1. Introduction
Thyroid cancer incidence has steadily increased worldwide, significantly affecting patients’ survival rates and quality of life [
1]. Enhanced diagnostic capabilities and increased screening efforts partly explain this trend; however, the rising incidence underscores the urgent need for improved diagnostic accuracy and personalized patient management [
2]. Fine-Needle Aspiration Biopsy (FNA) is the standard diagnostic approach for evaluating thyroid nodules, but its accuracy can be limited, especially in cases yielding non-diagnostic or indeterminate cytological results [
3]. Such inconclusive findings often lead to repeated biopsies or unnecessary surgical interventions, emphasizing the need for additional, reliable diagnostic tools to complement the conventional methods [
4].
To address these diagnostic challenges, Washout Thyroglobulin (Washout Tg) measurement has emerged as a promising adjunctive diagnostic method. Thyroglobulin (Tg), a protein exclusively produced by thyroid follicular cells, serves as an excellent marker for identifying thyroid tissue, particularly valuable in the postoperative setting to detect residual or recurrent disease and metastatic lymph nodes [
5]. Compared to conventional imaging techniques and serum Tg measurements alone, Washout Tg provides superior sensitivity in identifying small metastatic lymph nodes, differentiating between benign thyroid remnants and malignant tissue, and enabling the early detection and timely management of metastatic disease following thyroidectomy [
6,
7].
Previous studies have consistently demonstrated the diagnostic value of Washout Tg in detecting lymph node metastasis post-thyroidectomy. Cunha et al. highlighted its exceptional sensitivity and specificity, proposing Washout Tg as a critical diagnostic tool when cytological assessment via FNA remains inconclusive [
8]. Similarly, Pacini et al. emphasized its clinical importance in postoperative surveillance, aiding the identification of metastatic lymph nodes and informing more precise therapeutic strategies [
9]. Nonetheless, significant variability persists regarding optimal Washout Tg cutoff values, with various studies proposing different thresholds. Deacu et al. reported an optimal cutoff of 10 ng/mL [
10], while other researchers recommended higher values [
11,
12], reflecting the inherent differences in patient populations, methodological approaches, and diagnostic criteria [
13]. This variability highlights a substantial gap in the current literature, underscoring the necessity of further comprehensive evaluations to establish standardized clinical guidelines.
Moreover, the interpretation of Washout Tg significantly depends on the patient’s thyroid tissue status. Zhao et al. demonstrated that the diagnostic thresholds and interpretation of Washout Tg should be individualized based on the presence or absence of thyroid gland tissue [
14]. Previous research has generally categorized patients simplistically into binary groups (thyroid present vs. absent), neglecting more nuanced clinical scenarios. Recognizing this limitation, our study introduces a refined classification based on detailed thyroid gland status, differentiating between patients with an intact thyroid gland, those with unilateral thyroid remnants, and patients post-total thyroidectomy. This precise categorization facilitates the more accurate evaluation of Washout Tg levels and their diagnostic implications across diverse clinical contexts.
Finally, thyroid cancer metastasis can occur despite the surgical removal of the thyroid gland, manifesting primarily as lymph node or distant metastases. The early and accurate detection of these metastatic deposits is crucial for timely intervention and improved patient outcomes. Washout Tg measurement, particularly through the fine-needle aspiration of suspicious lymph nodes, has demonstrated exceptional utility in identifying microscopic metastatic disease, contributing significantly to enhanced postoperative patient surveillance and management [
15].
By refining Washout Tg classification, elucidating its advantages over conventional methods, and comprehensively analyzing its role in detecting metastatic disease, this study aims to improve diagnostic precision and optimize management strategies for patients with thyroid cancer. Ultimately, our research seeks to provide clinically meaningful insights to guide the development of effective diagnostic standards and enhance overall patient care practices.
2. Methods and Materials
This study retrospectively analyzed the clinical data from 706 patients who underwent thyroid cancer surgery at the Samsung Medical Center between 2013 and 2023. The patients were systematically categorized into three distinct groups according to their thyroid gland status to thoroughly evaluate the diagnostic utility of Washout Thyroglobulin (Washout Tg):
Four hundred and sixty-seven patients underwent total thyroidectomy with modified radical neck dissection (mRND);
Fifty-two patients underwent completion thyroidectomy with mRND;
One hundred and eighty-seven patients underwent mRND following previous total thyroidectomy.
All the enrolled patients underwent measurements of Washout Tg (fine-needle aspiration Washout Thyroglobulin) and serum thyroglobulin (Tg). Comprehensive patient data were collected, including Washout Tg and serum Tg levels and detailed clinical characteristics such as age, sex, cancer stage, tumor size, multifocality, extrathyroidal extension, lymphovascular invasion, and final pathological outcomes from surgical specimens. Clinical follow-up data were also collected to assess the recurrence rates and the long-term patient outcomes.
