, S.; Zhang, N.; Wang, F. Effects of citrate acid, solidum silicate and their compound on the properties of magnesium oxysulfate cement under carbonation condition. Constr. Build. Mater. 2022, 330, 127136. [Google Scholar] [CrossRef]
- Li, Q.; Su, A.; Gao, X. Preparation of durable magnesium oxysulfate cement with the incorporation of mineral admixtures and sequestration of carbon dioxide. Sci. Total Environ. 2022, 809, 152127. [Google Scholar] [CrossRef] [PubMed]
- TAPPI T 271 om-23, "Fiber length of pulp and paper by automated optical analyzer using polarized light" (Technical Association of the Pulp & Paper Industry, 2023). Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T271.aspx (accessed on 28 October 2023).
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: New York, NY, USA, 2018. [Google Scholar]
- Savastano, H.; Warden, P.G.; Coutts, R.S.P. Brazilian waste fibres as reinforcement for cement-based composites. Cem. Concr. Compos. 2000, 22, 379–384. [Google Scholar] [CrossRef]
- ASTM C 948-81; ASTM C 948-81 (Reapproved 2016) Standard Test Method for Dry and Wet Bulk Density, Water Absorption, and Apparent Porosity of Thin Sections of Glass-Fiber REINFORCED Concrete. ASTM-American Society for Testing Materials: West Conshohocken, PA, USA, 2016; Volume 81, pp. 1–2.
