A Three-Phase Model to Evaluate Effects of Phase Diffusivity and Volume Fraction upon the Crack Propagation in Concrete Subjected to External Sulphate Attack
Abstract
1. Introduction
2. Model Description
2.1. Underlying Chemical Mechanisms
2.2. Diffusion–Reaction Model
2.3. Three-Phase Model
2.4. Sulphate-Induced Tensile Strain
2.5. Durability-Based Limit State Function
3. Results and Discussion
3.1. Validation of Sulphate Penetration Based on Three-Phase Diffusion–Reaction Model
3.2. Parametric Analysis
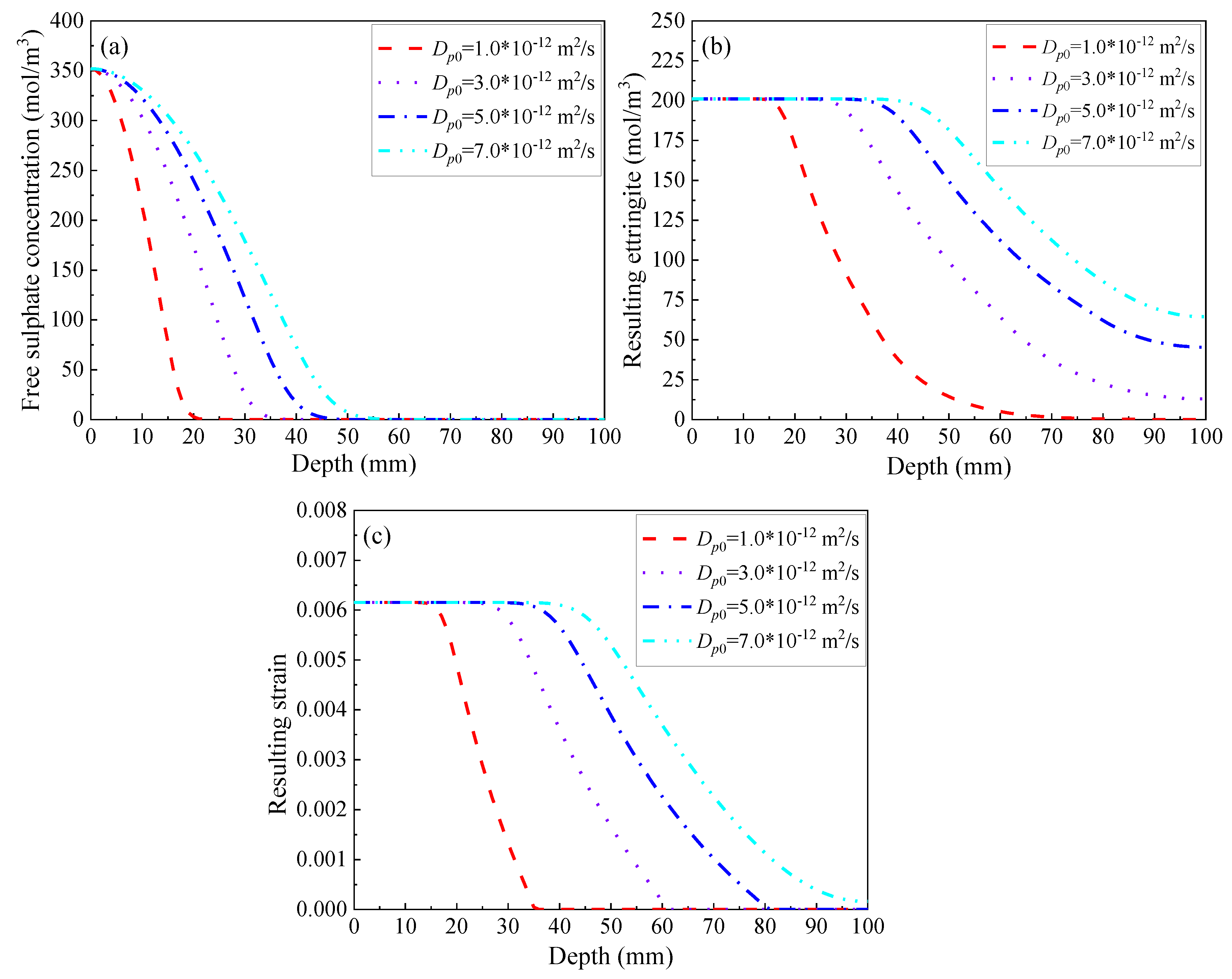
3.2.1. Diffusivity of Mortar
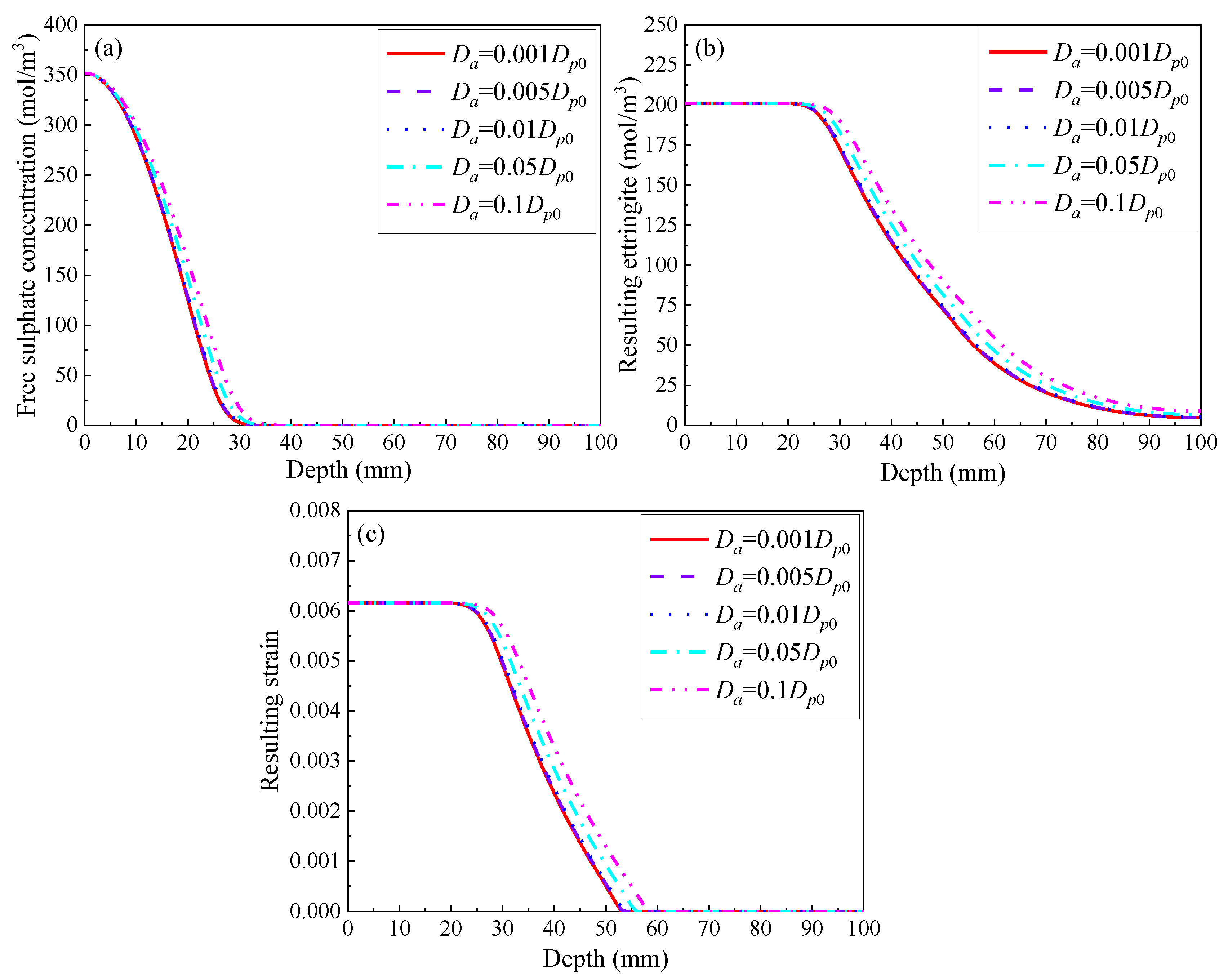
3.2.2. Diffusivity of Coarse Aggregate
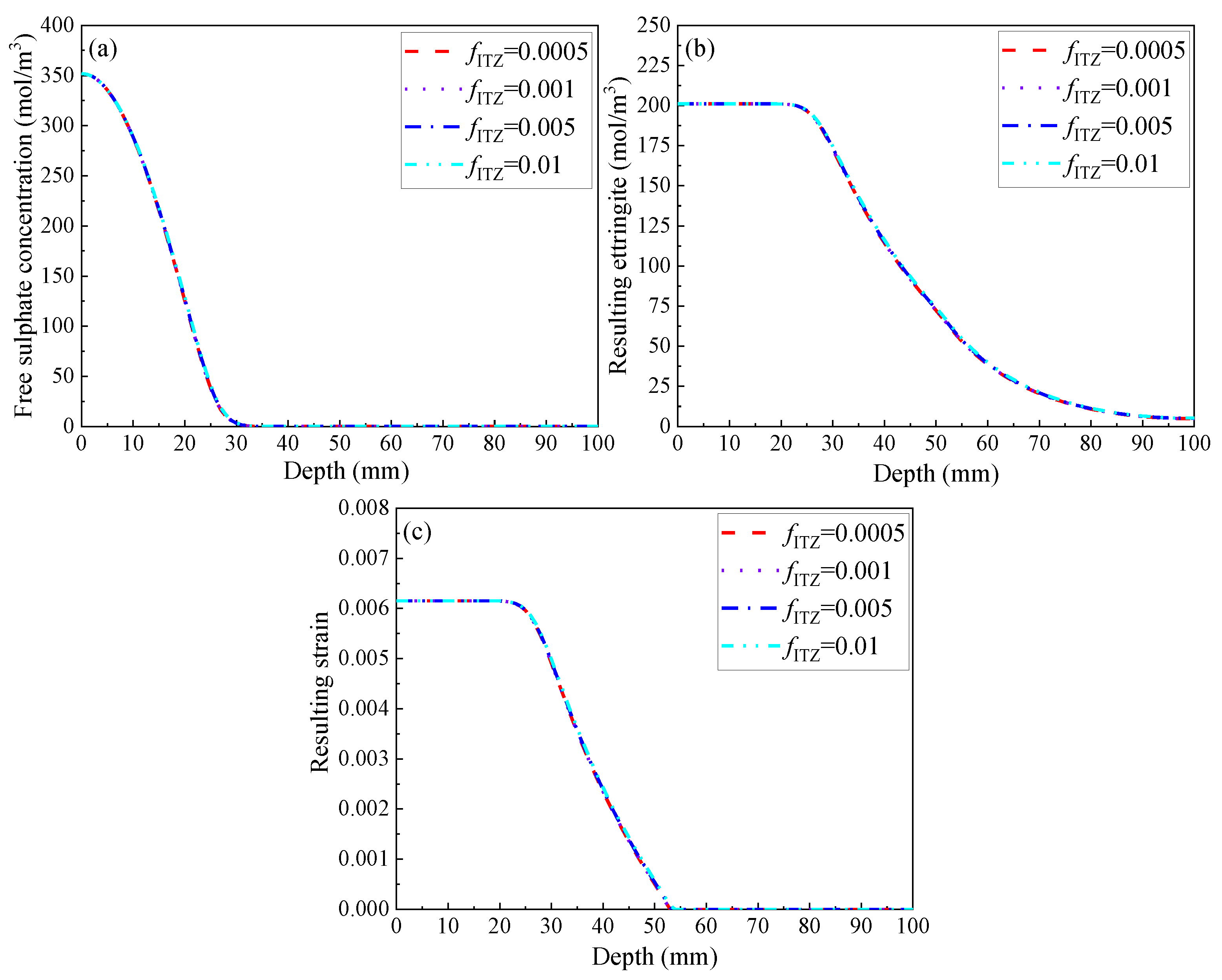
3.2.3. Diffusivity of ITZ
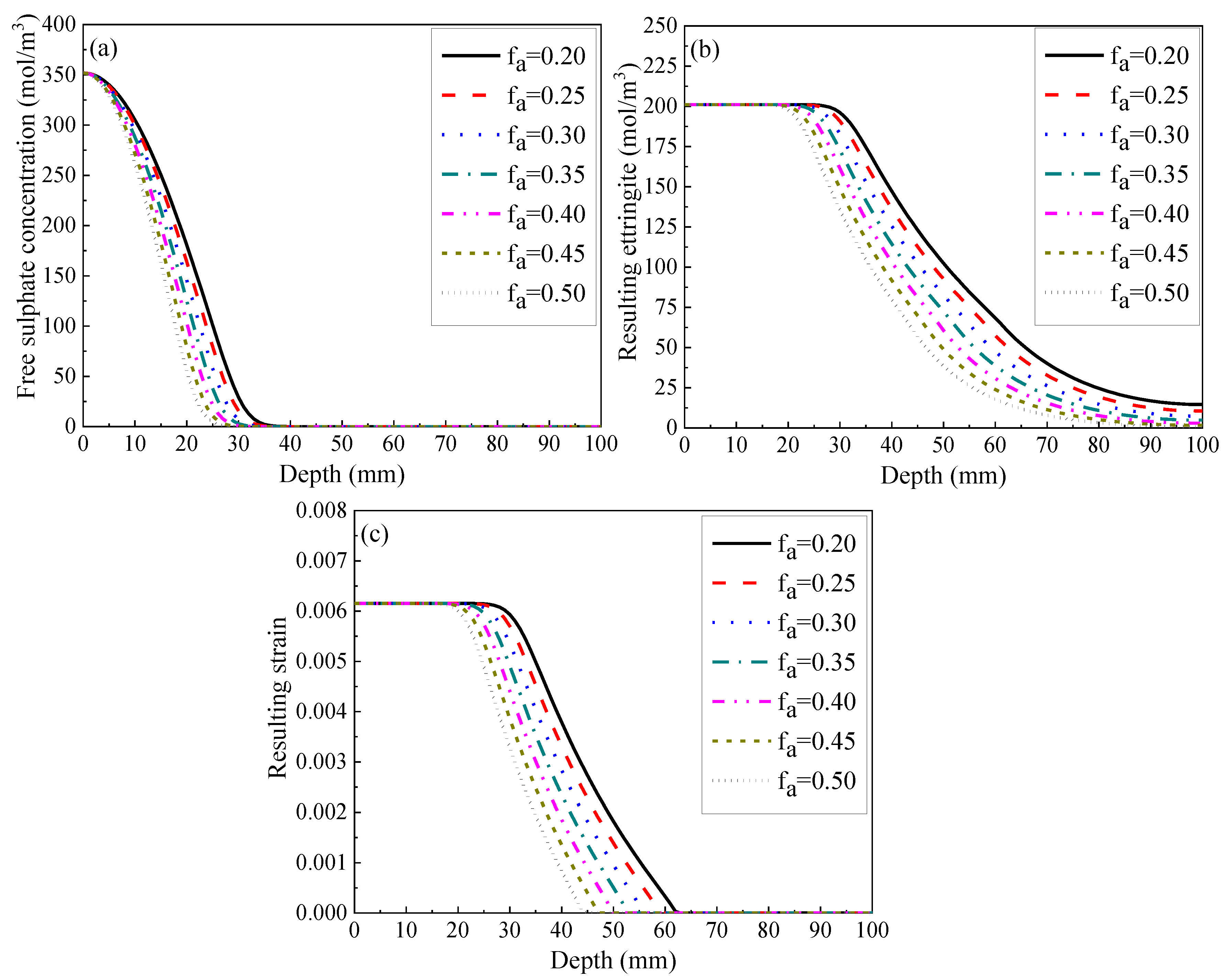
3.2.4. Volumetric Fraction of Coarse Aggregate
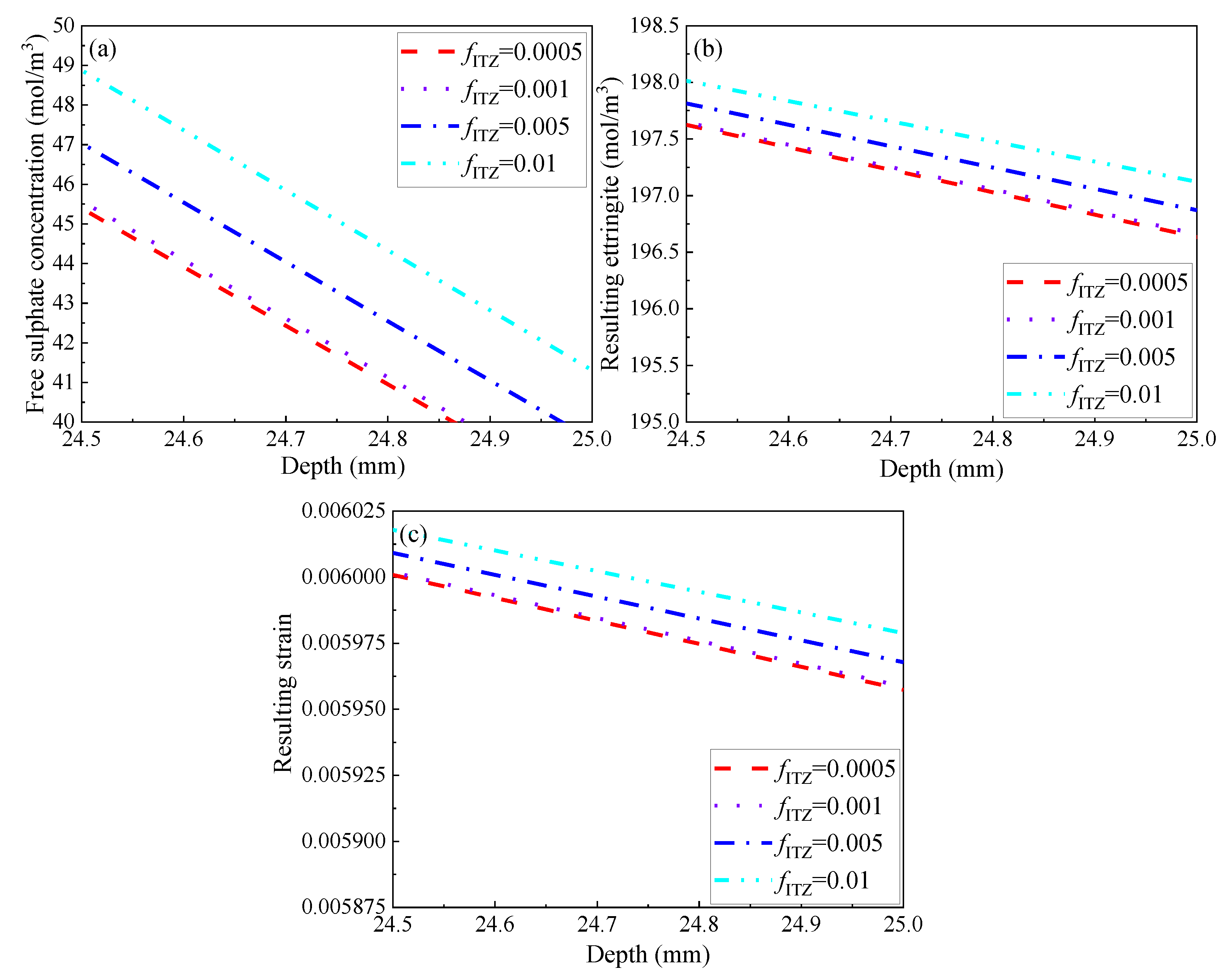
3.2.5. Volumetric Fraction of ITZ
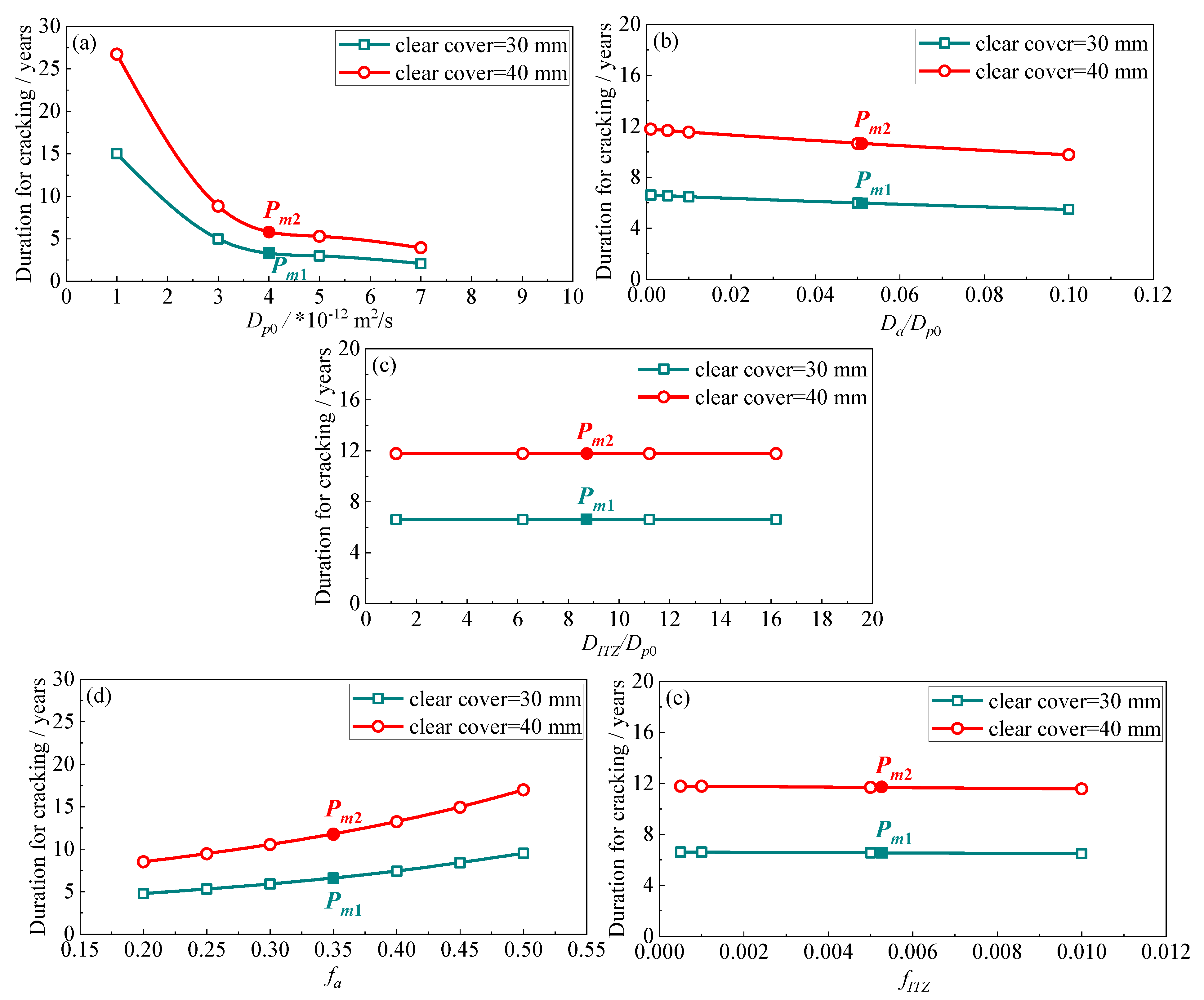
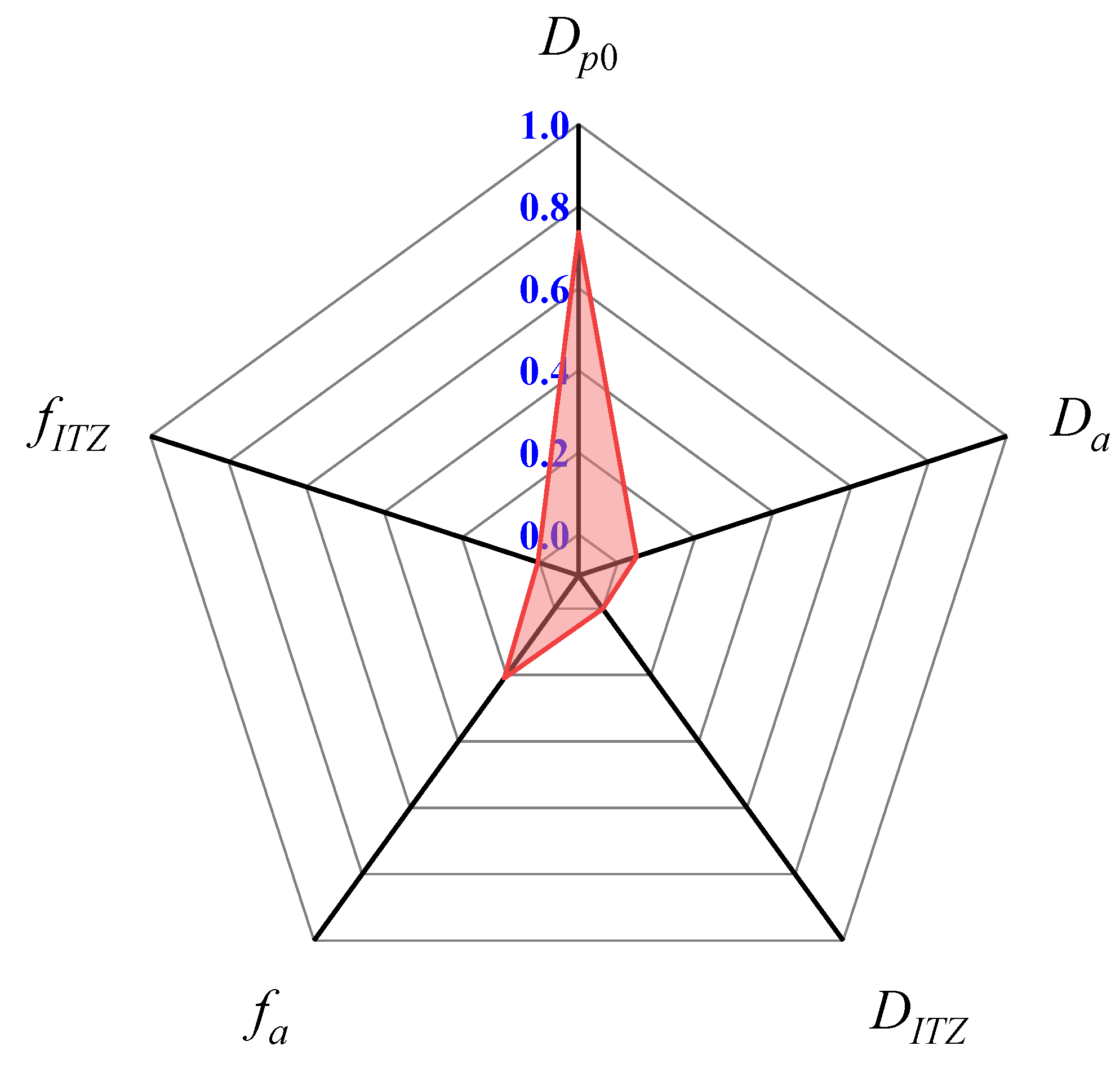
3.3. Crack Propagation Prediction and Sensitivity Analysis
4. Concluding Remarks
- The sulphate penetration and the ensuing crack propagation are found to be most sensitive to the diffusivity of mortar. This is specifically evident from its highest sensitivity index in terms of the sulphate-induced cracking. In addition, the correlation between the diffusivity of mortar and the sulphate-induced cracking is not linear. When the diffusion coefficient of sulphate ions in mortar is below a certain value, here found as 1.0 × 10−12 m2/s, the sulphate attack progress may be deterred significantly;