This study cohort included patients diagnosed with thyroid cancer, encompassing various histological subtypes like primarily papillary thyroid carcinoma (PTC). Tumor staging was conducted based on the 8th edition of the American Joint Committee on Cancer (AJCC) staging system. The patient histological diagnoses and clinical stages (TNM stages I-IV) were comprehensively recorded and analyzed to ensure the thorough characterization of the patient cohort.
Statistical Methods
Descriptive Statistical Analysis:
The baseline patient characteristics, including age, sex, cancer stage, tumor characteristics, and surgical outcomes, were summarized to comprehensively describe the study population. Descriptive statistics utilized included measures such as mean, median, range, standard deviation, and percentage distributions to provide a clear depiction of patient demographics and clinical profiles.
Receiver Operating Characteristic (ROC) Analysis:
ROC curve analysis was conducted to identify the optimal cutoff values for Washout Tg, aiming to effectively differentiate between metastatic and non-metastatic lymph nodes. The diagnostic performance of Washout Tg was evaluated by calculating sensitivity, specificity, the positive predictive value, the negative predictive value, and diagnostic accuracy at various cutoff points, with the Area Under the Curve (AUC) employed to quantify overall accuracy.
Logistic Regression Analysis:
Logistic regression was performed to assess the role of Washout Tg as an independent predictive factor for lymph node metastasis in the patients with thyroid cancer. Independent variables, such as patient demographics, clinical features, and pathological characteristics, were incorporated into the multivariate logistic regression model to control for potential confounding factors. Independent sample t-tests or Mann–Whitney U tests were utilized, as appropriate, for determining the statistical significance of continuous variables, while chi-square or Fisher’s exact tests were used for categorical variables.
Survival Analysis:
Kaplan–Meier survival analysis was conducted to evaluate recurrence-free survival based on the Washout Tg levels, and log-rank tests were applied to assess statistical significance. Additionally, multivariate Cox proportional hazards regression analysis was performed to explore the prognostic impact of Washout Tg levels, adjusting for confounding variables, such as patient demographics, tumor characteristics, and treatment-related factors.
All statistical analyses were performed using SPSS Statistics, version 27.0 (IBM Corp., Armonk, NY, USA), ensuring methodological rigor and the reliability of the findings.
3. Results
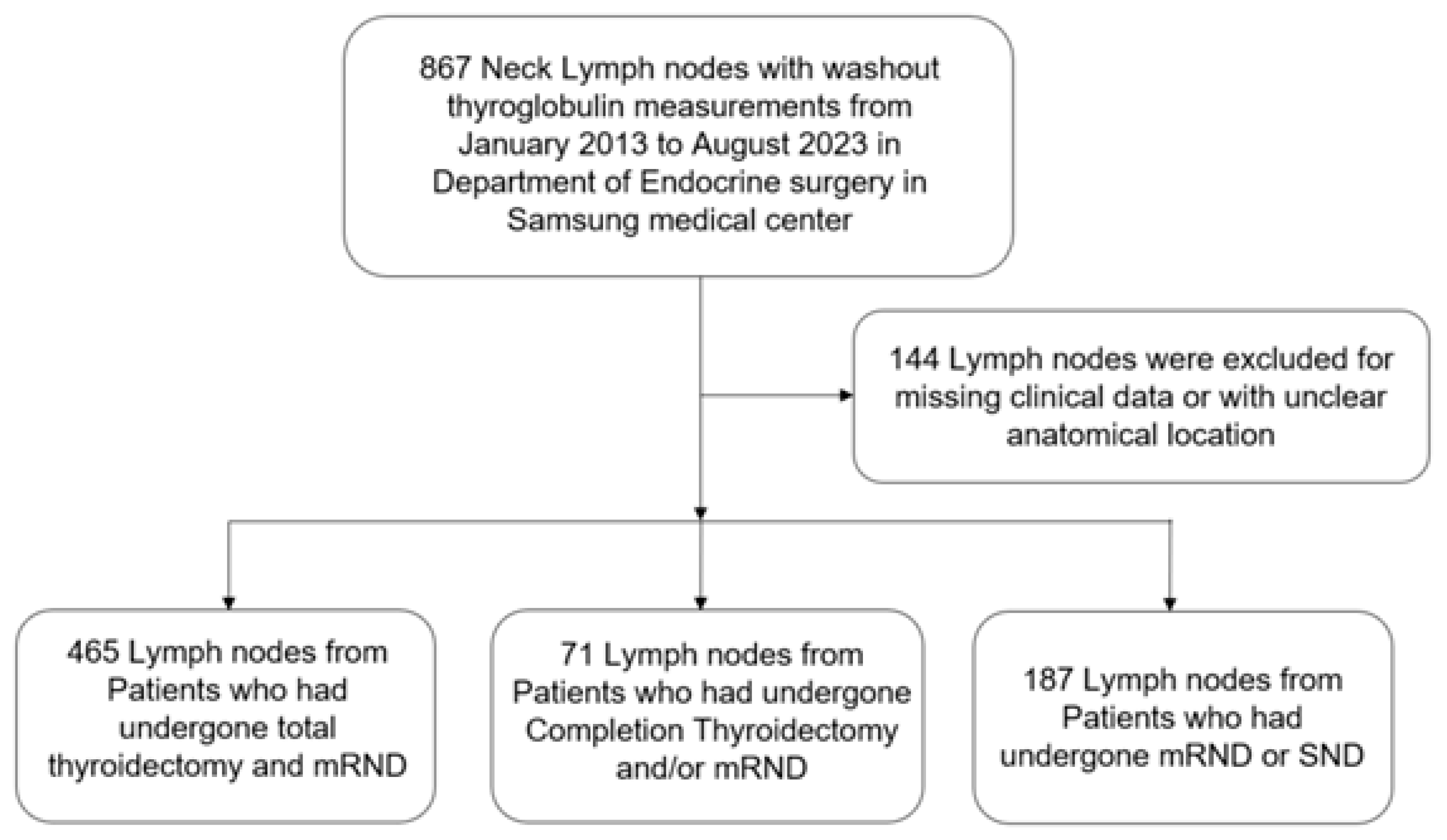
A total of 867 patients were initially included, but 144 were excluded due to incomplete data or inappropriate inclusion criteria. The remaining 723 patients were divided into three groups:
