1. Introduction
Pelvic organ prolapse (POP) is the most prevalent form of pelvic floor dysfunction, affecting up to 30% of women over 50 years old worldwide [
1,
2]. The development of POP is associated with factors such as aging, obesity, multiple childbirths, and vaginal delivery [
2,
3]. Almost half of women with symptomatic POP experience moderate to severe distress, significantly affecting their physical, social, and sexual activities [
4].
POP is a leading reason for gynecologic surgery, with an estimated 11–19% chance of women undergoing at least one surgical procedure for POP or incontinence by the age of eighty-five, and a reoperation rate of 30% [
5,
6]. Overall, perioperative morbidity ranges from 0.2% to 26% in general gynecologic surgery [
7,
8], while it is around 3.2% in primary POP surgery without concomitant urinary incontinence repair [
9].
Transvaginal mesh repair surgery was once popular for advanced pelvic organ prolapse but has faced scrutiny following the Food and Drug Administration (FDA) notification on mesh-related complications, especially mesh exposure [
10]. Nonetheless, there is ongoing research on second-generation mesh kits [
11,
12,
13] such as InGYNious mesh [
14]. These newer meshes are smaller and lighter than their predecessors and often do not require transcutaneous devices performing them through a single vaginal incision.
The Italian Association of Urogynecology (AIUG) supports using vaginal meshes in patients with anterior vaginal prolapse who have significant recurrence risk factors, such as obesity or a Pelvic Organ Prolapse Quantification (POP-Q) score ≥3, or for recurrent prolapse after native tissue surgery [
15]. In 2024, the Italian Federation of Obstetrics and Gynecology (FIGO) released their guidelines which aligned with the AIUG recommendations [
16]. All six groups participating in the study followed these guidelines, allowing for comparison between centers.
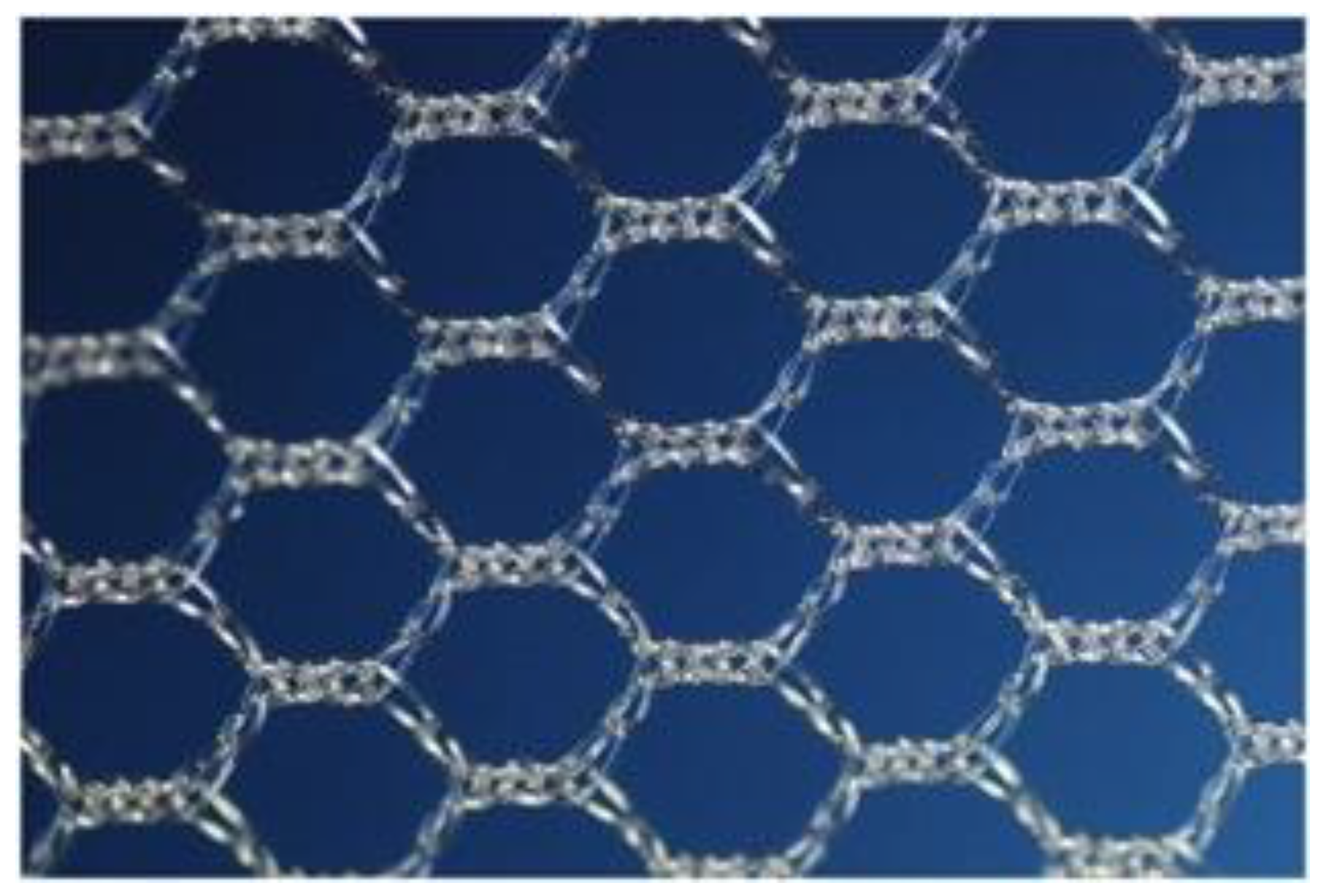
Compared to older mesh kits, the InGYNious system offers several technical and structural advantages. Its ultralight polypropylene mesh (21 g/m2) with hexagonal pore geometry is designed to balance tensile strength and elasticity while promoting optimal tissue ingrowth. This specific pore structure is designed to allow for tissue ingrowth while maintaining flexibility and strength. The presence of both micropores (100–150 μm) and macropores (1.9–2.8 mm) enhances host tissue integration and may reduce fibrotic encapsulation, a key factor in minimizing exposure and chronic pain. The single-incision, six-point fixation approach allows for precise anchoring without transcutaneous trocars, thereby simplifying the procedure and potentially reducing the risk of vascular, nerve, or organ injury. These features contribute to improved surgical ergonomics and may support faster recovery with lower rates of early complications.
