1. Introduction
Surgical site infections (SSIs) following pancreaticoduodenectomy remain a significant clinical challenge, with reported incidence ranging from 11% to 32%, substantially higher than rates observed in other clean-contaminated procedures [
1]. These infections contribute to increased morbidity, prolonged hospitalization, elevated healthcare costs, and diminished quality of life [
2,
3].
The heightened SSI risk in pancreaticoduodenectomy stems from multiple factors, including extended operative times, complex digestive tract reconstruction, potential contamination from biliary and pancreatic secretions, and frequent preoperative biliary drainage [
4]. Additionally, patients often present with risk factors, including malnutrition, jaundice, and immunosuppression from neoadjuvant therapy [
5,
6].
Negative pressure wound therapy (NPWT) has emerged as a potential intervention for SSI prevention. This technology applies controlled negative pressure to the wound surface, which may reduce edema, enhance tissue perfusion, promote granulation tissue formation, and decrease bacterial colonization. While NPWT has demonstrated efficacy in other surgical specialties, its role in pancreaticoduodenectomy requires further investigation [
2,
7,
8].
Despite growing evidence supporting NPWT in pancreatic surgery, critical implementation bottlenecks remain. Device heterogeneity across multiple NPWT systems operating at varying pressure settings (−100 to −125 mmHg) prevents standardized protocol development and direct efficacy comparisons. Validated patient selection criteria are lacking, though risk factors including preoperative biliary drainage, neoadjuvant therapy, elevated BMI, and hypoalbuminemia have been identified [
5,
6]. International consensus guidelines from professional societies such as the International Study Group of Pancreatic Surgery could standardize device selection, application timing, duration, and patient selection through systematic evidence review and expert consensus methodology. These research priorities reflect the field’s evolution from efficacy questions to implementation science challenges determining whether NPWT becomes standard perioperative care or remains a specialized intervention for selected high-risk patients.
Previous systematic reviews have examined NPWT in mixed surgical populations [
7,
8]. Lenet et al. conducted a meta-analysis in 2022 focusing on prophylactic NPWT in pancreatic resection, identifying three randomized clinical trials with 236 patients [
9]. Their analysis suggested a potential benefit (relative risk [RR], 0.70; 95% CI, 0.50–0.99), though with limited statistical power. Similarly, Zhang et al. briefly summarized four studies examining NPWT in pancreaticoduodenectomy, suggesting potential benefits but acknowledging significant limitations in the available evidence [
7].
Our systematic review and meta-analysis expand on previous work by including nine studies with 1247 patients. We analyzed both randomized and observational studies, providing a comprehensive assessment of real-world effectiveness. We conducted detailed subgroup analyses based on NPWT application timing, duration, and pressure settings while examining the relationship between patient risk factors and NPWT efficacy. Given the substantial morbidity associated with SSI following pancreaticoduodenectomy and the potential benefits of NPWT, this comprehensive analysis of the available evidence aims to guide clinical decision-making regarding NPWT implementation for SSI prevention in pancreatic surgery.