Group A: Total thyroidectomy and mRND (465 patients);
Group B: Completion thyroidectomy and mRND (71 patients);
Group C: mRND or selective neck dissection (SND) following previous total thyroidectomy (187 patients).
In this study, we conducted the comprehensive analysis of the 723 patients who underwent surgical treatment for thyroid cancer. The cohort was divided into three groups based on surgery type: group A, total thyroidectomy and modified radical neck dissection (TT and mRND) (465 patients); group C, modified radical neck dissection or selective neck dissection (mRND or SND) (187 patients); and, group B, completion thyroidectomy and modified radical neck dissection (71 patients). Analysis focused on the various clinical, radiological, and pathological aspects of the three groups (
Figure 1).
3.1. Patient Characteristics According to Neck Lymph Nodes
Table 1 presents the characteristics of the patients who underwent different types of thyroid surgery and neck lymph node dissection. The patients are categorized into three groups based on their surgical and pathological outcomes.
Group A consists of 465 lymph nodes, with 97.8% exhibiting metastasis according to permanent pathology, while 2.2% were non-metastatic. This group comprises 155 male and 310 female patients, with a mean age of 42.4 years. The surgical procedures performed include right mRND in 192 patients, left mRND in 167 patients, and bilateral mRND in 106 patients. Additionally, 10 cases of operative bed recurrence were observed.
Group B includes 71 lymph nodes, with 91.5% showing metastasis, and 8.5% classified as non-metastatic. This group consists of 30 male and 41 female patients, with a mean age of 46.5 years. Among these patients, right mRND and left mRND were performed in twenty-seven cases each, while bilateral mRND was conducted in seven patients.
Group C comprises 187 lymph nodes, with a 94.1% metastasis rate. This group includes 65 male and 122 female patients, with a mean age of 45 years. The specific surgical interventions performed were right mRND in 71 patients, left mRND in 33 patients, bilateral mRND in 6 patients, and SND in 77 patients.
Overall, this table provides a detailed breakdown of the patients with thyroid cancer based on gender, age, and the type of surgical intervention received, emphasizing the prevalence of lymph node metastasis within each group.
Table 2 shows the Washout Thyroglobulin levels. The mean Washout Tg levels (±standard deviation) were 19,708.10 ± 49,993.91 in the total thyroidectomy with mRND group, 15,384.59 ± 22,046.52 in the completion thyroidectomy with mRND group, and 17,704.84 ± 43,653.16 in the mRND or SND group.
3.2. Status of Washout Thyroglobulin
Table 3 presents the clinicopathologic status of lymph nodes in the patients who have undergone different thyroid surgery procedures, categorized into three groups: total thyroidectomy and mRND, completion thyroidectomy and mRND, and mRND or SND.
