Incidental Carcinomas and Lesions with Uncertain Malignant Potential (B3) Discovered During Symmetrization Mammoplasty in Breast Cancer Patients—Retrospective Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Procedure
2.2. Morphological Assessment
2.3. Data Analysis
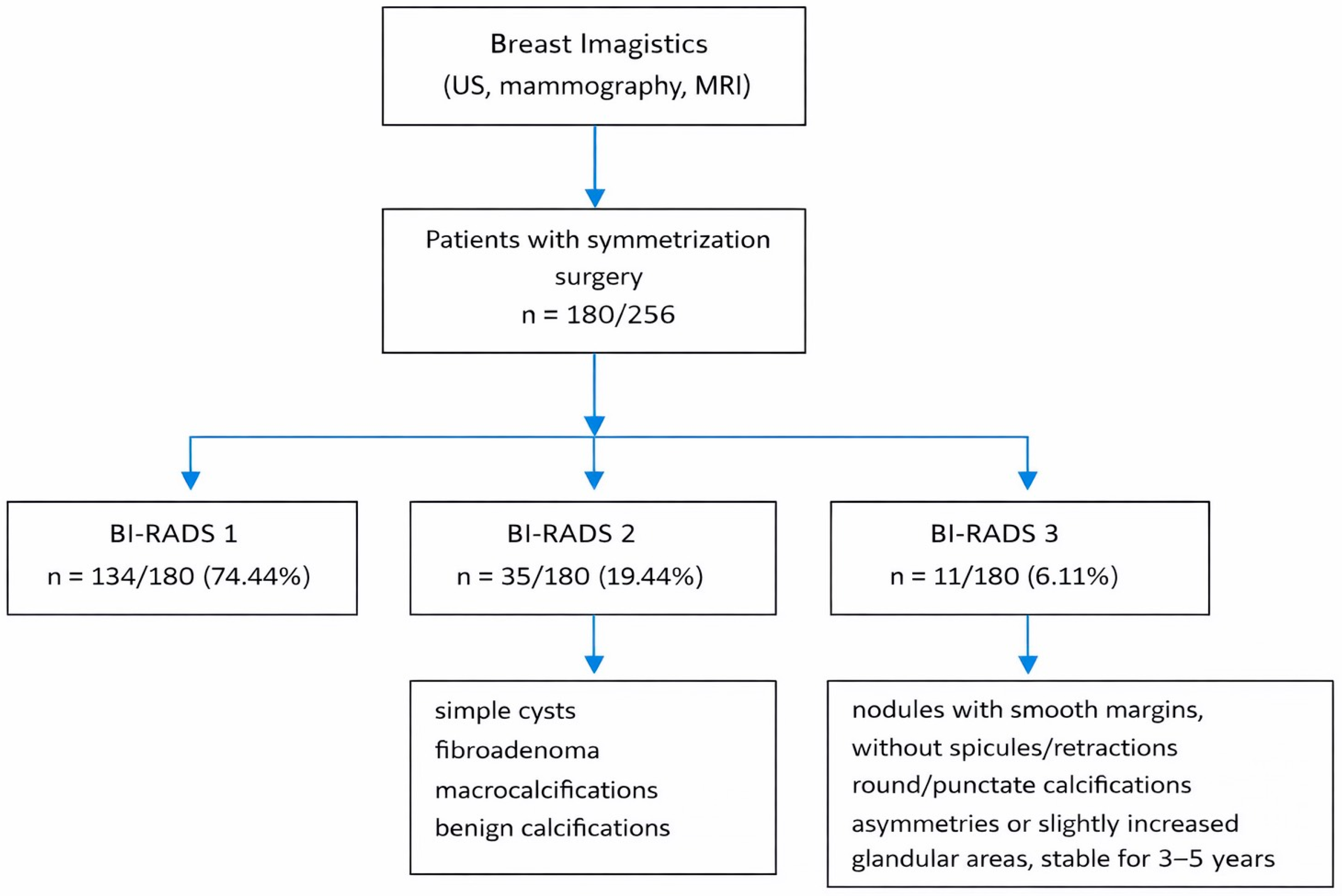
3. Results
4. Discussion
4.1. Imaging Considerations and BI-RADS 1–2 Malignant Findings
4.2. Pathological Findings and Histopathological Processing
4.3. B3 Lesions and Risk of Upgrade
4.4. Surgical Implications and Axillary Management
4.5. Risk Factors and Patient Selection Bias
4.6. Practical Implications and Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BI-RADS | Breast Imaging-Reporting and Data System |
| IHC | IHC |
| BMI | Body Mass Index |
| TNBC | Triple-Negative Breast Cancer |
| DCIS | Ductal Carcinoma in Situ |
| IDC | Invasive Ductal Carcinoma |
| ILC | Invasive Lobular Carcinoma |
| HE | Hematoxylin–Eozin |
| NOS | Not Otherwise Specified |
| NST | No Special Type |
| BC | Breast Cancer |
| AI | Artificial Intelligence |
| ER | Estrogen Receptor |
| cLCIS | Classic Lobular Carcinoma in Situ |
| FEA | Flat epithelial atypia |
| ADH | Atypical Ductal Hyperplasia |
| LN | Lobular Neoplasia |
| ALH | Atypical Lobular Hyperplasia |
| IDP | Intraductal Papilloma |
| RS | Radial Scar |
| CSLs | Complex Sclerosing Lesions |
| OBCs | Occult Breast Carcinomas |
| BRCA | Breast Cancer Gene |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| CHEK2 | Checkpoint Kinase 2 |
| US | Ultrasonography |
| MRI | Magnetic Resonance Imaging |
| TP53 | Tumor Protein P53 |
| PALB2 | Partner and Localizer of BRCA2 |
| PTEN | Phosphatase and Tensin Homolog Gene |
| CDH1 | Cadherin 1 |
| STK11 | Serine/Threonine Kinase 11 |
| SNLB | Sentinel Lymph Node Biopsy |
| T-DM1 | Trastuzumab Emtansine |
References
- Kuijlaars, Z.M.A.; Hillberg, N.S.; Kooreman, L.; Severens Rijvers, C.A.H.; Qiu, S.S. Breast Cancer in the Tissue of the Contralateral Breast Reduction. Cancers 2024, 16, 497. [Google Scholar] [CrossRef]
- Trabulsi, N.; Almaghrabi, S.; Bamakhrama, B.; Fadel, Z.; Shabkah, A.; Farsi, A.; Awan, B. Diagnosis, investigation, and treatment of occult breast cancer: A case report and systematic review of the literature. Curr. Probl. Cancer Case Rep. 2024, 14, 100296. [Google Scholar] [CrossRef]
- Ofri, A.; Moore, K. Occult breast cancer: Where are we at? Breast 2020, 54, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.K.; Bassett, L.W. Invasive lobular carcinoma of the breast: Spectrum of mammographic, US, and MR imaging findings. RadioGraphics 2009, 29, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Slezak, S.; Bluebond-Langner, R. Occult carcinoma in 866 reduction mammaplasties: Preserving the choice of lumpectomy. Plast. Reconstr. Surg. 2011, 127, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Camarneiro, R.; Ferreira, Á.; Barros, M.; e Melo, M.B. Occult breast cancer presenting as axillary lymphadenopathy—Case Report. Int. J. Surg. Case Rep. 2022, 99, 107677. [Google Scholar] [CrossRef]