2. Methods
2.1. Search Strategy and Study Selection
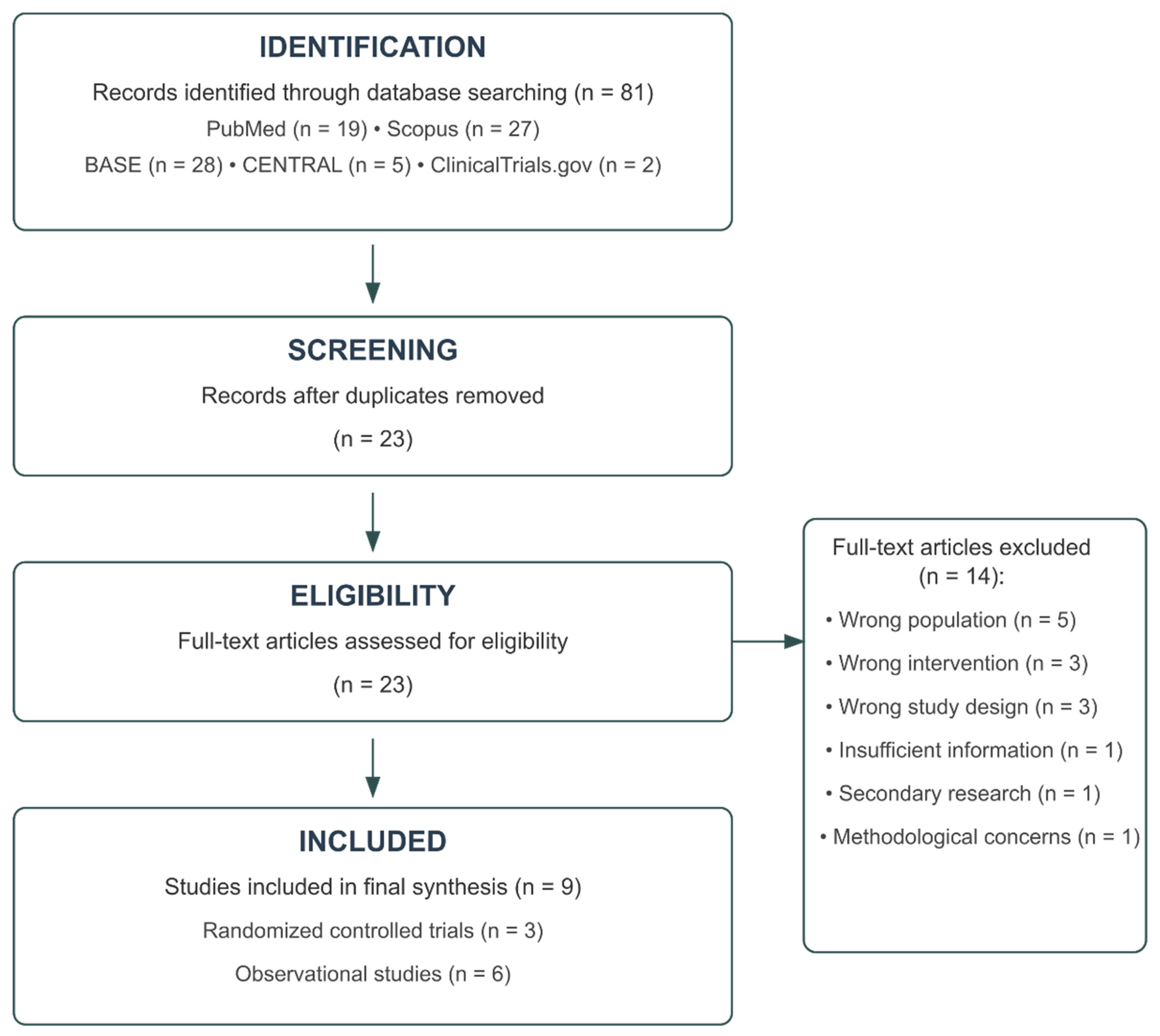
Following PRISMA guidelines, a comprehensive literature search was conducted on 2 April 2025, across multiple electronic databases, including PubMed, Scopus, BASE, Cochrane CENTRAL, and ClinicalTrials.gov. Search terms combined concepts related to negative pressure wound therapy (e.g., “negative pressure wound therapy,” “NPWT,” “vacuum assisted closure”), pancreaticoduodenectomy (e.g., “pancreaticoduodenectomy,” “Whipple,” “pancreatic surgery”), and surgical site infections (e.g., “surgical site infection,” “SSI,” “wound complication”). The complete search strategy for each database is presented in
Table S1. The protocol was not registered.