First-generation vaginal mesh kits, such as the trocar-guided Prolift system, were introduced to improve anatomical outcomes in advanced POP. These devices typically used heavyweight polypropylene mesh and required blind passage of trocars through the obturator or sacrospinous regions. While effective in reinforcing prolapsed compartments, these systems were associated with higher risks of mesh exposure, erosion, hematoma, nerve and vascular injuries, and postoperative pain. Such complications contributed to regulatory restrictions in many countries.
In response to these safety concerns, second-generation vaginal meshes were developed. These systems are lighter in weight, use macroporous and more elastic structures to improve tissue integration, and are inserted through a single vaginal incision without transcutaneous trocars. The InGYNious system, used in this study, exemplifies these advances: it consists of an ultralight polypropylene mesh (21 g/m2) with hexagonal pore geometry, combining tensile strength with elasticity while minimizing foreign body load. The presence of both micropores (100–150 μm) and macropores (1.9–2.8 mm) supports host tissue ingrowth and may reduce fibrotic encapsulation. The standardized single-incision, six-point fixation technique allows for secure placement while avoiding blind trocar passage, thereby aiming to decrease the risk of major vascular or organ injury compared with earlier systems.
This study specifically aimed to evaluate the perioperative safety profile of the standardized single-incision, six-point fixation InGYNious mesh system in primary anterior and/or central POP surgery. The primary objective was to assess intra- and perioperative complications, while secondary objectives included evaluating early and late postoperative pain and the duration of catheterization. We also explored associations between baseline clinical characteristics, POP-Q parameters, and perioperative outcomes in order to identify potential risk factors. We hypothesized that, when performed by experienced urogynecologists using a uniform technique, this procedure would result in a low rate of complications and predictable postoperative recovery.
2. Materials and Methods
2.1. Study Design
This retrospective observational study investigated morbidity and postoperative pain after a single-incision, six-point fixation vaginal ultra-light mesh approach for the primary treatment of anterior/central prolapse. The study was conducted at St. Camillo Hospital in Trento, San Luca Clinic in Pecetto Torinese, San Camillo de Lellis Hospital in Rieti, Moriggia Pelascini Hospital in Gravedona, Carate Brianza Hospital in Carate Brianza, Padua Hospital, Basso Ionio Hospital in Soverato and Maggiore della Carità University Hospital in Novara (Italy). The protocol was approved by the Ethical Committee of the leading group and adopted by the other members. The study followed the International Ethical Guidelines for Biomedical Research Involving Human Subjects, with the good clinical practice guidelines and with the Declaration of Helsinki. Written informed consent was obtained from the included patients.
Further details on Institutional Review Board approval, patient informed consent, confidentiality safeguards, and conflict-of-interest disclosures are provided in
Section 2.2.
2.2. Patients
All women undergoing pelvic reconstructive surgery for anterior/apical POP from May 2016 and February 2024 were eligible for inclusion in the study. Inclusion criteria were single-incision vaginal mesh repair with InGYNious Anterior Mesh (A.M.I., Feldkirch, Austria) (
Figure 1); age 30 years or older; POP with the leading edge at or beyond the hymen according to pelvic organ prolapse quantification (POPQ) scores [
17]. Exclusion criteria were prior or concomitant hysterectomy or surgical procedures for stress urinary incontinence or for POP, planned or present pregnancy, earlier oncological surgery and previous pelvic irradiation. Preoperative urodynamic was not performed routinely but only in case of manifest incontinence or if otherwise indicated. Transvaginal ultrasound was performed always before surgery and after it only in case of abnormal course. As the information was gathered during a hospital admission, this research has no missing data for all 426 patients, unlike what will happen when we present the follow-up data.
At our institution, patients with Stage III–IV pelvic organ prolapse are evaluated for multiple surgical options, including sacrocolpopexy and native tissue repair with or without mesh augmentation. This specific mesh device is typically offered to patients who desire uterine preservation or who are not ideal candidates for abdominal or laparoscopic approaches, after thorough counseling regarding the risks, benefits, and alternatives.
We acknowledge that the inclusion criteria may appear broad and that patient selection for mesh repair was based primarily on adherence to AIUG/FIGO guidelines, which recommend transvaginal mesh in patients with advanced (stage III–IV) anterior or apical POP and at high risk for recurrence. All included women were considered eligible for mesh repair based on clinical criteria and patient preference following preoperative counseling.
However, the retrospective design precluded systematic documentation of patients who declined mesh or were offered alternative treatments (e.g., native tissue repair or laparoscopic sacrocolpopexy). As a result, we were unable to conduct a sensitivity analysis comparing eligible but excluded patients. We recognize that this limits the generalizability of our findings and recommend that future prospective studies address selection processes and patient choice more transparently.
The choice of the InGYNious mesh system over other commercially available meshes was based on several considerations. First, this system is consistent with the AIUG/FIGO guidelines supporting the use of transvaginal mesh in women with stage III–IV anterior or apical POP who are at high risk of recurrence. Second, the InGYNious device offers specific technical advantages: an ultralight polypropylene structure (21 g/m2), hexagonal pore geometry allowing both tissue integration and elasticity, and a single-incision, six-point fixation technique that avoids the need for transcutaneous trocars. These features were considered favorable for minimizing foreign body load, reducing perioperative risks, and facilitating reproducibility across multiple centers.