- The lifetime of concrete structures is believed to extend with a decrease in the diffusivity of the coarse aggregate and with an increase in its volume fraction with respect to the entire mixture. This is due to its extremely low diffusion coefficient, as well as its negligible calcium aluminate content. Furthermore, increasing its fraction is a more promising method to improve the sulphate resistance of concrete;
- Although the ITZ is the weakest phase in the modelled concrete, varying its diffusivity and fraction may affect the progress of sulphate attack only very slightly. This is attributed to its extremely low volumetric fraction in comparison to that of mortar or coarse aggregates. Compared to the diffusivity of ITZ, the sulphate attack may be more sensitive to its fraction.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamun, M.; Bindiganavile, V. Sulphate resistance of fibre reinforced cement-based foams. Constr. Build. Mater. 2011, 25, 3427–3442. [Google Scholar] [CrossRef]
- Al-Dulaijan, S.U.; Maslehuddin, M.; Al-Zahrani, M.M.; Sharif, A.M.; Shameem, M.; Ibrahim, M. Sulphate resistance of plain and blended cements exposed to varying concentrations of sodium sulphate. Cem. Concr. Compos. 2003, 25, 429–437. [Google Scholar] [CrossRef]
- El-Hachem, R.; Rozière, E.; Grondin, F.; Loukili, A. New procedure to investigate external sulphate attack on ce-mentitious materials. Cem. Concr. Compos. 2012, 34, 357–364. [Google Scholar] [CrossRef]
- Müllauer, W.; Beddoe, R.E.; Heinz, D. Sulphate attack expansion mechanisms. Cem. Concr. Res. 2013, 52, 208–215. [Google Scholar] [CrossRef]
- Jiang, L.; Niu, D.; Yuan, L.; Fei, Q. Durability of concrete under sulphate attack exposed to freeze–thaw cycles. Cold Reg. Sci. Technol. 2015, 112, 112–117. [Google Scholar] [CrossRef]
- Gollop, R.S.; Taylor, H.F.W. Microstructural and microanalytical studies of sulphate attack. I. Ordinary Portland cement paste. Cem. Concr. Res. 1992, 22, 1027–1038. [Google Scholar] [CrossRef]