- RILEM Reunion Internationale des Laboratooires d’essais et des Recheches sur les Materiaux et les Constructions. RILEM Technical Committee 49TFR: Testing methods for fibre reinforced cement-based composites. Mater. Constr. 1984, 17, 441–456. [Google Scholar]
- Hay, R.; Celik, K. Hydration, carbonation, strength development and corrosion resistance of reactive MgO cement-based composites. Cem. Concr. Res. 2020, 128, 105941. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. Green Construction with carbonating reactive magnesia porous blocks: Effect of cement and water contents. In Proceedings of the International Conference on Future Concrete, Dubai, United Arab Emirates, 12–14 December 2011. [Google Scholar]
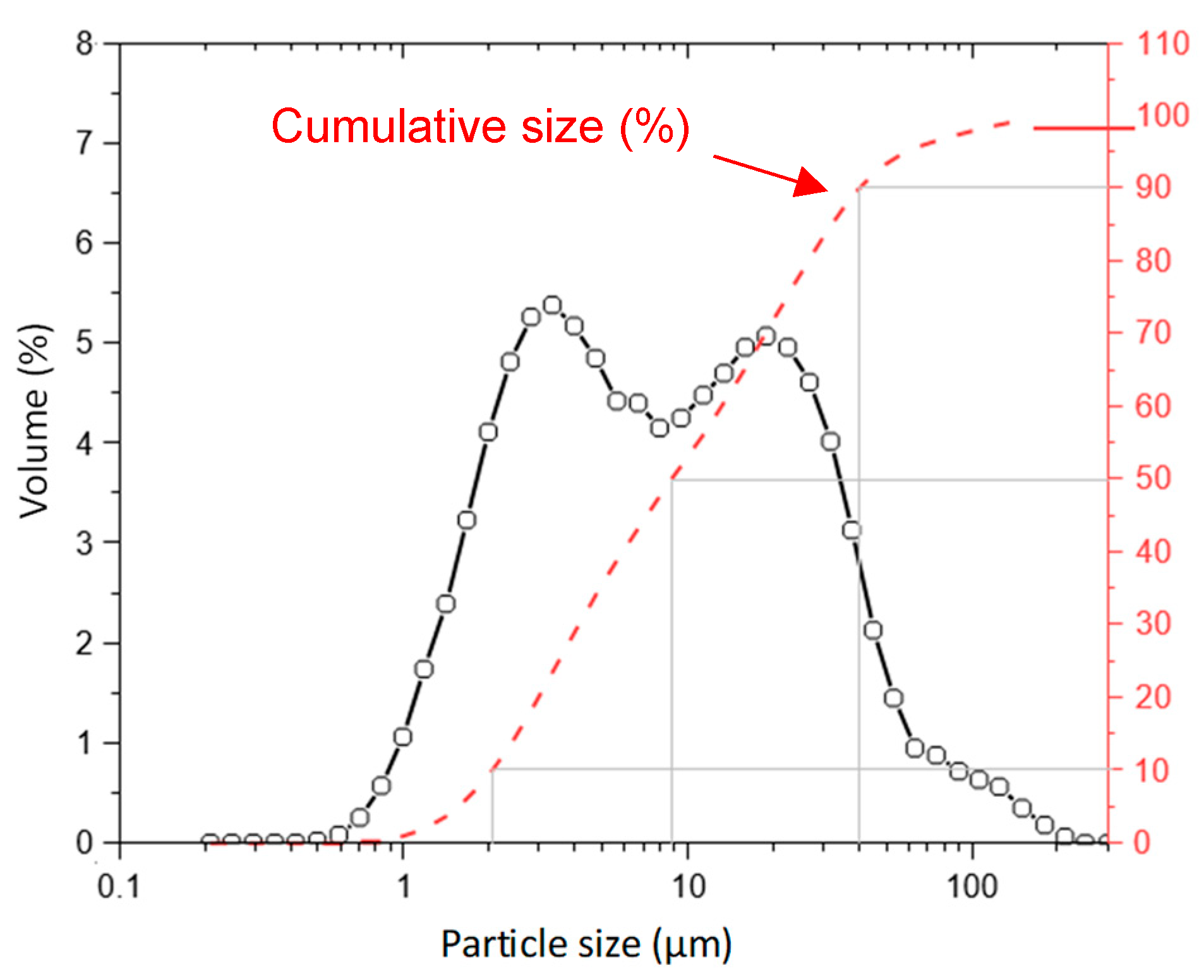
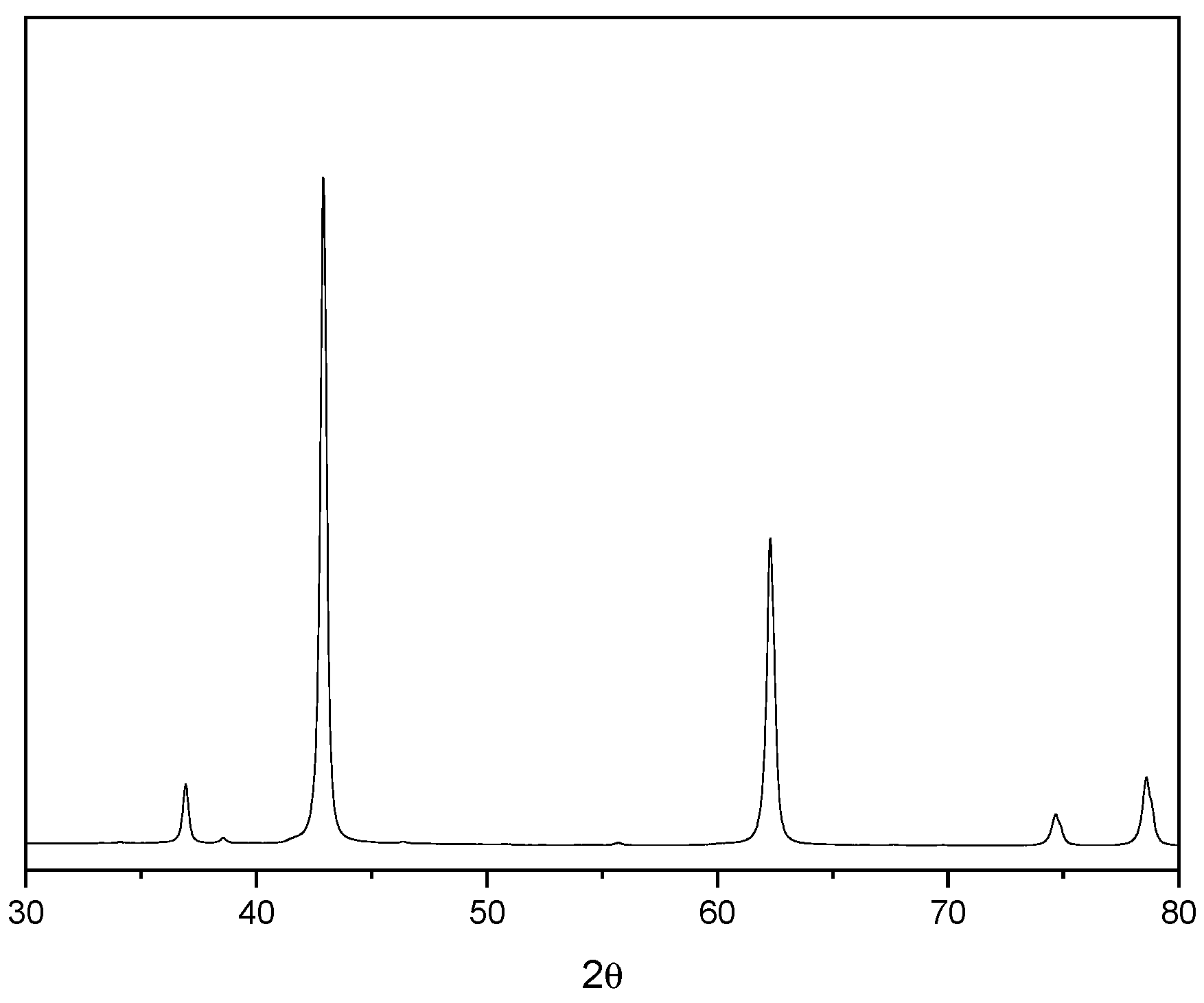


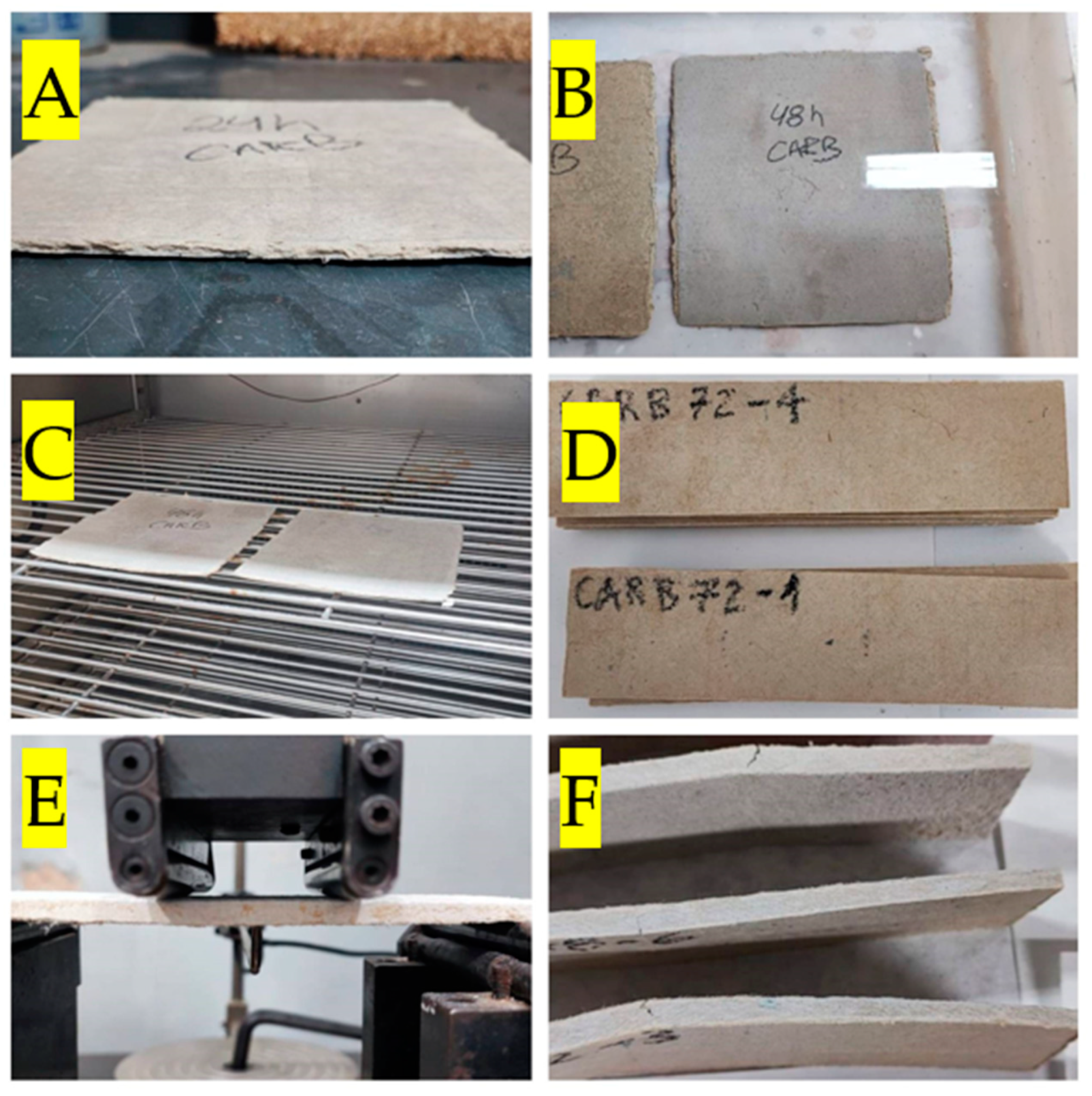



| Compound | Dolomitic Limestone (wt%) | MgO(wt%) |
|---|---|---|
| MgO | 7.6 | 97.4 |
| Al2O3 | 1.03 | <0.10 |
| SiO2 | 3.99 | 0.1 |
| P2O5 | 0.05 | _ |