Patient selection for the InGYNious system also took into account individual clinical characteristics. Women with advanced prolapse, desire for uterine preservation, or contraindications to abdominal/laparoscopic approaches (e.g., severe comorbidities, frailty, or prior extensive abdominal surgery) were preferentially considered for this mesh-based repair. Importantly, women with previous pelvic irradiation, prior POP or continence surgery, or planned hysterectomy were excluded, as outlined in the eligibility criteria. While no single comorbidity automatically indicated mesh use, conditions such as obesity, collagenopathies, or chronic constipation—recognized recurrence risk factors—were additional elements considered during patient counseling. Ultimately, the decision reflected both guideline-based criteria and individualized surgical judgment following detailed preoperative counseling.
In summary, the inclusion criteria were as follows:
(1) women ≥30 years old;
(2) stage III–IV anterior or apical POP with the leading edge at or beyond the hymen (POP-Q system);
(3) primary single-incision vaginal mesh repair with the InGYNious system.
Exclusion criteria were as follows:
(1) prior or concomitant hysterectomy;
(2) prior POP or stress urinary incontinence surgery;
(3) planned or ongoing pregnancy;
(4) history of oncologic pelvic surgery or pelvic irradiation;
(5) concomitant laparoscopic or abdominal POP repair;
(6) refusal of mesh surgery following counseling.
2.3. Procedures
The InGYNious mesh (A.M.I., Feldkirch, Austria) was inserted through a single vaginal incision using the i-Stitch device (Presurgy SL, Calle Pollensa, 2, 28290, Las Rodas, Madrid, Spain) for six-point fixation to the sacrospinous ligament, tendinous arch of the endopelvic fascia, and paravesical space. After hydrodissection and full-thickness dissection, the mesh was positioned beneath the pubocervical fascia and secured with braided polyester sutures, followed by closure of the vaginal incision.
The patients underwent general anesthesia or spinal anesthesia and were placed in a gynecological position. Standard preoperative preparation included vaginal cleansing with povidone–iodine solution, a single dose of intravenous broad-spectrum antibiotic administered 30 min before incision, and bladder catheterization to facilitate surgical exposure. Bowel preparation was not routinely performed. Thromboprophylaxis followed institutional protocols based on individual risk assessment. An antibiotic prophylaxis was given 30 min prior to surgery, followed by urinary tract disinfectants for 5 days after the surgery. A Foley trans urethral catheter was inserted and left in place and, after emptying the bladder, 10 mL of a vital dye was introduced into the bladder.
The mesh (
Figure 1 and
Figure 2) used in this study was isoelastic with hexagonal structure, ultralight weight (21 g/m
2), made from monofilament polypropylene mesh and consisted of large micropores of 100 to 150 μm and macropores of 1.9 to 2.8 mm.
Figure 1 and
Figure 2 illustrate the mesh and the i-Stitch instrument used in this procedure. The mesh is constructed with a hexagonal pore geometry and features both micropores (100–150 μm) and macropores (1.9–2.8 mm), which facilitate optimal tissue ingrowth while minimizing fibrotic encapsulation. The ultralight polypropylene structure (21 g/m
2) reduces the foreign body load, which may contribute to lower rates of postoperative pain and mesh exposure. Previous studies on newer generation meshes have reported reduced exposure and erosion rates compared to earlier trocar-guided kits [
2,
3].
The hexagonal design provides multidirectional elasticity, improving adaptability to anatomical movement and reducing shear forces on surrounding tissues.
The microporous architecture of the device is designed to promote tissue ingrowth while maintaining flexibility and strength. While direct clinical evidence for this specific design is limited, previous studies have shown that pore size and mesh structure significantly influence host tissue integration and the inflammatory response [
18]. Thus, the current design reflects principles established in prior mesh development aimed at optimizing biocompatibility and mechanical performance.
The i-Stitch device allows for accurate and minimally invasive placement of fixation sutures, improving consistency in anchoring the mesh to critical pelvic structures such as the sacrospinous ligament and tendinous arch of the endopelvic fascia. The safety and reproducibility of suture fixation using this dedicated device have been documented in prior surgical reports [
2,
3,
17,
18]. This contributes to a reproducible surgical technique with potentially reduced risk of injury and postoperative complications.
The mesh is anchored at four points along the arcus tendineus fascia pelvis (ATFP), providing stable lateral fixation to support the anterior and apical compartments
After a longitudinal incision, the mesh was positioned in the paravesical space beneath the pubocervical fascia, following hydrodissection with adrenaline and saline solution and full-thickness dissection (the ‘Lychee layer’), to minimize the risk of exposure [
17,
18].
The mesh was secured with six braided polyester sutures using the i-Stitch instrument (
Figure 1) to the sacro-spinous ligament, the tendinous arch of the endopelvic fascia and the para bladder neck space, bilaterally. The vaginal incision was closed with braided resorbable sutures, and the patients were managed postoperatively with a vaginal pack and urinary catheter for 48 h. Only the perineorrhaphy procedure was allowed at physician’s discretion. All procedures were performed by an experienced urogynecologist (>100 cases of POP surgery performed annually in the study period) and following manufacturer’s instructions. Preoperative urodynamic tests were not routinely performed. Over the data collection period (2016–2024) there were no changes in surgical and clinical practice.
Across the seven participating centers, the surgical approach was highly standardized. All surgeons adhered strictly to the manufacturer’s instructions and the AIUG/FIGO guidelines regarding patient selection and mesh placement. The single-incision, six-point fixation technique was uniformly adopted, including identical steps for hydrodissection, paravesical dissection, mesh positioning, and anchoring with the i-Stitch device.
Minor variations occurred in perioperative management, such as the choice of anesthesia (general vs. spinal) and the use of vaginal packing, which were left to the surgeon’s discretion. However, these did not affect the core surgical technique. To ensure consistency, all procedures were performed by senior urogynecologists with a high annual case volume (>100 POP surgeries/year). Additionally, prior to study initiation, representatives from each center participated in joint workshops and hands-on training sessions to standardize the operative steps. Periodic peer-to-peer reviews and cross-center discussions were also conducted to confirm adherence to the protocol. Any intraoperative deviation or unexpected finding was documented, but no significant departures from the standardized technique occurred during the study period.