- Gu, Y.; Martin, R.P.; Metalssi, O.O.; Fen-Chong, T.; Dangla, P. Pore size analyses of cement paste exposed to external sulfate attack and delayed ettringite formation. Cem. Concr. Res. 2019, 123, 105766. [Google Scholar] [CrossRef]
- Ragoug, R.; Metalssi, O.O.; Barberon, F.; Torrenti, J.M.; Roussel, N.; Divet, L.; de Lacaillerie, J.B.D.E. Durability of cement pastes exposed to external sulfate attack and leaching: Physical and chemical aspects. Cem. Concr. Res. 2019, 116, 134–145. [Google Scholar] [CrossRef]
- Tian, B.; Cohen, M.D. Does gypsum formation during sulphate attack on concrete lead to expansion? Cem. Concr. Res. 2000, 30, 117–123. [Google Scholar] [CrossRef]
- Yang, D.Y.; Guo, R. Experimental Study on Modulus and Hardness of Ettringite. Exp. Tech. 2014, 38, 6–12. [Google Scholar] [CrossRef]
- Tian, B.; Cohen, M.D. Expansion of Alite Paste Caused by Gypsum Formation during Sulfate Attack. J. Mater. Civ. Eng. 2000, 12, 24–25. [Google Scholar] [CrossRef]
- Chen, Z.; Yi, C.; Bindiganavile, V. Investigating the two-dimensional diffusion-reaction behaviour of sulphate ions in cement-based systems. Constr. Build. Mater. 2018, 187, 791–802. [Google Scholar] [CrossRef]
- Rozière, E.; Loukili, A.; El Hachem, R.; Grondin, F. Durability of concrete exposed to leaching and external sulphate attacks. Cem. Concr. Res. 2009, 39, 1188–1198. [Google Scholar] [CrossRef]
- Kandasamy, S.; Shehata, M.H. Durability of ternary blends containing high calcium fly ash and slag against sodium sulphate attack. Constr. Build. Mater. 2014, 53, 267–272. [Google Scholar] [CrossRef]
