Minimally Invasive Subcutaneous Adipose Tissue Biopsy in a Nonhuman Primate Model: Approach and Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Demographics
2.2. Site Selection and Biopsy
2.3. Post Biopsy Care
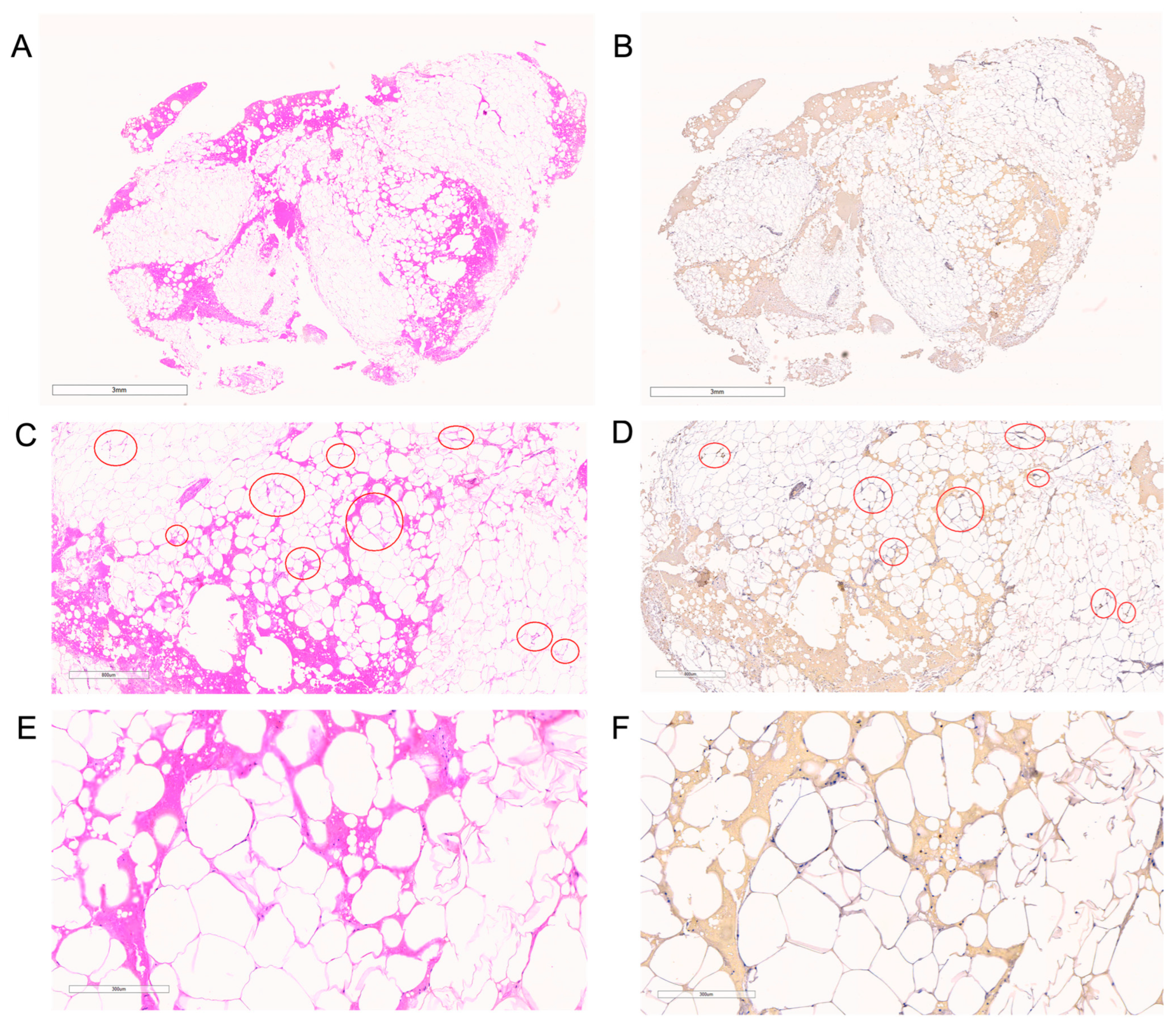
2.4. Tissue Processing for Immunohistochemistry and Adipocyte Morphology