2.4. Outcome Measures
The variables of interest included baseline demographic and clinical characteristics (age, BMI, menopause status, comorbidities, smoking, chronic constipation, and POP-Q measurements) and surgical parameters (duration, blood loss, and hospital stay). Anticipated complications were predefined as hematomas, bladder or ureter injuries, bowel or vascular injury, fever > 38 °C, blood transfusion, and urinary retention (catheterization > 48 h). Secondary outcomes were early and late postoperative pain and catheter duration. Correlation of these outcomes with patient and surgical factors was also explored.
In addition, functional urinary outcomes were assessed. Urinary incontinence (urge and stress) was measured using validated questionnaires (ICIQ-UI SF and OAB-q SF), and the occurrence of postoperative urinary tract infections (UTIs) was prospectively recorded.
The primary outcome was the intra- and perioperative complication rate, including hematomas, bladder and ureter injuries, fever, and vascular or bowel injury.
Secondary outcomes included early postoperative pain (within 48 h), late postoperative pain (assessed at the first follow-up visit between 1 week and 1 month), and duration of postoperative catheterization.
“Intra/early postoperative morbidity” was defined as any adverse event occurring intraoperatively or during the initial hospital stay (within 48 h postoperatively). These included hematomas, bladder or ureter injuries, postoperative fever (>38 °C), significant bleeding (requiring transfusion or embolization), and urinary complications necessitating catheterization beyond the standard 48 h. Complications were recorded prospectively by the surgical team and confirmed through clinical records and imaging, when indicated.
The early postoperative pain was measured within 48 h after the intervention using a Visual Analogue Scale ranging from 0 (no pain) to 10 (a lot of pain). The late postoperative pain was measured at the first postoperative visit (between 1 week and 1 month after the intervention) using three categories: tolerable pain (medication as per patient’s request), moderate pain (lasting few days and requiring NSAIDs), and severe pain (lasting from 1 week to 1 month and requiring cortisone drugs).
Early postoperative pain was assessed using the Visual Analogue Scale (VAS), which, while widely used in clinical practice, is a subjective tool and not specific to pelvic floor surgery. Late postoperative pain was categorized into three severity groups based on duration and need for medication; these categories were not based on a validated scale but were chosen to reflect common clinical practice and facilitate pragmatic data collection across centers. These categories were pragmatic and not based on validated instruments, reflecting routine clinical practice across the centers. This approach facilitated feasibility but may have reduced comparability and reliability of late pain assessment We acknowledge that this approach may lack precision and limits the interpretability of pain outcomes. Future studies should consider using validated pelvic-specific pain instruments and predefined, clinically meaningful thresholds to improve standardization and comparability.
Early postoperative pain was assessed within 48 h of surgery, before discharge, using a Visual Analogue Scale (VAS) ranging from 0 (no pain) to 10 (maximum pain). Late postoperative pain was recorded at the first follow-up visit, scheduled between 7 and 30 days after surgery, and was categorized based on duration and analgesic requirements. Perioperative complications were documented during the hospital stay. Catheter duration was measured in days from surgery until successful voiding.
2.5. Data Collection
All data were collected using a dedicated form and entered into an anonymized database for the analysis. Data collection included demographics, medical history, clinical characteristics, information about the intervention, length of hospital stay, and outcome measures.
The data collection form was developed collaboratively by investigators from all participating centers and piloted on an initial subset of 20 patients to ensure clarity and feasibility. Following this pilot phase, minor revisions were made to improve uniformity in recording perioperative complications, pain scores, and POP-Q measurements. To ensure accuracy, all data were double-checked by a second investigator at each site before being entered into the anonymized central database. Automated range and logic checks (e.g., detection of out-of-range POP-Q values or inconsistencies between questionnaire scores and clinical findings) were applied during database entry. Completeness was ensured by mandatory fields in the electronic form, which prevented saving incomplete records. Any discrepancies identified during routine cross-center audits were resolved by reviewing original patient charts. This process minimized the risk of missing or inaccurate data and guaranteed consistency across centers.
Among the clinical characteristics, the urinary incontinence was assessed using the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UI SF) [
19] and the symptom score of the Overactive Bladder Questionnaire Short Form (OAB-q SF) [
20].
2.6. Statistical Analysis
Numerical data were summarized as median and interquartile range (IQR), and categorical data as absolute and relative frequency (percentage). Numerical data were compared between groups using the Mann–Whitney test or Kruskal–Wallis test. Categorical data were compared between groups using the Chi-Square test or Fisher’s test. Correlation between numerical data was assessed using Spearman’s rank correlation coefficient. The internal consistency of ICIQ-UI SF and OAB-q SS was tested using the Cronbach’s alpha coefficient. All tests were two-sided and a p-value of less than 0.05 was considered statistically significant. Adjustment for multiple testing was not performed given the exploratory—rather than confirmatory—nature of the study.
Because this was a retrospective, hypothesis-generating study including all eligible patients across participating centers, no formal sample size calculation or power analysis was conducted.
The analyses should therefore be interpreted as exploratory and descriptive, aimed at generating hypotheses for future prospective studies rather than drawing definitive causal inferences.
Variance of surgical results among the 6 groups was tested using Anova test Statistical analyses were performed using R 4.4 (R Foundation for Statistical Computing, Vienna, Austria) [
21].
Given the exploratory nature of this study, no adjustment for multiple comparisons was performed. All statistical tests were two-sided, and p-values < 0.05 were considered indicative of potentially meaningful associations rather than definitive conclusions.
We did not perform multivariate regression analyses to identify independent predictors of outcomes due to the limited number of events, particularly for perioperative complications. Including too many variables in a regression model would risk overfitting. Future analyses with larger cohorts and longer follow-up will incorporate multivariable modeling to better characterize independent risk factors.