- El-Hachem, R.; Rozière, E.; Grondin, F.; Loukili, A. Multi-criteria analysis of the mechanism of degradation of Port-land cement based mortars exposed to external sulphate attack. Cem. Concr. Res. 2012, 42, 1327–1335. [Google Scholar] [CrossRef]
- Yi, C.; Chen, Z.; Bindiganavile, V. A non-homogeneous model to predict the service life of concrete subjected to external sulphate attack. Constr. Build. Mater. 2019, 212, 254–265. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, L.; Bindiganavile, V.; Yi, C. Coupled models to describe the combined diffusion-reaction behaviour of chloride and sulphate ions in cement-based systems. Constr. Build. Mater. 2020, 243, 118232. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Chen, W.-K.; Mu, S.; Šavija, B.; Liu, Q.-F. Numerical investigation of external sulfate attack and its effect on chloride binding and diffusion in concrete. Constr. Build. Mater. 2021, 285, 122806. [Google Scholar] [CrossRef]
- Tixier, R.; Mobasher, B. Modeling of Damage in Cement-Based Materials Subjected to External Sulfate Attack. I: Formulation. J. Mater. Civ. Eng. 2003, 15, 305–313. [Google Scholar] [CrossRef]
- Tixier, R.; Mobasher, B. Modeling of Damage in Cement-Based Materials Subjected to External Sulfate Attack. II: Comparison with Experiments. J. Mater. Civ. Eng. 2003, 15, 314–322. [Google Scholar] [CrossRef]
- Marchand, J. Modeling the behavior of unsaturated cement systems exposed to aggressive chemical environments. Mater. Struct. 2001, 34, 195–200. [Google Scholar] [CrossRef]
- Marchand, J.; Samson, E.; Maltais, Y.; Beaudoin, J. Theoretical analysis of the effect of weak sodium sulfate solutions on the durability of concrete. Cem. Concr. Compos. 2002, 24, 317–329. [Google Scholar] [CrossRef]