2.5. Tissue Processing for Stromal Vascular Fraction Component Analysis
2.6. Outcomes and Endpoints
2.7. Statistical Analysis
3. Results
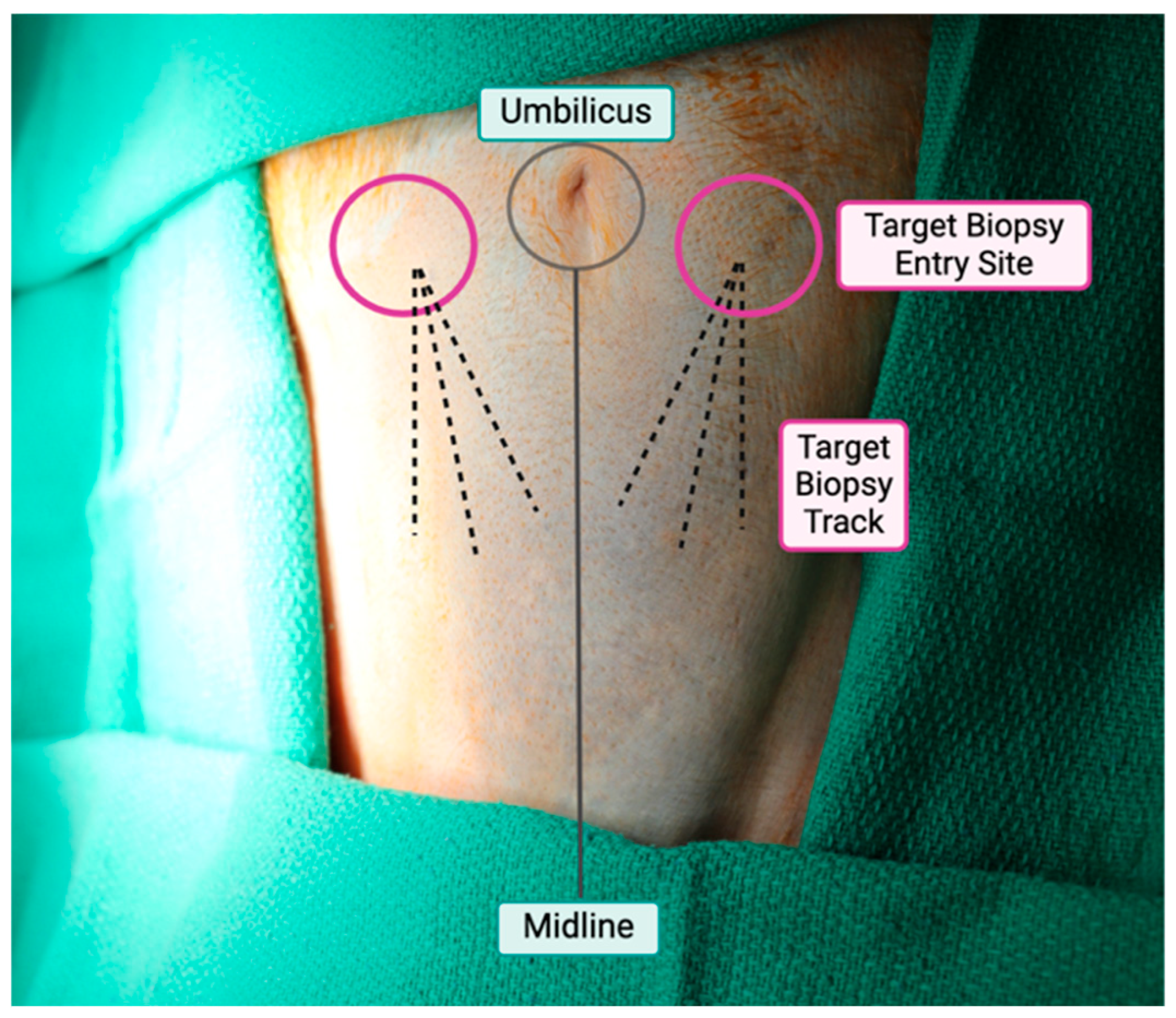
3.1. Site and Biopsy Approach
3.2. Positioning and Preparation
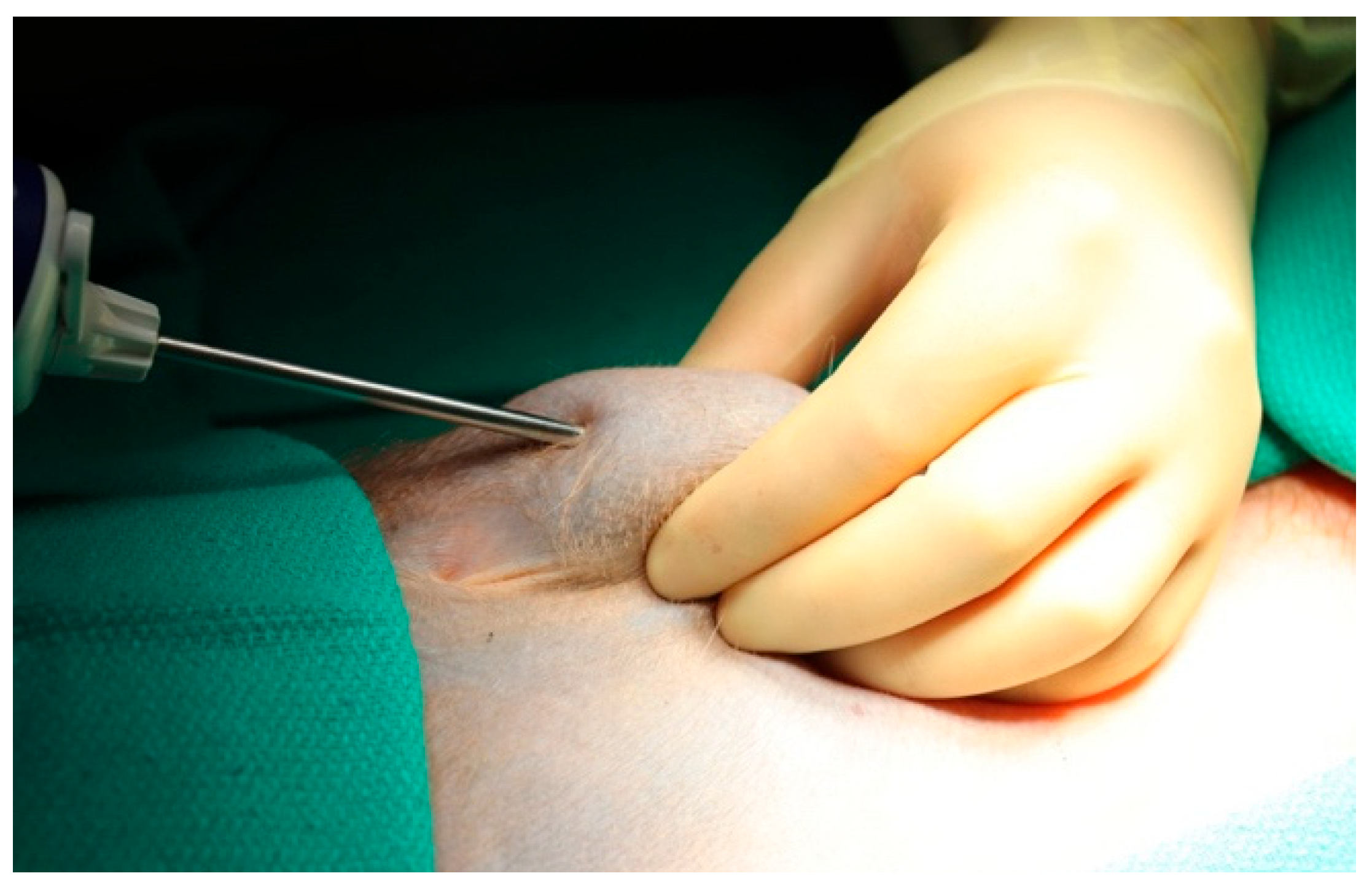
3.3. Biopsy Technique
3.4. Post-Operative Care
3.5. Special Considerations
- (1)
- When using the biopsy device, special attention should be made to the angle of the biopsy device. When positioned near compliant structures, such as skin, activation of the vacuum may inadvertently pull these tissues into the device, resulting unintended excision.