The absence of multivariate regression analyses limits our ability to disentangle independent predictors of perioperative morbidity, pain, or urinary retention from potential confounding factors. As a result, the associations reported here should be interpreted as exploratory and hypothesis-generating rather than confirmatory. For example, while higher POP-Q values and lower BMI were associated with early postoperative pain, it is unclear whether these relationships would persist after adjusting for other variables such as age, comorbidities, or surgical duration.
Future studies with larger cohorts and higher event rates will allow for robust multivariable modeling. Such analyses would provide greater insight into independent risk factors, quantify their relative contributions, and potentially inform patient selection and risk stratification. Prospective multicenter registries with standardized outcome reporting may help generate sufficiently powered datasets for this purpose.
3. Results
The analysis included 426 women satisfying the inclusion criteria. As the Anova analysis did not show any significative differences among the groups concerning the distribution of cases and surgical results (both success and complication) data are collected and analyzed as one group. The demographics and background information of the study population are reported in
Table 1. Overall, 285 (67.6%) had stage III anterior compartment prolapse and 92 (21.8%) stage IV; and 117 (27.7%) and 125 (29.6%) had stage III or stage IV apical prolapse, respectively. No patient had POP-Q A e C stages together equal to 1.
The assessment of the urinary incontinence is reported in
Table 2. Median ICIQ score was 5 out of max 21 points (IQR 0; 8). Overall, 122/420 women (29.0%) declared no urine leakage. The most frequent occasions of urine leakage were when coughing or sneezing (54.9%) and before getting to the toilet (53.1%). Median OAB-q symptom score was 14 out of max 36 points (IQR 10; 18). The internal consistency was adequate for both scores (ICIQ-UI SF: Cronbach’s alpha 0.85; OAB-q SS: Cronbach’s alpha 0.88).
Age was not correlated with ICIQ score (Spearman correlation coefficient −0.06, p = 0.20) or OAB-q symptom score (Spearman correlation coefficient 0.02, p = 0.65). Higher OAB-q symptom score was correlated with higher POP-Q Aa (Spearman correlation coefficient 0.12, p = 0.02), POP-Q Ba (Spearman correlation coefficient 0.10, p = 0.03) and POP-Q C (Spearman correlation coefficient 0.15, p = 0.002). This finding was also confirmed by the high percentage (53.1%) of patients leaking before they can get to the toilet.
The incontinence data presented in
Table 2 reflect baseline preoperative symptoms, as measured by the ICIQ-UI SF and OAB-q SF. These findings were not related to perioperative complications such as bladder or ureteral injury. During the perioperative period, no cases of de novo stress urinary incontinence or urgency incontinence were observed. Similarly, none of the women who sustained urinary tract injuries developed new incontinence symptoms
Overall morbidity rate was 31/426 (7.3%), including postoperative hematomas (n = 19, 4.5%), bladder injury (n = 6, 1.4%), fever (n = 5, 1.2%), ureteral injury (n = 3, 0.7%), and vascular injury (n = 1, 0.2%). Blood transfusion was required only in one case. Bowel injury, skin/muscle injury, and abscess did not occur. The surgery strategy was not changed, with a median duration of the intervention of 40 min (IQR 35; 40), and over the years, the complication rates did not change.
No cases of de novo stress urinary incontinence or urinary tract infection were reported during the perioperative period. Urgency symptoms were evaluated with OAB-q SF and did not significantly worsen postoperatively.
Details on patients with postoperative hematomas, bladder injury e ureteral injury are reported in
Supplementary Table S1. The most frequent complication was the formation of hematomas, which was highlighted by symptoms or by ultrasound at discharge. Anemia occurred in two cases. In one of these, the most serious complication was the bilateral compression of the ureters which required the application of two stents, blood transfusion and embolization of an arterial branch (lower gluteal artery). In 12 of the 19 cases of hematomas, the diagnosis was accidentally made by ultrasound, complemented in two patients by modest fever, in three by more intense pain and in one by a modest drop in the hemoglobin level, which did not require transfusion. These 12 cases did not present any other relevant factors and had average intraoperative blood loss. In the remaining six cases, the hematoma compressed the urethra, hindering urination, and the ultrasound explained the reason in the absence of other disorders. The average diameter was 6.6 cm and required the persistence of a bladder catheter for 15 days.
Five of the six bladder injuries involved minor leakage and were treated with intraoperative suturing followed by mesh implantation. In all these patients, the anterior defect was central, without lateral detachment. Only one case had significant bleeding (500 mL), but the diagnosis was made in the postoperative period and would have required corrective surgery that the elderly patient refused, preferring permanent catheterization.
The ureteral complications caused the greatest discomfort to the patients as they required the application of a stent kept for 2 months. One stenosis was indirectly caused by hematoma compression and not by direct injury, hence it was considered a hematoma complication. All ureteral complications were solved with a stent, and none required reimplantation.
The analysis of factors associated with morbidity is reported in
Table 3. As expected, the length of hospital stay over 2 days was more frequent in women with complications compared to those without complications (17.2% vs. 5.7%,
p = 0.03), while the other factors of interest were not statistically different between women with or without complications (
Table 3).
Among patients with bladder injuries (n = 6), catheterization was maintained for 7–14 days, and none developed urinary tract infections or de novo incontinence. Ureteral injuries (n = 3) were managed with stent placement for approximately 60 days, without subsequent incontinence. Hematomas (n = 19) were managed conservatively in most cases, although 6 patients required prolonged catheterization (mean 15 days, range 10–21) due to bladder outlet compression.
Median early postoperative pain was 4 out of max 10 points (IQR 3; 5). The analysis of factors associated with early postoperative pain is reported in
Table 4. Higher early postoperative pain was associated with lower BMI (
p = 0.3), higher POP-Q at the points: Aa (
p = 0.03), Ba (
p = 0.0003), and D (
p = 0.0004), and longer duration of the intervention (
p = 0.0002). The other factors of interest were not associated with early postoperative pain (
Table 4). Median early postoperative pain was 4 (IQR 3; 5) in women with 1–2 days of hospital stay and 5 (IQR 4; 5) in women with >2 days of hospital stay (
p = 0.39).