- Marchand, J.; Samson, E.; Maltais, Y.; Lee, R.J.; Sahu, S. Predicting the performance of concrete structures exposed to chemically aggressive environment—Field validation. Mater. Struct. 2002, 35, 623–631. [Google Scholar] [CrossRef]
- Ikumi, T.; Cavalaro, S.H.; Segura, I.; Aguado, A. Alternative methodology to consider damage and expansions in external sulfate attack modeling. Cem. Concr. Res. 2014, 63, 105–116. [Google Scholar] [CrossRef]
- Zuo, X.-B.; Sun, W.; Yu, C. Numerical investigation on expansive volume strain in concrete subjected to sulfate attack. Constr. Build. Mater. 2012, 36, 404–410. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Zhu, J.; Zhang, M.; Ye, J. A new diffusion model of sulfate ions in concrete. Constr. Build. Mater. 2013, 39, 39–45. [Google Scholar] [CrossRef]
- Idiart, A.E.; López, C.M.; Carol, I. Chemo-mechanical analysis of concrete cracking and degradation due to external sulfate attack: A meso-scale model. Cem. Concr. Compos. 2011, 33, 411–423. [Google Scholar] [CrossRef]
- Li, M.; Chen, H.; Qing, L.; Lin, J. Generalized implicit solution of ITZ percolation threshold and its effect on the diffusivity of concrete: Influence of aggregate shape-and size-polydispersities. Int. J. Heat Mass Transfer 2023, 200, 123514. [Google Scholar] [CrossRef]
- Xiao, J.; Ying, J.; Shen, L. FEM simulation of chloride diffusion in modeled recycled aggregate concrete. Constr. Build. Mater. 2012, 29, 12–23. [Google Scholar] [CrossRef]
- Chen, X.; Gu, X.; Xia, X.; Li, X.; Zhang, Q. A Chemical-Transport-Mechanics Numerical Model for Concrete under Sulfate Attack. Materials 2021, 14, 7710. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, X.; Zhou, X.; Du, L. Diffusion model of sulfate ions in concrete based on pore change of cement mortar and its application in mesoscopic numerical simulation. Struct. Concr. 2022, 23, 3786–3803. [Google Scholar] [CrossRef]