- Mansel, R.E.; Fallowfield, L.; Kissin, M.; Goyal, A.; Newcombe, R.G.; Dixon, J.M.; Yiangou, C.; Horgan, K.; Bundred, N.; Monypenny, I.; et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J. Natl. Cancer Inst. 2006, 98, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Iwuagwu, O.C.; Drew, P.J. Reduction mammoplasty specimens and occult breast carcinomas. Eur. J. Surg. Oncol. 2005, 31, 806. [Google Scholar] [CrossRef]
- Mann, R.M.; Kuhl, C.K.; Kinkel, K.; Boetes, C. Breast MRI: Guidelines from the European Society of Breast Imaging. Eur. Radiol. 2008, 18, 1307–1318. [Google Scholar] [CrossRef]
- Rubio, I.T.; Wyld, L.; Marotti, L.; Athanasiou, A.; Regitnig, P.; Catanuto, G.; Schoones, J.W.; Zambon, M.; Camps, J.; Santini, D.; et al. European guidelines for the diagnosis, treatment and follow-up of breast lesions with uncertain malignant potential (B3 lesions) developed jointly by EUSOMA, EUSOBI, ESP (BWG) and ESSO. Eur. J. Surg. Oncol. 2024, 50, 107292. [Google Scholar] [CrossRef]
- Sorin, T.; Fyad, J.; Delay, E.; Rouanet, P.; Rimareix, F.; Houpeau, J.; Classe, J.; Garrido, I.; De Lara, C.T.; Dauplat, J.; et al. Occult cancer in specimens of reduction mammaplasty aimed at symmetrization. A multicentric study of 2718 patients. Breast 2015, 24, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Sitges, C.; Mann, R.M. Breast MRI to Screen Women with Extremely Dense Breasts. J. Magn. Reson. Imaging 2025, 62, 58–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, K.M.; Millen, J.-C.; Orozco, J.I.J.; Stern, S.L.; Fancher, C.E.; Grumley, J.G. A look at the other side: High risk lesions and occult contralateral malignancy in symmetry procedures of patients undergoing oncoplastic breast-conserving surgery. Ann. Surg. Oncol. 2023, 30, 6159–6166. [Google Scholar] [CrossRef] [PubMed]
- Demirdover, C.; Geyik, A.; Vayvada, H.; Menderes, A. Is Histological Evaluation of Reduction Mammaplasty Specimens Worthwhile? Aesthetic Surg. J. 2019, 39, NP178–NP184. [Google Scholar] [CrossRef] [PubMed]
- D’Archi, S.; Carnassale, B.; Accetta, C.; Belli, P.; De Lauretis, F.; Di Guglielmo, E.; Di Leone, A.; Franco, A.; Gambaro, E.; Magno, S.; et al. Assessing Malignant Risk in B3 Breast Lesions: Clinical Insights and Implications. J. Clin. Med. 2025, 14, 70. [Google Scholar] [CrossRef]
- Liao, T.; Yang, Y.; Lin, X.; Ouyang, R.; Deng, Y.; Ma, J. High-risk breast lesions: A combined intratumoral and peritumoral radiomics nomogram model to predict pathologic upgrade and reduce unnecessary surgical excision. Front. Oncol. 2024, 14, 1479565. [Google Scholar] [CrossRef]
- Le, J.; O’Keefe, T.J.; Khan, S.; Grossi, S.M.; Choi, H.Y.; Ojeda-Fournier, H.; Armani, A.; Wallace, A.M.; Blair, S.L. Distance of Biopsy-Confirmed High-Risk Breast Lesion from Concurrently Identified Breast Malignancy Associated with Risk of Carcinoma at the High-Risk Lesion Site. Cancers 2024, 16, 2268. [Google Scholar] [CrossRef]
- D’Archi, S.; Carnassale, B.; Sanchez, A.M.; Accetta, C.; Belli, P.; De Lauretis, F.; Di Guglielmo, E.; Di Leone, A.; Franco, A.; Magno, S.; et al. Navigating the Uncertainty of B3 Breast Lesions: Diagnostic Challenges and Evolving Management Strategies. J. Pers. Med. 2025, 15, 36. [Google Scholar] [CrossRef]