Late postoperative pain (after 2 days) was small in 70 women (16.4%), tolerable (a few days and NSAIDs) in 270 women (63.4%) and severe (1 week–1 month and cortisone) in 86 women (20.2%). The analysis of factors associated with the pain at the first visit is reported in
Table 5. The factors of interest were not associated with the pain at the first visit (
Table 5).
The median postoperative urinary retention was 2 days (IQR 2; 2). In patients with prolonged retention, the mean duration of catheterization was 15 days (range 10–21). Women with bladder injuries required catheterization for 7–14 days, whereas those with ureteral injuries underwent ureteral stenting for a median of 60 days. None of these patients developed perioperative urinary tract infections. The analysis of associated factors is reported in
Table 6. A higher number of days with a catheter was associated with higher POP-Q at the points C (
p = 0.0007) and D (
p = 0.0007), while other factors of interest were not associated with early postoperative pain (
Table 6).
A total of 426 women underwent primary transvaginal mesh repair for anterior and/or apical pelvic organ prolapse. Most patients (67.6%) had stage III anterior prolapse, and nearly 60% had advanced apical prolapse (stage III–IV). The overall intra- and early postoperative morbidity rate was 7.3% (31/426), with hematomas being the most frequent complication (4.5%), followed by bladder injuries (1.4%), fever (1.2%), and ureteral injuries (0.7%). No bowel, abscess, or skin/muscle injuries were reported. One case required a blood transfusion and embolization due to arterial bleeding.
Early postoperative pain, assessed within 48 h of surgery, had a median VAS score of 4 (IQR 3–5) and was significantly associated with higher POP-Q values at points Aa, Ba, and D, longer operative time, and lower BMI. Late pain, assessed at the first postoperative visit (7–30 days), was reported as tolerable in most cases (63.4%), with 20.2% reporting severe pain. No baseline or surgical variables were significantly associated with late pain.
Postoperative urinary retention, measured by catheter duration, had a median of 2 days. Extended catheterization was associated with higher POP-Q values at points C and D.
No conversions or changes in surgical strategy were required, and complication rates remained stable across centers. Complete case analysis was possible due to in-hospital data collection. Detailed case descriptions and extended tables have been included in the
Supplementary Materials.
4. Discussion
Vaginal prosthetic surgery for the treatment of pelvic organ prolapse (POP) has proven superior anatomical outcomes compared to repairs utilizing native tissue [
22,
23]. This is particularly clear in the treatment of anterior prolapse, where mesh surgery shows higher success rates than traditional colporrhaphy, with recurrence rates of 13% in mesh-based procedures compared to 32–45% in native tissue repair [
24]. The primary objective of mesh use is to provide more durable support [
23]. However, there is substantial debate in the literature regarding how to optimize long-term outcomes, reduce complication rates, and enhance patient quality of life while minimizing risks [
24,
25].
This initial report is deliberately confined to perioperative outcomes and should be interpreted as the first phase of an ongoing longitudinal investigation designed to capture late mesh-related complications over a minimum five-year follow-up.
In 2008, the FDA issued a Public Health Notification (PHN) highlighting severe complications associated with mesh use, which was reinforced by an updated notice in 2011 [
26]. This led to the discontinuation of transvaginal mesh (TVM) for POP in 2019, following the FDA’s Executive Summary [
27]. Several countries, including the United States, the United Kingdom, Australia, New Zealand, and France, have restricted or banned TVM for POP repair. Nonetheless, data from countries such as Taiwan [
12], Japan [
18], Germany [
28], and Austria [
28] suggest potential benefits of TVM, which remains an option in Italy as well [
13] for carefully selected patients with appropriate preoperative counseling.
This paper focuses exclusively on perioperative complications, with a lower observed overall morbidity rate (7.3%) compared to other studies reporting mesh-unrelated complications rates (up to 22.5%) [
27].
While direct comparisons with other mesh systems were beyond the scope of this study, these design attributes—combined with standardization of technique and high-volume surgical experience—likely contributed to the favorable perioperative outcomes observed in our series.
The most frequent complication was the presence of hematomas, often due to small vessel injuries during dissection of the para-vesical and pararectal spaces. A single case had injuries of the inferior gluteal artery and required embolization and a transfusion due to anemia. Another patient received a blood transfusion and had a ureter compression treated with stent insertion. Other notable complications of vaginal, retropubic, or perineal hematomas in the postoperative period included intense pain in two patients and compression of the bladder neck/urethra in six others, leading to urinary difficulties. An earlier study [
28] on 694 patients undergoing to pelvic floor reconstructive procedures reported 68 patients with 200 mL blood loss (9.8%) and three (0.1%) with 500 mL blood loss. Another study [
27] on 677 patients who had surgery for POP with trocar-guided Prolift mesh reported an overall complication rate of 22.5%, including bleeding over 500 cc in 2.2% of patients, pelvic hematomas in 5.5%, and perineal hematomas in 2.5%.
The second most common complication was urinary tract damage. All but one case of bladder injuries was intraoperatively detected, most of which were without wide bladder mucosa damage, but only with leakage, and were treated with intraoperative suture, and mesh was implanted. It is noteworthy that the anterior defect was central in all these patients, without lateral detachment. The full-thickness dissection of the vaginal wall is useful to decrease the risk of mesh exposure, but deeper dissection might have increased the risk of injuries to the bladder. An earlier study [
18], using vaginal mesh, reported bladder injury in 1.6% of cases, which was comparable with Western studies (0.7–2.1) [
29,
30,
31] and our experience. Another study [
32] reported 3.3%.