In the total thyroidectomy and mRND group, the median Washout Tg level is 1742 ng/mL, with an interquartile range (IQR) of 10,229.9 ng/mL, indicating substantial variability in Washout Tg levels among the patients.
In the completion thyroidectomy and mRND group, the median Washout Tg level is 7550 ng/mL, with a notably larger IQR of 17,598.5 ng/mL. This suggests even greater variability in the Washout Tg levels compared to the previous group.
For the patients in the mRND or SND group, this table reports only the serum Tg levels, with a median of 0.4 ng/mL and an IQR of 1.35 ng/mL. This is significantly lower than the Washout Tg levels, highlighting the greater consistency and lower magnitude of the serum Tg values in comparison.
Additionally, this table provides a comparison between the Washout Tg and serum Tg levels.
In the mRND or SND group, the median difference between the washout and serum Tg levels is 2202.5 ng/mL, with an IQR of 16,431.9 ng/mL. This substantial range suggests a high degree of variability in the differences between the washout and serum Tg levels among these patients.
These measurements play a critical role in assessing the presence and extent of thyroid cancer metastasis in lymph nodes. Elevated Washout Tg levels may serve as potential indicators of more significant disease involvement. Furthermore, the large interquartile ranges observed reflect considerable heterogeneity in the clinicopathologic characteristics of the patient groups.
3.3. Receiver Operating Characteristic (ROC) Curve Analysis of Washout Thyroglobulin (Tg) for Lymph Node Metastasis
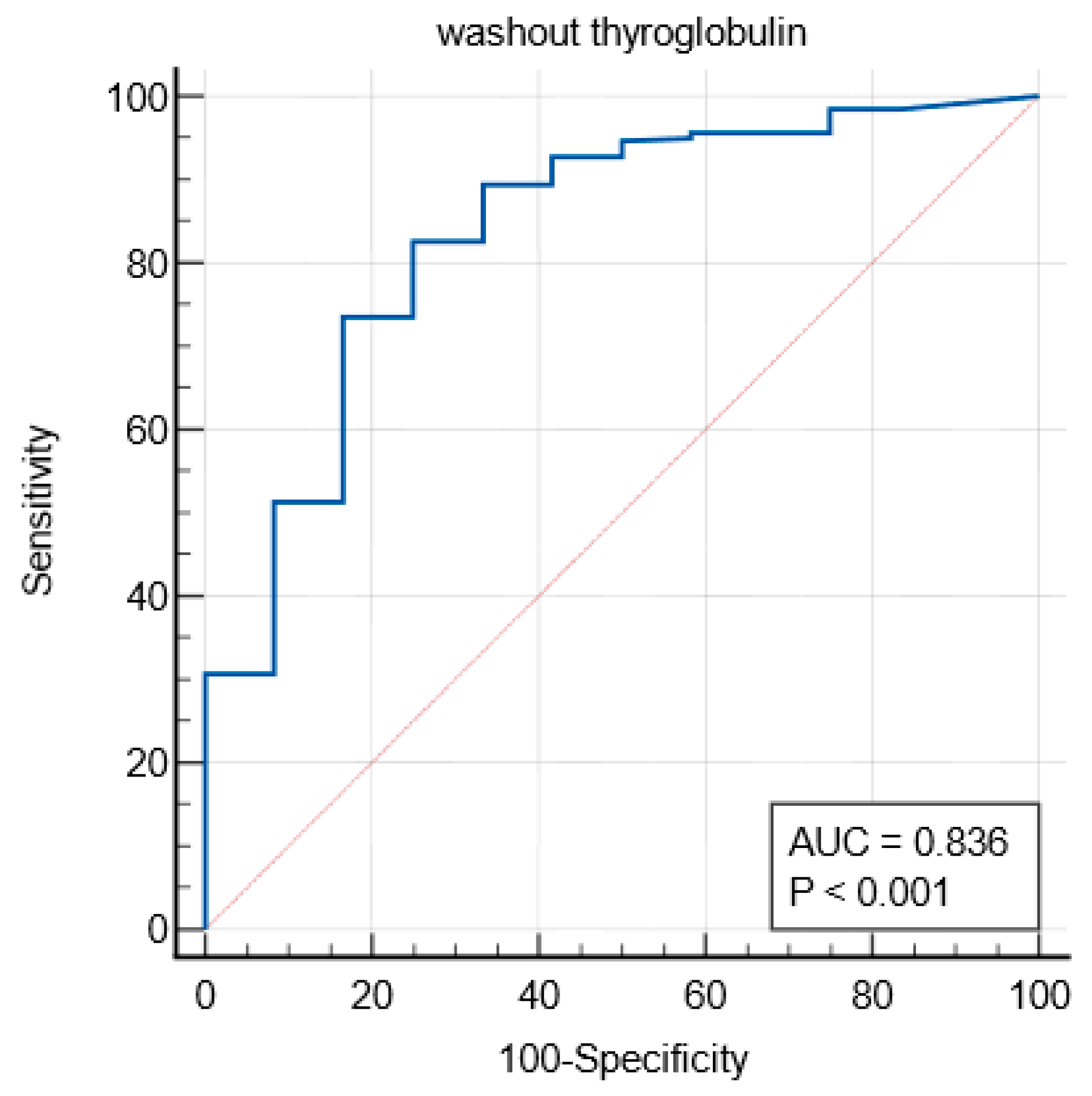
The ROC (Receiver Operating Characteristic) curve for Washout Thyroglobulin (Tg) analysis was plotted based on the sensitivity and specificity values with various threshold criteria. This curve illustrates the diagnostic performance of Washout Tg in identifying lymph node metastasis in the patients with thyroid cancer.