- (2)
- In regions of interest with limited fat, ultrasound guidance may be used to confirm appropriate needle placement, above the fascial plane and below the skin.
- (3)
- When applying manual pressure after the procedure, pressure should be maintained along the entire needle track and rather than only the entry site. Because AT is vascular, minor oozing may occur post-tissue collection; additional or repeated pressure may be applied as needed to achieve hemostasis.
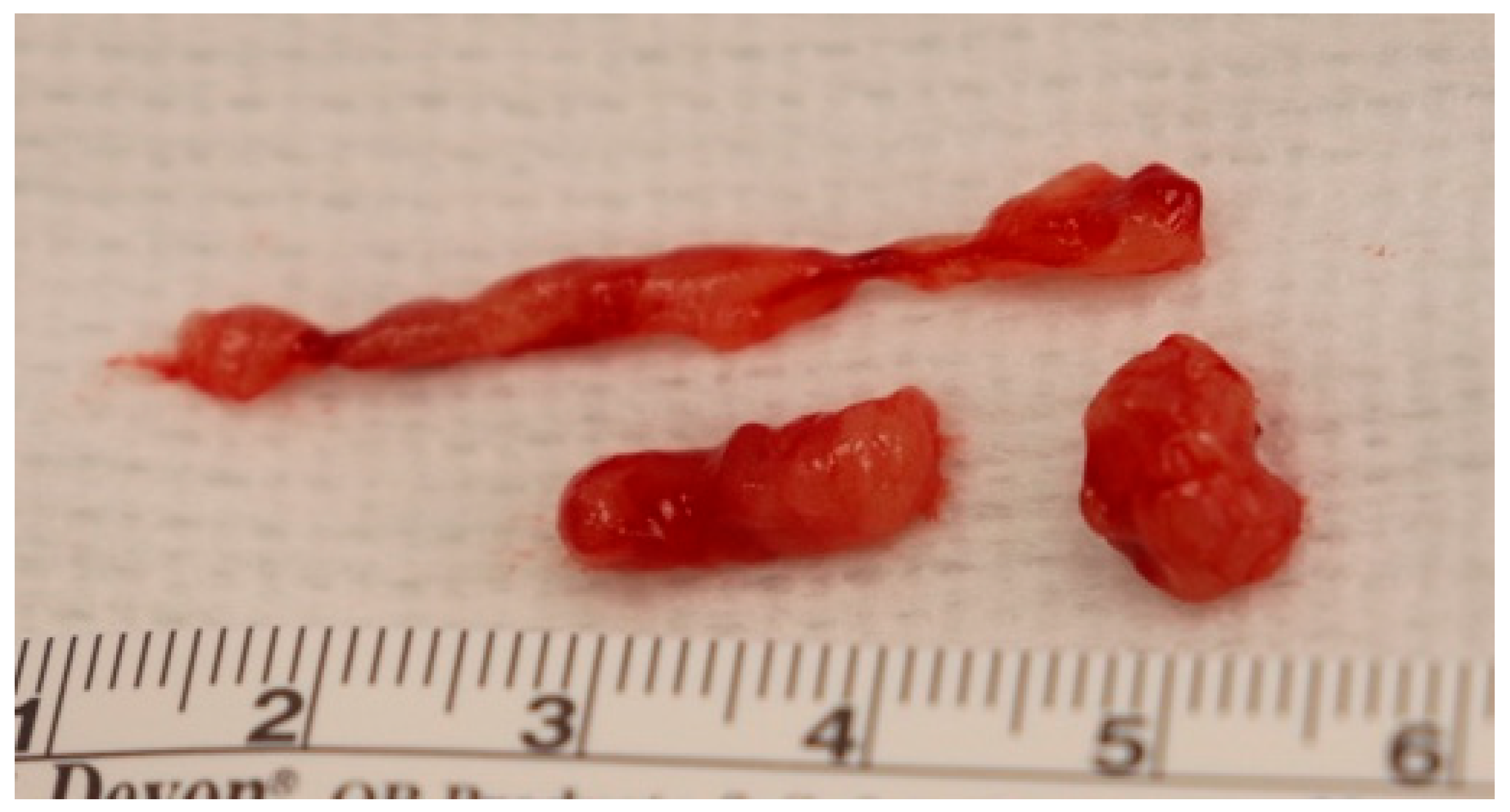
3.6. Biopsy Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Cottam, M.A.; Caslin, H.L.; Winn, N.C.; Hasty, A.H. Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice. Nat. Commun. 2022, 13, 2950. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Jun, H.-S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediat. Inflamm. 2016, 2016, 3094642. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Bradley, D.; Deng, T.; Shantaram, D.; Hsueh, W.A. Orchestration of the Adipose Tissue Immune Landscape by Adipocytes. Annu. Rev. Physiol. 2024, 86, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Ikramuddin, S.; Korner, J.; Lee, W.J.; Thomas, A.J.; Connett, J.E.; Bantle, J.P.; Leslie, D.B.; Wang, Q.; Inabnet, W.B., 3rd; Jeffery, R.W.; et al. Lifestyle Intervention and Medical Management With vs Without Roux-en-Y Gastric Bypass and Control of Hemoglobin A1c, LDL Cholesterol, and Systolic Blood Pressure at 5 Years in the Diabetes Surgery Study. JAMA 2018, 319, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Pipek, L.Z.; Moraes, W.A.F.; Nobetani, R.M.; Cortez, V.S.; Condi, A.S.; Taba, J.V.; Nascimento, R.F.V.; Suzuki, M.O.; do Nascimento, F.S.; de Mattos, V.C.; et al. Surgery is associated with better long-term outcomes than pharmacological treatment for obesity: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 9521. [Google Scholar] [CrossRef]