The ureteral complications were rare in our series but may cause the greatest discomfort for the patients as they require the application of a stent. However, none required reimplantation in our patients, suggesting that it might be due to stretching with kinking. Of note, in those cases, the ipsilateral tendinous arch of the endopelvic fascia, useful for the reconstruction of the second level of DeLancey, was hard to find. Anatomical landmarks are essential in pelvic organ prolapse repair. During hands-on training for mesh surgeries, it is necessary to pay special attention to safety landmarks and anatomical topography. Our patients did not experience any urethral injuries but only compression of the bladder neck and urethra by some hematomas, with difficult urination. Neither bowel/rectum damage nor abscess was present in our series. Another study [
18] reported a 0.28% rate of rectum injuries, 1.5% of bladder injuries and 0.02% ureteral ones. In another series [
33] of 75 geriatric patients, four (5.6%) of the patients had a severe intra-, peri- or postoperative complication (two bowel injuries, one bleeding requiring blood transfusion, one resuscitation). However, all women with severe complications showed no persistent problem at the time of discharge or during the follow-up period. No significant correlation between age and complications was identified in our study.
In contrast to laparoscopic approaches, where conversions to vaginal surgery have been reported (around 6.6%) [
34], no switching techniques during operations occurred in our series. The surgery duration for the vaginal approach was significantly shorter (median 40 min) than the laparoscopic approach (mean 119 min) [
34], with comparable morbidity rate. On our series higher grade of prolapse are associated with longer surgery duration and an early postoperative pain, but not with a prolonged pain after a month.
Late complications such as mesh exposure, vaginal exposure, dyspareunia, prolapse recurrence, or functional outcomes (e.g., urinary or sexual function) were not assessed in this initial report. Additionally, patient-reported satisfaction and quality of life measures were not collected systematically during the perioperative period. These patient-centered outcomes are critical to fully evaluate the benefit-risk profile of transvaginal mesh and will be addressed in the ongoing follow-up of this cohort.
Using the same mesh (InGYNious), an earlier study [
35] reported comparable morbidity rates, such as overall morbidity of 7%, heavy bleeding causing a hematoma (5.5%), bladder injuries (0.8%) and ureteral injury (0.4%).
Higher early postoperative pain was associated with higher POP-Q at the points Aa, Ba and D, longer duration of the intervention and low BMI. Notably, a longer period with a catheter was associated with higher POP-Q at the points C and D. Other factors of interest were not associated with early postoperative pain, and none was associated with late postoperative pain. One patient reported postoperative pain associated with the presence of large hematoma without other symptoms. A degree of late pain may be associated with chronic pain that sometimes exists, but this also occurs in vaginal surgeries without an implant, as highlighted in a meta-analysis of 161,536 patients [
36].
Notably, early postoperative pain was significantly associated with lower BMI.
Although statistically significant associations were observed between certain POP-Q points (Aa, Ba, D) and early postoperative pain, the magnitude of these differences was small and likely not clinically relevant. These findings should be interpreted with caution, and further study is needed to determine whether anatomical severity meaningfully influences acute postoperative discomfort.
An elevated BMI is a reported important lifestyle factor affecting pelvic prolapse [
37]. The most likely mechanism of POP development among obese women is the increased intra-abdominal pressure that causes weakening of pelvic floor muscles and fascia. A sacrocolpopexy serie [
38] assessing obese women reported a higher laparocconversion rate, rate of infection, estimated blood loss and a longer operative duration. This was not confirmed in our experience as in a cadaveric study the distances from the fixation point to the pudendal artery and nerve were directly proportional to the BMI and this could explain the reduce risk for early pain associated with low BMI [
39]. Obesity was not associated with one-day postoperative complications after POP surgery, when controlling for possible confounders, using the American College of Surgeons National Surgical Quality Improvement Program.
An earlier study [
40] found that pain after pelvic surgery was significantly associated with increased rates of postoperative urinary retention. In sacrospinous ligament fixation, injury to the inferior gluteal nerve is an unlikely cause of postoperative gluteal pain [
41]. Instead, it is likely that pain arises from the S3 and/or S4 nerve branches, which innervate the coccygeus muscles, or from nerves passing between the sacrospinous and Sacro tuberous ligaments to supply the gluteus maximus muscles [
42]. A detailed understanding of the surrounding anatomy of the sacrospinous ligament, combined with careful control of needle penetration depth and avoidance of needle entry or exit beyond the ligament upper margin, may help to minimize the risk of nerve entrapment, and reduce the incidence of postoperative gluteal pain.
A relevant factor that may have influenced outcomes is the surgeon’s experience. All procedures were performed by urogynecologic surgeons with a high annual surgical volume (>100 POP surgeries/year), using a uniform technique across centers. This consistency likely contributed to the observed low complication rates. However, surgeon-specific variables such as years of experience, technical nuances, or learning curve effects were not captured in our dataset and could not be analyzed. Future studies should consider these surgeon-related factors as potential confounders or effect modifiers in evaluating surgical outcomes.
The strength of our studies included the newer mesh use in the first surgery for a continuous series, the standardized way of mesh insertion which benefited from a substantial number of interventions, and an improved knowledge of anatomical landmarks [
33] and a multi-center approach. As surgeon’s experience is a key factor in implanting meshes [
34], we have shown that if this knowledge is widespread uniform results can be obtained.
Our study also has some limitations such as the retrospective data collection, which potentially limits the quality of the data. In this first analysis of the series, our study does not evaluate mesh-related complication, as exposure. In scientific literature the percentage reported [
34] was 2.3%, with the newer mesh, implanted by expert surgeons and easily resolved.
Moreover, this study has several limitations inherent to its retrospective and observational nature, including the absence of a control group undergoing native tissue repair. The lack of a comparative arm was a deliberate choice, as the study’s primary objective was to evaluate perioperative morbidity and early postoperative pain in women undergoing a standardized six-point fixation vaginal ultra-light mesh procedure. Given that the participating centers adhere to the Italian Association of Urogynecology (AIUG) guidelines, which still support the use of vaginal mesh in selected patients with anterior vaginal prolapse at high risk of recurrence, our study reflects real-world clinical practice in Italy.
We acknowledge that synthetic transvaginal mesh (TVM) is restricted or banned in several countries due to long-term complications. However, our study does not intend to challenge existing regulatory decisions; rather, it aims to provide data on the short-term safety profile of this procedure in a population where its use remains an option. It is critical to emphasize that long-term complications were beyond the scope of this study and will be addressed in ongoing follow-up analyses.