3.4. Total Thyroidectomy (TT) and Modified Radical Neck Dissection (mRND) Group
Among the patients who underwent TT and mRND, the Area Under the Curve (AUC) was 0.836, indicating the high diagnostic accuracy of the Washout Tg test (
Figure 2). The Youden Index was calculated as 0.5764, with an optimal threshold of >23.3 ng/mL. At this cutoff, the test demonstrated a sensitivity of 82.64% and a specificity of 75.00%, suggesting its strong predictive value for lymph node metastasis.
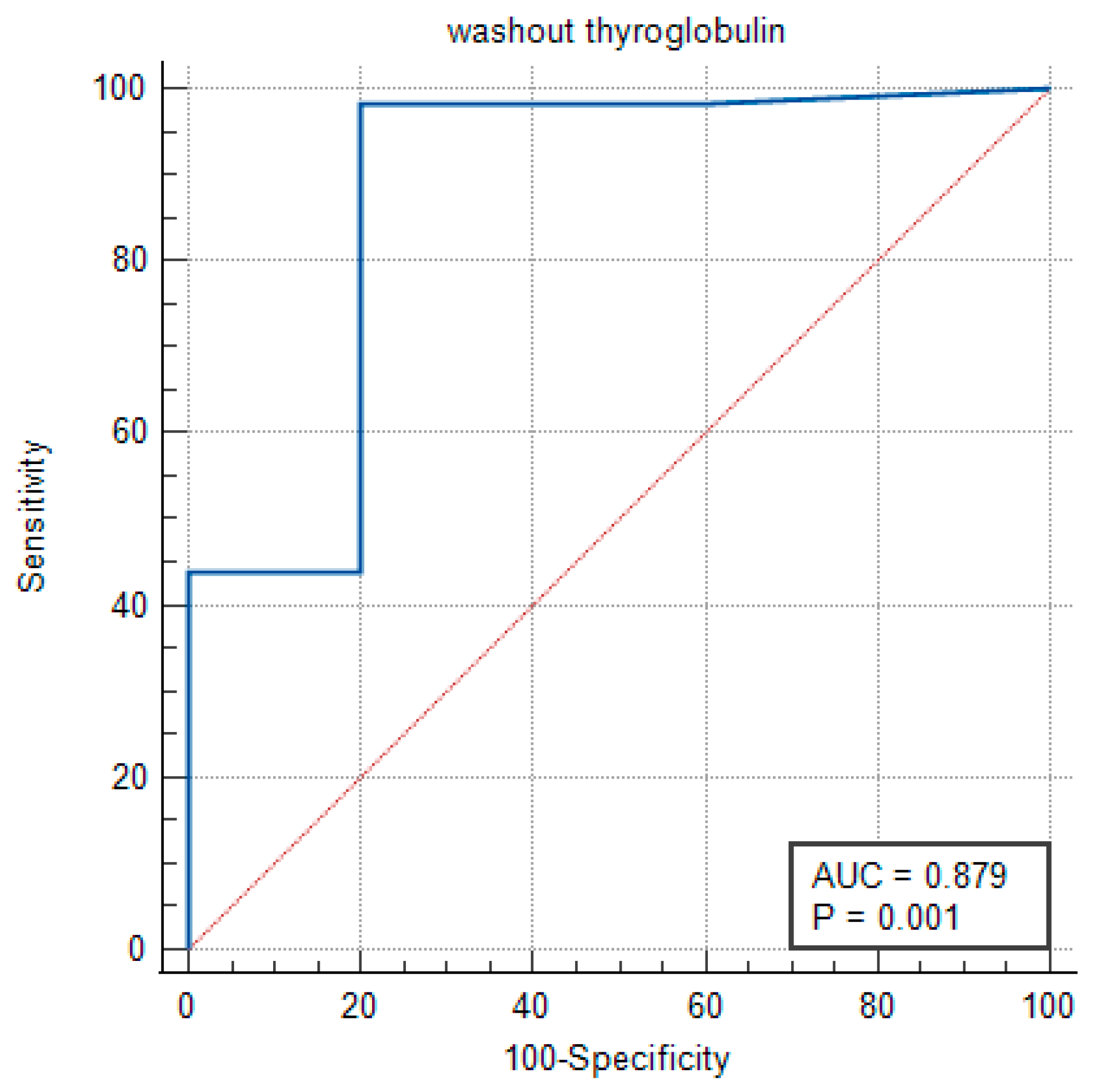
3.5. Completion Thyroidectomy and mRND Group
In the completion thyroidectomy and mRND group, the AUC was determined to be 0.879, reflecting a moderate-to-high level of accuracy in distinguishing between the positive and negative clinical outcomes. The statistical significance of this result was confirmed with
p = 0.006, reinforcing the reliability of the Washout Tg measurement (
Figure 3).