- Collepardi, M.; Marcialis, A.; Turriziani, R. The kinetics of penetration of chloride ions into the concrete. II Cemento 1970, 67, 157–164. [Google Scholar]
- Collepardi, M.; Marcialis, A.; Turriziani, R. Penetration of Chloride Ions into Cement Pastes and Concretes. J. Am. Ceram. Soc. 1972, 55, 534–535. [Google Scholar] [CrossRef]
- Hanshin, Z.; Shtrikman, S. A variational approach to the theory of the elastic behaviour of multi-phase materials. J. Mech. Phys. Solids 1963, 11, 127–140. [Google Scholar] [CrossRef]
- Hobbs, D.W. Aggregate influence on chloride ion diffusion into concrete. Cem. Concr. Res. 1999, 29, 1995–1998. [Google Scholar] [CrossRef]
- Wang, J.G. Sulfate attack on hardened cement paste. Cem. Concr. Res. 1994, 24, 735–742. [Google Scholar] [CrossRef]
- Hibbeler, R.C.; Hibbeler, R.C. Engineering Mechanics: Statics & Dynamics; Pearson Education India: Englewood Cliffs, NJ, USA, 2007. [Google Scholar]
- Sarkar, S.; Mahadevan, S.; Meeussen, J.C.L.; van der Sloot, H.; Kosson, D.S. Numerical simulation of cementitious materials degradation under external sulfate attack. Cem. Concr. Compos. 2010, 32, 241–252. [Google Scholar] [CrossRef]
- Gao, Y.; De Schutter, G.; Ye, G.; Tan, Z.; Wu, K. The ITZ microstructure, thickness and porosity in blended cementi-tious composite: Effects of curing age, water to binder ratio and aggregate content. Compos. Part B Eng. 2014, 60, 1–13. [Google Scholar] [CrossRef]
- Canadian Standards Association. Concrete Materials and Methods of Concrete Construction: Test Methods and Standard Practices for Concrete; Canadian Standards Association: Toronto, ON, Canada, 2009. [Google Scholar]