- Schrenk, P.; Wölfl, S.; Bogner, S.; Huemer, G.M.; Wayand, W. Symmetrization reduction mammaplasty combined with sentinel node biopsy in patients operated for contralateral breast cancer. J. Surg. Oncol. 2006, 94, 9–15. [Google Scholar] [CrossRef]
- Brown, K.A. Metabolic pathways in obesity-related breast cancer. Nat. Rev. Endocrinol. 2021, 17, 350–363. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D.; Million Women Study Collaboration. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 2007, 335, 1134. [Google Scholar] [CrossRef] [PubMed]
- Danikas, D.; Theodorou, S.J.; Kokkalis, G.; Vasiou, K.; Kyriakopoulou, K. Mammographic findings following reduction mammoplasty. Aesthetic Plast. Surg. 2001, 25, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Li, C.; Liu, M.; Wang, Y.; Feng, Z.; Li, J.; Wang, W.; Wu, F.; Zhang, S.; Zhao, X. Prognostic Models Using Machine Learning Algorithms and Treatment Outcomes of Occult Breast Cancer Patients. J. Clin. Med. 2023, 12, 3097. [Google Scholar] [CrossRef] [PubMed]




| Symmetrization Techniques | No Cases (%) |
|---|---|
| Dermoglandular flaps based on the inferior pedicle | 51 (28.33) |
| Dermoglandular flaps based on the superomedial pedicle | 64 (35.55) |
| Dermoglandular flaps based on the superolateral pedicle | 10 (5.55) |
| Dermoglandular flaps based on the superior pedicle | 34 (18.88) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Grujic, D.; Cristian, H.; Dema, A.; Glaja, M.I.; Hoinoiu, T.; Simion, F.; Pit, D.; Caizer-Găitan, I.; Oprean, C. Incidental Carcinomas and Lesions with Uncertain Malignant Potential (B3) Discovered During Symmetrization Mammoplasty in Breast Cancer Patients—Retrospective Single-Center Experience. Surgeries 2026, 7, 10. https://doi.org/10.3390/surgeries7010010
Grujic D, Cristian H, Dema A, Glaja MI, Hoinoiu T, Simion F, Pit D, Caizer-Găitan I, Oprean C. Incidental Carcinomas and Lesions with Uncertain Malignant Potential (B3) Discovered During Symmetrization Mammoplasty in Breast Cancer Patients—Retrospective Single-Center Experience. Surgeries. 2026; 7(1):10. https://doi.org/10.3390/surgeries7010010
Chicago/Turabian StyleGrujic, Daciana, Horia Cristian, Alis Dema, Mihai Iliescu Glaja, Teodora Hoinoiu, Fabiana Simion, Daniel Pit, Isabela Caizer-Găitan, and Cristina Oprean. 2026. "Incidental Carcinomas and Lesions with Uncertain Malignant Potential (B3) Discovered During Symmetrization Mammoplasty in Breast Cancer Patients—Retrospective Single-Center Experience" Surgeries 7, no. 1: 10. https://doi.org/10.3390/surgeries7010010
APA StyleGrujic, D., Cristian, H., Dema, A., Glaja, M. I., Hoinoiu, T., Simion, F., Pit, D., Caizer-Găitan, I., & Oprean, C. (2026). Incidental Carcinomas and Lesions with Uncertain Malignant Potential (B3) Discovered During Symmetrization Mammoplasty in Breast Cancer Patients—Retrospective Single-Center Experience. Surgeries, 7(1), 10. https://doi.org/10.3390/surgeries7010010