The Youden Index was 0.766, with an optimal threshold of >7.2 ng/mL.
At this cutoff, the test exhibited a sensitivity of 98.48%, indicating a strong ability to correctly identify positive cases, while the specificity was 80.00%.
These findings suggest that the Washout Thyroglobulin cutoff values differ between the patients with an intact thyroid lobe and those who have undergone complete thyroidectomy.
3.6. mRND or Selective Neck Dissection (SND) Group
In the mRND or SND group, the AUC was 0.766, with a statistical significance of
p = 0.01, indicating that the diagnostic utility of Washout Tg in the patients without a thyroid is relatively lower (
Figure 4).
The Youden Index was 0.5886, with an optimal threshold of >0.1 ng/mL.
At this cutoff, the test exhibited a sensitivity of 98.86%, but the specificity was comparatively lower at 60.00%, highlighting the reduced diagnostic precision of Washout Tg in this patient group.
ROC analysis confirms the diagnostic value of Washout Tg in predicting lymph node metastasis, with varying levels of accuracy depending on the thyroid gland status. The optimal cutoff values differ among the patient groups, reinforcing the necessity of tailored interpretation strategies for Washout Tg in clinical practice.
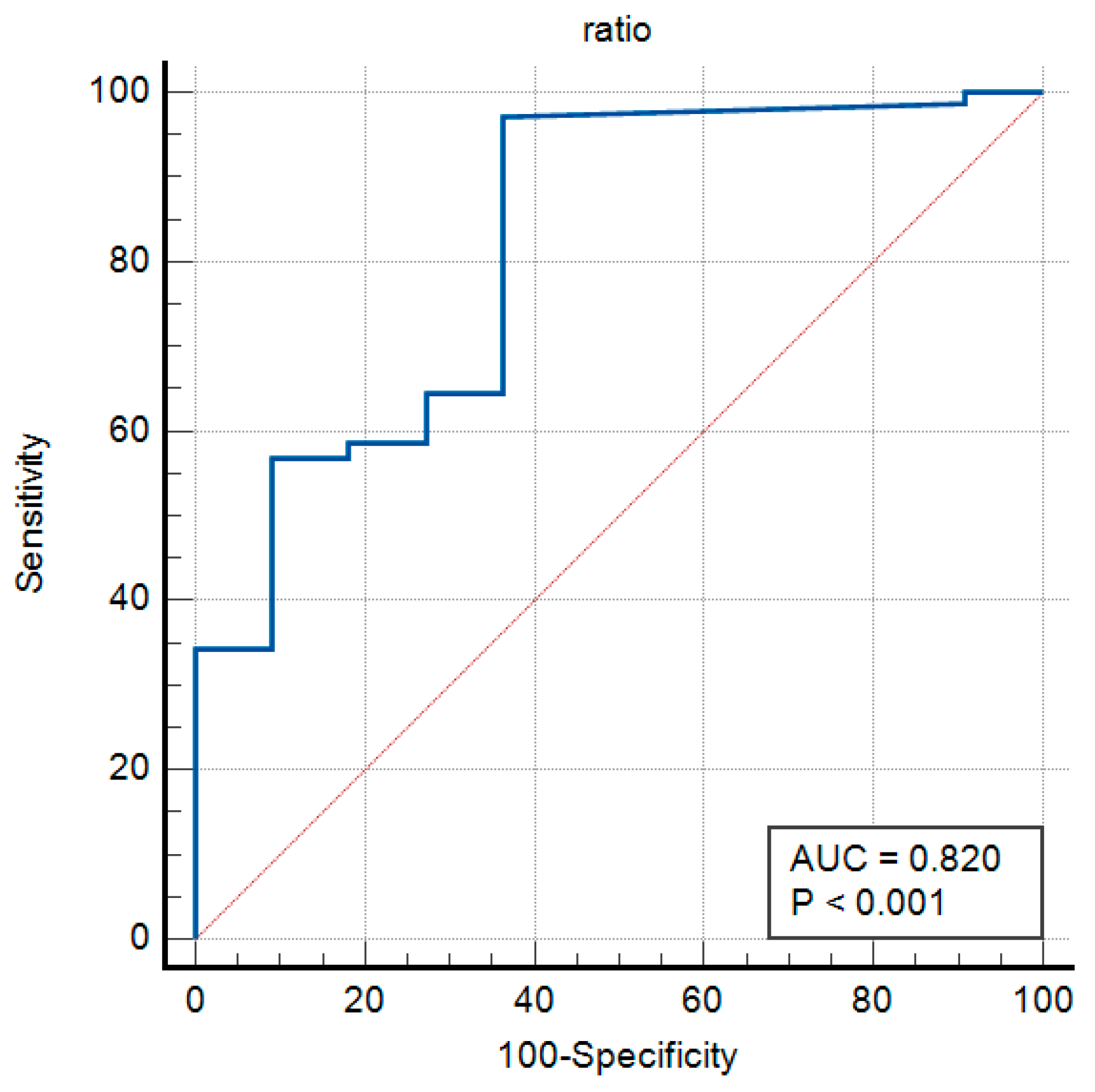
3.7. Washout Tg/Serum Tg Ratio as a Diagnostic Marker
In patients who have undergone thyroidectomy, the Washout Tg/serum Tg ratio serves as a significant factor in diagnosing lymph node metastasis. This is because in the absence of a thyroid gland, the serum Tg levels are expected to be low. Consequently, an elevated Washout Tg/serum Tg ratio may suggest the presence of metastatic tissue in the lymph nodes.
The Receiver Operating Characteristic (ROC) curve for the Washout Tg/serum Tg ratio was plotted, demonstrating its diagnostic performance (
Figure 5). The Area Under the Curve (AUC) for this ratio was calculated as 0.820, indicating a high level of diagnostic accuracy.
The Youden Index was 0.6073, with a significance level of p < 0.001, confirming the statistical significance of the AUC.
The optimal Youden Index value was 0.6154, representing a balance between sensitivity and specificity.
The associated threshold for this index was determined to be >1, yielding a sensitivity of 97.09% and a specificity of 63.64% at this cutoff (