- Yi, C.; Chen, Z.; Bindiganavile, V. Crack growth prediction of cement-based systems subjected to two-dimensional sulphate attack. Constr. Build. Mater. 2019, 222, 814–828. [Google Scholar] [CrossRef]




| Oxide | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | R2O | Na2O | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement [12] | 62.7% | 20.4% | 4.6% | 3.4% | 2.7% | 2.8% | 0.5% | - | - | - |
| Cement [17] | 58.1% | 22.1% | 6.7% | 4.43% | 2.22% | 0.87% | 0.37% | 0.54% | 0.3% | - |
| Fly ash | 11.0% | 55.5% | 23.2% | 3.6% | 0.2% | 1.2% | 0.8% | - | 2.8% | 0.7% |
| Mixture | W/B | Fly Ash | Water | Cement | Fine Aggregate | Coarse Aggregate | Admixture |
|---|---|---|---|---|---|---|---|
| Reference [12] | 0.485 | 160.5 kg | 260 kg | 374.5 kg | 1470 kg | - | - |
| Reference [17] | 0.45 | 80 kg | 180 kg | 320 kg | 710 kg | 1060 kg | 0.383% |
| Factor | xik | yik (30 mm) | yik (40 mm) | xim | yim (30 mm) | yim (40 mm) | αkm | Si1 (%) (30 mm) | Si2 (%) (40 mm) | Si (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Dp0 | 1 | 15.01 | 26.72 | 4 | 3.40 | 5.80 | 0.75 | 73.351 | 73.939 | 73.645 |
| 3 | 4.99 | 8.85 | 0.25 | |||||||
| 5 | 2.99 | 5.28 | 0.25 | |||||||
| 7 | 2.10 | 3.95 | 0.75 | |||||||
| Da | 0.001 | 6.60 | 11.77 | 0.0505 | 5.965 | 10.64 | 0.980198 | 4.956 | 4.843 | 4.899 |
| 0.005 | 6.55 | 11.67 | 0.90099 | |||||||
| 0.01 | 6.47 | 11.54 | 0.80198 | |||||||
| 0.05 | 5.97 | 10.65 | 0.009901 | |||||||
| 0.1 | 5.47 | 9.76 | 0.980198 | |||||||
| DITZ | 1.2 | 6.61 | 11.78 | 8.7 | 6.60 | 11.77 | 0.862069 | 0.057 | 0.031 | 0.044 |
| 6.2 | 6.60 | 11.77 | 0.287356 | |||||||
| 11.2 | 6.60 | 11.77 | 0.287356 | |||||||
| 16.2 | 6.60 | 11.77 | 0.862069 | |||||||