Two independent reviewers (initials blinded for review) screened the titles and abstracts of the identified records. The full texts of potentially eligible studies were then assessed against predetermined inclusion and exclusion criteria (
Table S2). Disagreements were resolved through discussion with a third reviewer. The study selection process followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Studies were included if they (1) enrolled patients undergoing pancreaticoduodenectomy or other pancreatic resections with subgroup analysis for pancreaticoduodenectomy; (2) compared negative-pressure wound therapy with conventional dressings; (3) reported surgical site infection rates or related complications; and (4) employed randomized controlled trial, cohort, or case–control study designs. Studies were excluded if they (1) did not focus on pancreaticoduodenectomy patients; (2) did not examine NPWT as the primary intervention; (3) used inappropriate study designs (case reports, editorials, and animal studies); (4) provided insufficient methodological details; (5) contained duplicate data; or (6) were secondary research publications.
2.2. Data Extraction and Quality Assessment
Data extraction was performed independently by two reviewers using a standardized form. The following information was collected: study characteristics (author, year, country, design), patient demographics (sample size, age, sex, comorbidities), intervention details (NPWT device, pressure settings, duration), comparison group characteristics, outcomes (surgical site infections, pancreatic fistula, seroma, incisional hernia, and readmission rates), and risk factors for complications.
Surgical site infections were classified according to the Centers for Disease Control and Prevention criteria as superficial, deep, or organ/space infections when such a distinction was provided in the original studies. Pancreatic fistula was defined according to the International Study Group of Pancreatic Surgery (ISGPS) criteria when reported.
The methodological quality of the included randomized controlled trials was assessed using the Cochrane Risk of Bias Tool 2.0, which evaluates bias across five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results. Each domain was rated as “low risk,” “some concerns,” or “high risk” of bias, with an overall risk of bias judgment derived from domain-level assessments.
For observational studies, the Newcastle-Ottawa Scale (NOS) was employed, which evaluates studies based on the selection of study groups, comparability of groups, and ascertainment of exposure or outcome. The NOS assigns up to 9 stars, with studies receiving 7–9 stars considered high quality, 4–6 stars moderate quality, and 0–3 stars low quality.
The overall quality of evidence for each outcome was evaluated using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach, which considers the risk of bias, inconsistency, indirectness, imprecision, and publication bias. Evidence quality was categorized as high, moderate, low, or very low.
2.3. Data Synthesis and Statistical Analysis
A random-effects meta-analysis was performed using the Mantel-Haenszel method to calculate pooled relative risks (RR) with 95% confidence intervals (CI) for dichotomous outcomes. The primary outcome was the incidence of surgical site infections. The secondary outcomes included pancreatic fistula, seroma formation, incisional hernia, and readmission rates. Heterogeneity was assessed using the I2 statistic, with values of 25%, 50%, and 75% considered low, moderate, and high heterogeneity, respectively. The τ2 statistic was calculated to estimate the between-study variance. Potential sources of heterogeneity were explored through subgroup analyses based on the study design (RCT vs. observational), NPWT application timing (prophylactic vs. therapeutic), NPWT duration (≥5 days vs. <5 days), and NPWT pressure settings (−125 mmHg vs. −100 mmHg). Publication bias was assessed through visual inspection of funnel plots and Egger’s test, with p < 0.05 indicating significant publication bias. Sensitivity analyses were conducted by sequentially excluding individual studies to evaluate their influence on the pooled effect estimate. All statistical analyses were performed using Review Manager (RevMan) version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark) and R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) with the “meta” package. Statistical significance was set at p < 0.05, and all tests were two-sided.