Figure 5).
These findings suggest that the Washout Tg/serum Tg ratio is a valuable diagnostic marker for lymph node metastasis, particularly in patients post-thyroidectomy. The high sensitivity of this parameter underscores its potential clinical utility, while the moderate specificity highlights the need for further validation and refinement in its application.
4. Discussion
The findings of this study emphasize the critical diagnostic role of Washout Thyroglobulin (Washout Tg) measurement in detecting lymph node metastases in patients treated for thyroid cancer, particularly those who have undergone thyroidectomy. The observed high sensitivity at the optimal cutoff values underscores the potential of Washout Tg as a reliable and valuable biomarker in clinical practice. However, the relatively lower specificity at certain cutoff values highlights the increased likelihood of false positive results, necessitating careful clinical interpretation and validation through prospective studies [
16].
Receiver Operating Characteristic (ROC) curve analysis and the Youden Index calculations further confirm the diagnostic utility of Washout Tg, supporting its routine clinical use. Specifically, the high sensitivity achieved at the lower Washout Tg thresholds in thyroid-absent patients demonstrates significant efficacy in early lymph node metastasis detection. Nevertheless, the associated reduction in specificity at these thresholds underscores a clinical challenge, highlighting the importance of balancing sensitivity and specificity to minimize false positive results [
17,
18].
Our study provides a comprehensive evaluation of the diagnostic capability of Washout Tg and the Washout Tg/serum Tg ratio across diverse patient populations, with a specific emphasis on variations in thyroid gland status. A notable observation was the considerable variability in the optimal cutoff values, influenced by whether the patients had an intact thyroid gland, partial thyroid remnants, or no thyroid gland. These findings align with and extend prior research. For example, Pacini et al. have similarly stressed the necessity of adjusting diagnostic strategies according to residual thyroid tissue status, emphasizing tailored diagnostic approaches [
9].