| fa | 0.20 | 4.77 | 8.51 | 0.35 | 6.60 | 11.77 | 0.428571429 | 21.184 | 20.711 | 20.948 |
| 0.25 | 5.30 | 9.45 | 0.285714286 | |||||||
| 0.30 | 5.91 | 10.53 | 0.142857143 | |||||||
| 0.40 | 7.41 | 13.22 | 0.142857143 | |||||||
| 0.45 | 8.40 | 14.93 | 0.285714286 | |||||||
| 0.50 | 9.52 | 16.98 | 0.428571429 | |||||||
| fITZ | 0.0005 | 6.61 | 11.78 | 0.00525 | 6.547 | 11.674 | 0.904761905 | 0.452 | 0.476 | 0.464 |
| 0.001 | 6.60 | 11.77 | 0.80952381 | |||||||
| 0.005 | 6.55 | 11.68 | 0.047619048 | |||||||
| 0.01 | 6.49 | 11.56 | 0.904761905 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, C.; Chen, Z.; Yu, J.; Bindiganavile, V. A Three-Phase Model to Evaluate Effects of Phase Diffusivity and Volume Fraction upon the Crack Propagation in Concrete Subjected to External Sulphate Attack. CivilEng 2023, 4, 12-33. https://doi.org/10.3390/civileng4010002
Yi C, Chen Z, Yu J, Bindiganavile V. A Three-Phase Model to Evaluate Effects of Phase Diffusivity and Volume Fraction upon the Crack Propagation in Concrete Subjected to External Sulphate Attack. CivilEng. 2023; 4(1):12-33. https://doi.org/10.3390/civileng4010002
Chicago/Turabian StyleYi, Chaofan, Zheng Chen, Jiamin Yu, and Vivek Bindiganavile. 2023. "A Three-Phase Model to Evaluate Effects of Phase Diffusivity and Volume Fraction upon the Crack Propagation in Concrete Subjected to External Sulphate Attack" CivilEng 4, no. 1: 12-33. https://doi.org/10.3390/civileng4010002
APA StyleYi, C., Chen, Z., Yu, J., & Bindiganavile, V. (2023). A Three-Phase Model to Evaluate Effects of Phase Diffusivity and Volume Fraction upon the Crack Propagation in Concrete Subjected to External Sulphate Attack. CivilEng, 4(1), 12-33. https://doi.org/10.3390/civileng4010002