4. Discussion
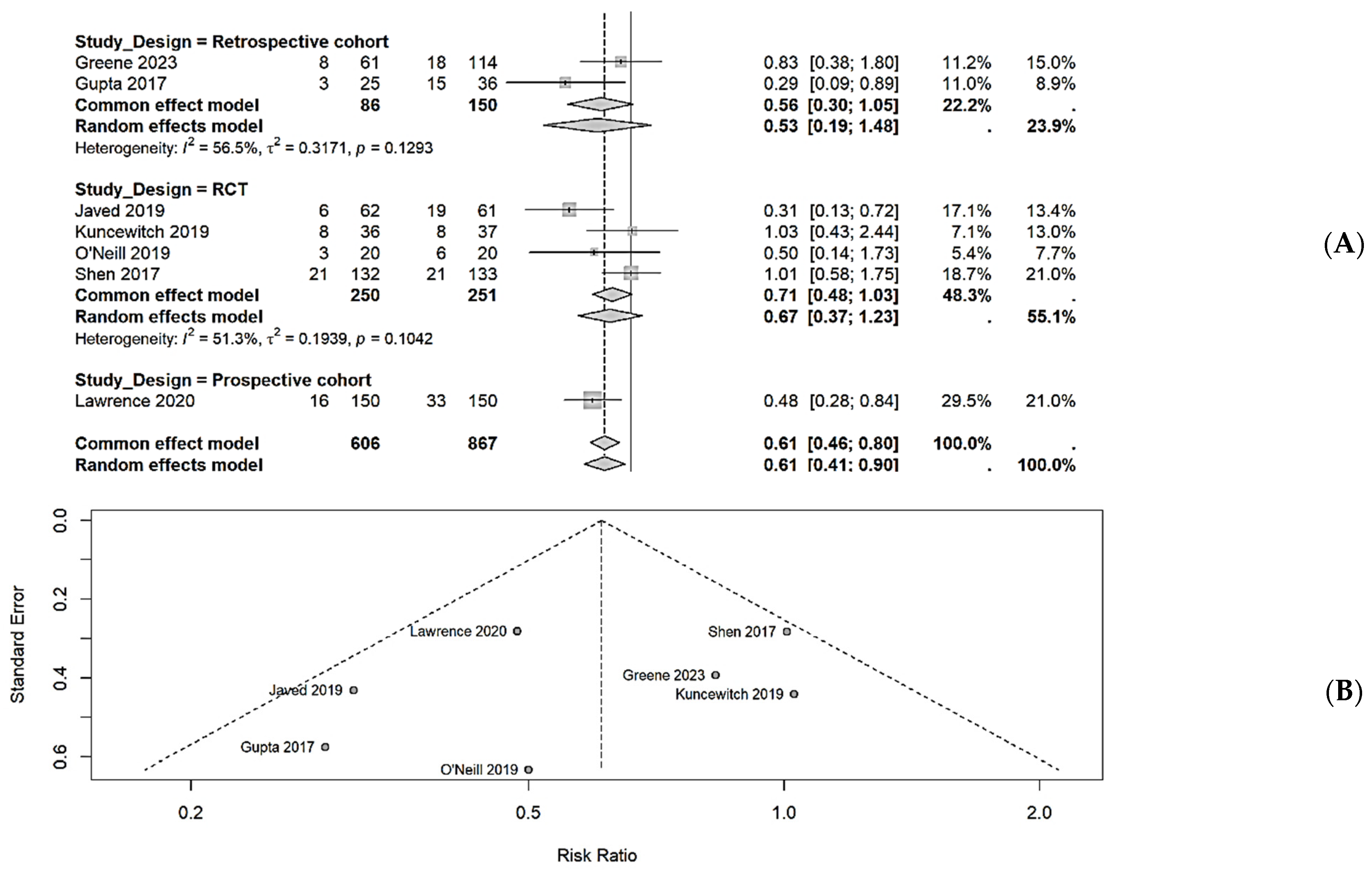
This systematic review and meta-analysis provide evidence that NPWT is associated with a significant reduction in surgical site infections following pancreaticoduodenectomy compared with conventional dressings. The overall meta-analysis demonstrated a 39% reduction in SSI risk (RR, 0.61; 95% CI, 0.41–0.90), although individual study results varied by design and methodology.