| SO3 | 0.13 | _ |
| Cl | 0.01 | _ |
| K2O | 0.22 | <0.10 |
| Na2O | _ | <0.10 |
| CaO | 43.9 | 0.81 |
| TiO2 | 0.06 | _ |
| MnO | 0.09 | _ |
| Fe2O3 | 0.42 | _ |
| ZnO | <0.01 | _ |
| Rb2O | <0.02 | _ |
| SrO | <0.03 | _ |
| LOI | 42.4 | 1.6 |
| Type of Fiber | Chemical Composition (%) | |||
|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | Others | |
| Eucalyptus (unbleached) | 82.55 | 8.39 | 1.73 | 7.33 |
| Sample | Boards Produced |
|---|---|
| Ref | Without rehydration and carbonation |
| Ref-RE | Rehydrated-uncarbonated boards |
| Carb24 | Carbonated after 24 h without rehydration |
| Carb48 | Carbonated after 48 h without rehydration |
| Carb72 | Carbonated after 72 h without rehydration |
| Carb24-RE | Carbonated after 24 h with rehydration |
| Carb48-RE | Carbonated after 48 h with rehydration |
| Carb72-RE | Carbonated after 72 h with rehydration |
| Sample | MOR | LOP | MOE | SE |
|---|---|---|---|---|
| (MPa) | (MPa) | (GPa) | (KJ/m2) | |
| Ref | 15.16 ± 0.63 | 12.40 ± 1.95 | 9.89 ± 1.24 | 3.44 ± 0.48 |
| Carb24 | 13.79 ± 1.16 | 10.23 ± 1.47 | 9.90 ± 1.60 | 2.71 ± 0.33 |
| Carb48 | 18.47 ± 0.95 | 14.66 ± 0.90 | 10.52 ± 1.37 | 2.93 ± 0.1 |