Importantly, our analysis highlights the Washout Tg/serum Tg ratio as a particularly effective diagnostic parameter for patients post-total thyroidectomy. This ratio provides a normalized metric that significantly enhances diagnostic accuracy by adjusting for variations in the serum Tg levels, typically low or undetectable in thyroid-absent patients. Wang Y et al. have reported similar findings, supporting the ratio’s reliability for identifying metastatic lymph nodes [
18]. This ratio effectively addresses the limitations of serum Tg alone, which often lacks sensitivity in detecting small or subclinical metastases, thus proving especially beneficial in these clinical scenarios [
19,
20].
Moreover, this study emphasizes the necessity of individualized diagnostic thresholds for Washout Tg to optimize clinical decision making. Tailored thresholds can markedly improve diagnostic precision, reduce unnecessary clinical interventions, and alleviate patient anxiety related to false positive diagnoses. Furthermore, we recognize that the variability in Washout Tg measurements may stem from multiple factors, including the assay methods, patient demographics, tumor characteristics, the surgical approaches, and the follow-up protocols. Standardizing methodologies and conducting prospective validation studies are essential steps toward enhancing diagnostic consistency and clinical applicability [
4,
15].
Additionally, integrating Washout Tg measurements with other clinical indicators, such as imaging findings, cytopathological evaluation, and molecular profiling, may substantially enhance diagnostic specificity and predictive accuracy. Combined diagnostic approaches, incorporating multiple biomarkers and modalities, potentially provide a superior diagnostic performance compared to that of Washout Tg alone, thus eliminating false positive outcomes and improving patient outcomes [
19].
Although our current analysis primarily investigates Washout Tg as a diagnostic biomarker, we acknowledge the rising importance of molecular profiling—such as BRAF V600E mutation and TERT promoter mutations—in predicting lymph node and distant metastases in thyroid cancer. Our findings, particularly regarding tailored Washout Tg thresholds, could complement these molecular markers. Prospective studies integrating molecular profiles with Washout Tg assessments are highly recommended, as combined strategies may offer a robust predictive framework for metastatic risk, further refining patient-specific management.
This study’s notable strength lies in its substantial sample size, derived from a single institution, significantly enhancing statistical reliability. A large dataset not only supports robust statistical outcomes, but also reinforces clinical applicability. Our findings align closely with the existing guidelines from organizations like the American Thyroid Association (ATA) and the European Thyroid Association (ETA), emphasizing the accurate and early detection of residual or recurrent thyroid cancer post-surgery [
4,
20]. Recent meta-analyses and systematic reviews further stress the importance of extensive studies for establishing precise Washout Tg cutoff values and diagnostic standards. Thus, our results offer both statistical validation and substantial theoretical support, bolstering the clinical relevance of Washout Tg in thyroid cancer surveillance.
Overall, our study contributes significantly to the evolving evidence advocating personalized and precise diagnostic approaches in thyroid cancer management. The observed variability in diagnostic performance, depending on thyroid gland status, highlights the complexity of postoperative thyroid cancer surveillance. Our results strongly support personalized diagnostic strategies, emphasizing the need for tailored interpretation criteria for Washout Tg and related biomarkers to enhance patient outcomes and clinical management [
19].
5. Conclusions
Based on the comprehensive analysis of a dataset covering a decade from Samsung Medical Center, this study establishes optimized Washout Tg cutoff values for detecting lymph node metastases in thyroid cancer, differentiated by thyroid gland status:
Total thyroidectomy and mRND: >23.3 ng/mL;
Completion thyroidectomy and mRND: >7.2 ng/mL;
mRND or SND: >0.1 ng/mL;
Washout Tg/serum Tg ratio: >1.
These findings underscore the diagnostic significance of Washout Tg measurement, particularly when thyroid tissue is absent, and emphasize the clinical value of the Washout Tg/serum Tg ratio. By refining the interpretation criteria for Washout Tg levels, this research substantially contributes to the more accurate detection and optimized management of thyroid cancer metastases. The results highlight the need for individualized assessment based on the Washout Tg thresholds to better distinguish between true positive cases and false positives, enhancing clinical decision-making processes. Additionally, this study draws attention to potential influences of various clinical factors, such as patient demographics, surgical histories, and assay methodologies, on the Washout Tg measurement outcomes, indicating areas requiring further methodological standardization and validation. Continued research, particularly through prospective, large-scale, multicenter studies with comprehensive follow-up data, is essential to reinforce these conclusions, standardize diagnostic practices, and further refine patient management strategies. Such research efforts will likely contribute to establishing universally accepted cutoff values, reducing inter-institutional variability, and ultimately improving the clinical outcomes in thyroid cancer care by enabling more precise and reliable detection, prognosis assessment, and treatment decision-making processes [
15,
19].