Our findings align with those of previous meta-analyses examining NPWT in other surgical specialties. A recent network meta-analysis by Zhu et al. demonstrated that NPWT significantly reduced SSI in clean-contaminated and contaminated wounds across various surgical procedures. Similarly, Sahebally et al. found that prophylactic NPWT reduced SSI by 50% following abdominal surgery [
15,
16]. The present analysis extends these findings specifically to pancreatic surgery, which has inherently high wound complication rates due to prolonged operative times, extensive dissection, and frequent preoperative biliary drainage.
The observed heterogeneity in the treatment effect across studies warrants careful interpretation. Only four of the nine included studies demonstrated statistically significant SSI reduction with NPWT, while five showed no significant difference. This variability can be explained by several factors. First, patient risk profiles differed substantially across studies. Javed et al. and Greene et al. specifically targeted high-risk patients (those with preoperative biliary drainage or neoadjuvant therapy), while other studies included all-comers. Second, NPWT application protocols varied considerably in duration (3–10 days) and pressure settings (−100 to −125 mmHg). Third, different NPWT devices were employed across the studies, including PICO, Prevena, and traditional polyurethane foam systems, which may have had different efficacy profiles.
Our subgroup analyses suggest that prophylactic NPWT application immediately after wound closure may be more effective than therapeutic application upon the detection of complications. Additionally, longer NPWT duration (≥5 days) and higher negative pressure settings (−125 mmHg) appeared to yield greater SSI reduction, although these findings should be interpreted cautiously given the limited number of studies in each subgroup.
The lack of a significant effect on pancreatic fistula rates in the pooled analysis is noteworthy. While Andrianello et al. reported a significant reduction in severe pancreatic fistula with NPWT, this finding was not consistently observed across other studies. This suggests that while NPWT may effectively manage the wound environment [
17,
18], it may not influence deeper intra-abdominal complications that arise from pancreatic anastomotic failure. The paradoxically higher fistula rates in the NPWT group observed by Kuncewitch et al. highlight the complex relationship between wound management and anastomotic healing.
Several patient-specific risk factors for SSI have been consistently identified across studies, including preoperative biliary drainage, neoadjuvant therapy, elevated BMI, and lower preoperative albumin. These findings are consistent with previous literature and suggest that targeted NPWT application in high-risk patients may yield the greatest benefit. Gupta et al. demonstrated that NPWT remained significantly protective against SSI (OR 0.15, p = 0.036) even after adjusting for these risk factors in multivariate analysis, suggesting an independent protective effect.
The quality of evidence, as assessed by the GRADE methodology, was moderate for total SSI and pancreatic fistula outcomes, with inconsistency and heterogeneity as the main limiting factors. Evidence for other outcomes, including superficial SSI, seroma formation, and readmission rates, was of lower quality due to imprecision and inconsistent reporting across studies. This underscores the need for larger, well-designed trials with standardized reporting of outcomes.
This meta-analysis had several limitations that warrant consideration. First, the included studies employed different NPWT devices and protocols, which limited their direct comparability. Second, the definition and assessment of SSI varied across studies, potentially introducing measurement bias. Third, the relatively small number of RCTs (n = 3) limits the strength of the conclusions that can be drawn. Fourth, most studies were conducted at high-volume centers with expertise in pancreatic surgery, potentially limiting the generalizability to lower-volume settings.
Despite these limitations, our findings have several important clinical implications. The significant reduction in SSI with NPWT suggests that this intervention may be beneficial in pancreaticoduodenectomy, particularly for high-risk patients. The cost-effectiveness of routine NPWT application remains uncertain; however, only Greene et al. performed a formal cost analysis, finding that NPWT was cost-effective despite its higher upfront cost due to reduced complication management expenses.
Future research should focus on standardizing NPWT protocols, identifying optimal patient selection criteria, and conducting larger multicenter RCTs with longer follow-up periods. Additionally, cost-effectiveness analyses incorporating both direct and indirect costs are needed to inform policy decisions regarding routine NPWT implementation after pancreaticoduodenectomy.