| Carb72 | 12.48 ± 1.22 | 10.79 ± 2.88 | 8.26 ± 1.16 | 2.63 ± 0.37 |
| Ref-RE | 13.81 ± 1.63 | 8.75 ± 0.29 | 9.48 ± 0.34 | 4.41 ± 1.11 |
| Carb24-RE | 16.16 ± 0.97 | 12.03 ± 1.65 | 13.44 ± 1.69 | 3.60 ± 1.63 |
| Carb48-RE | 18.51 ± 1.18 | 12.73 ± 1.02 | 14.83 ± 1.48 | 3.28 ± 0.31 |
| Carb72-RE | 19.31 ± 0.70 | 12.39 ± 1.22 | 13.57 ± 0.25 | 4.08 ± 0.62 |
| Sample | Water Absorption (%) | Apparent Porosity (%) | Bulk Density (g/cm3) |
|---|---|---|---|
| Ref | 19.98 ± 1.48 | 30.13 ± 0.76 | 1.46 ± 0.01 |
| Carb24 | 18.08 ± 1.76 | 27.59 ± 0.78 | 1.53 ± 0.05 |
| Carb48 | 19.09 ± 1.87 | 28.18 ± 1.03 | 1.52 ± 0.05 |
| Carb72 | 19.38 ± 1.40 | 29.47 ± 1.13 | 1.50 ± 0.05 |
| Sample | Water Absorption (%) | Apparent Porosity (%) | Bulk Density (g/cm3) |
|---|---|---|---|
| Ref-RE | 17.21 ± 0.93 | 27.31 ± 0.91 | 1.59 ± 0.91 |
| Carb24-RE | 16.03 ± 1.10 | 25.62 ± 1.04 | 1.601 ± 0.045 |
| Carb48-RE | 14.63 ± 1.10 | 23.42 ± 0.93 | 1.603 ± 0.056 |
| Carb72-RE | 14.34 ± 0.78 | 23.36 ± 0.84 | 1.631 ± 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, A.G.S.; Molano, J.C.A.; Parente, I.; Freitas, T.O.G.; Camões, A.; Faria, P.; Savastano, H., Jr. Effect