The study population included all women undergoing primary mesh surgery for anterior/central prolapse without additional selection criteria beyond AIUG recommendations. The lack of a comparative group consisting of patients who opted for native tissue repair limits the ability to draw conclusions regarding the superiority of one approach over another. Additionally, while preoperative counseling was conducted at each center, specific details on how patients were informed about alternative surgical options and why some declined mesh surgeries were not captured in this analysis.
The study was conducted across seven centers that voluntarily contributed to data collection and followed the same surgical protocol. These centers were selected based on their adherence to AIUG guidelines and experience in pelvic reconstructive surgery. The continuation of the study beyond 2019 aligns with the Italian Federation of Obstetrics and Gynecology (FIGO) and AIUG recommendations, which still permit TVM use in well-selected cases. Importantly, the study was not funded by any external organization, and no financial incentives influenced data collection.
From a clinical perspective, these findings support a selective approach to mesh use. The patients most likely to benefit are those with advanced (stage III–IV) anterior or apical POP, particularly when associated with recurrence risk factors such as obesity, collagenopathies, or chronic constipation, or when abdominal/laparoscopic approaches are contraindicated. Thorough preoperative counseling and individualized decision-making remain essential to ensure appropriate patient selection.
Regarding specific methodological concerns, the study did not include routine urodynamic testing before surgery unless clinically indicated, which may limit insights into baseline functional bladder status. The classification of urinary retention as a complication was based on the requirement for catheterization beyond 48 h, though we acknowledge that this duration was protocol-driven rather than purely pathological. Additionally, postoperative pain assessment utilized different scales for early and late pain, which could affect comparability. The influence of perineorrhaphy and vaginal packing on pain outcomes was not systematically analyzed, and future studies should consider these confounding factors.
Moreover, urinary incontinence is a critical functional outcome in pelvic reconstructive surgery, and anatomical correction of prolapse may unmask previously asymptomatic stress urinary incontinence. While preoperative continence symptoms were assessed using validated questionnaires (ICIQ-UI SF and OAB-q SF), postoperative continence outcomes were not systematically captured in this perioperative analysis. This is an important limitation, and future studies from this cohort will include longitudinal assessment of urinary function. In addition, patients undergoing concomitant anti-incontinence procedures were excluded from this series to preserve procedural uniformity.
Another limitation is the absence of a control group undergoing native tissue repair or alternative mesh procedures, such as sacrocolpopexy. While our study was designed specifically to assess perioperative complications and early postoperative outcomes associated with a standardized six-point fixation transvaginal mesh technique, the lack of a comparator arm precludes direct conclusions about the relative safety or efficacy of mesh versus non-mesh procedures. This design choice limits interpretability regarding comparative benefits and harms, making the present study best classified as a large multicenter case series. However, this limitation is mitigated by the study’s focus on real-world outcomes in centers operating under uniform national guidelines (AIUG/FIGO), offering valuable data on the short-term safety profile of mesh-based POP surgery in a carefully selected patient population. Future prospective or comparative studies are needed to address this gap.
Another major limitation is the lack of long-term follow-up, which prevents us from evaluating critical mesh-related complications such as exposure, dyspareunia, or chronic pelvic pain. Therefore, our findings pertain only to perioperative safety, and conclusions about the overall safety profile of mesh use must be interpreted with caution. Long-term follow-up for this cohort is ongoing and will be reported separately.
To address this limitation, a structured long-term follow-up program is currently underway for the entire cohort. Patients are being systematically re-evaluated at 6 months, 12 months, and annually thereafter, with a planned minimum follow-up of 5 years. Follow-up assessments include pelvic examination with POP-Q staging, transvaginal ultrasound when indicated, and evaluation of mesh-related complications such as exposure, erosion, dyspareunia, and chronic pelvic pain. Patient-reported outcomes are being collected through validated questionnaires covering urinary, bowel, sexual function, and quality of life domains.
The long-term data will be integrated with the present perioperative analysis to provide a comprehensive safety and efficacy profile of the InGYNious mesh system. This will allow us to assess not only short-term perioperative morbidity but also the durability of anatomical correction, functional outcomes, and the incidence of mesh-related adverse events over time. We expect that this longitudinal approach will clarify the overall benefit–risk balance of the procedure and inform guideline recommendations regarding the use of contemporary vaginal mesh systems in carefully selected patients.
We also acknowledge potential underestimation of bleeding-related complications. While all patients received a postoperative hemoglobin check on day 1, an ultrasound assessment for hematoma was not performed systematically, but only in patients with clinical signs or hemoglobin drop. As a result, asymptomatic or mild hematomas may have gone undetected.
In addition, no power calculation was performed, since the study was retrospective and exploratory. Pain assessment methods were not fully standardized, with VAS used for early pain and non-validated categories for late pain. Furthermore, preoperative urodynamic testing and postoperative ultrasound were only performed when clinically indicated, rather than systematically. This may have led to an underestimation of occult incontinence, voiding dysfunction, or subclinical hematomas.
Moreover, a major limitation of our study is the relatively short follow-up period (1 month). While this timeframe was adequate to capture perioperative morbidity, it is insufficient to evaluate mesh-related complications such as exposure, erosion, or dyspareunia, which often manifest later. Long-term follow-up of this cohort is ongoing, with systematic assessments at 6 months, 12 months, and annually thereafter, to address this critical issue.
Finally, although rare, certain complications, such as prolonged catheterization and one case of permanent catheterization due to an uncorrected bladder injury, are clinically significant.
Despite these limitations, this study contributes valuable insights into the perioperative safety of contemporary TVM procedures in a structured, multi-center setting. The findings highlight the importance of surgeon experience, standardized techniques, and patient selection in optimizing short-term outcomes. However, we fully recognize the necessity of long-term follow-up data to assess the durability and safety of vaginal mesh surgery over time.