of the Rehydration Method on the Physical–Mechanical Properties of CO2-Cured Magnesium-Based Fiber Cement Boards. CivilEng 2024, 5, 247-264. https://doi.org/10.3390/civileng5010013
Azevedo AGS, Molano JCA, Parente I, Freitas TOG, Camões A, Faria P, Savastano H Jr. Effect of the Rehydration Method on the Physical–Mechanical Properties of CO2-Cured Magnesium-Based Fiber Cement Boards. CivilEng. 2024; 5(1):247-264. https://doi.org/10.3390/civileng5010013
Chicago/Turabian StyleAzevedo, Adriano G. S., Juan Camilo Adrada Molano, Igor Parente, Taís O. G. Freitas, Aires Camões, Paulina Faria, and Holmer Savastano, Jr. 2024. "Effect of the Rehydration Method on the Physical–Mechanical Properties of CO2-Cured Magnesium-Based Fiber Cement Boards" CivilEng 5, no. 1: 247-264. https://doi.org/10.3390/civileng5010013
APA StyleAzevedo, A. G. S., Molano, J. C. A., Parente, I., Freitas, T. O. G., Camões, A., Faria, P., & Savastano, H., Jr. (2024). Effect of the Rehydration Method on the Physical–Mechanical Properties of CO2-Cured Magnesium-Based Fiber Cement Boards. CivilEng, 5(1), 247-264. https://doi.org/10.3390/civileng5010013