- Adams, T.D.; Davidson, L.E.; Litwin, S.E.; Kim, J.; Kolotkin, R.L.; Nanjee, M.N.; Gutierrez, J.M.; Frogley, S.J.; Ibele, A.R.; Brinton, E.A.; et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N. Engl. J. Med. 2017, 377, 1143–1155. [Google Scholar] [CrossRef]
- Stenberg, E.; Cao, Y.; Ottosson, J.; Hedberg, S.; Naslund, E. Glycaemic and weight effects of metabolic surgery or semaglutide in diabetes dosage for patients with type 2 diabetes. Diabetes Obes. Metab. 2024, 26, 5812–5818. [Google Scholar] [CrossRef]
- Ghanem, O.M.; Abi Mosleh, K.; Kerbage, A.; Lu, L.; Hage, K.; Abu Dayyeh, B.K. Continued Diabetes Remission Despite Weight Recurrence: Gastric Bypass Long-Term Metabolic Benefit. J. Am. Coll. Surg. 2024, 238, 862–871. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Davies, M.; Van Gaal, L.F.; Kandler, K.; Konakli, K.; Lingvay, I.; McGowan, B.M.; Oral, T.K.; Rosenstock, J.; et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obes. Metab. 2022, 24, 1553–1564. [Google Scholar] [CrossRef]
- Hruska, P.; Kucera, J.; Pekar, M.; Holeczy, P.; Mazur, M.; Buzga, M.; Kuruczova, D.; Lenart, P.; Fialova Kucerova, J.; Potesil, D.; et al. Proteomic Signatures of Human Visceral and Subcutaneous Adipocytes. J. Clin. Endocrinol. Metab. 2022, 107, 755–775. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef]
- Nugent, J.L.; Singh, A.; Wirth, K.M.; Oppler, S.H.; Hocum Stone, L.; Janecek, J.L.; Sheka, A.C.; Kizy, S.; Moore, M.E.G.; Staley, C.; et al. A nonhuman primate model of vertical sleeve gastrectomy facilitates mechanistic and translational research in human obesity. iScience 2021, 24, 103421. [Google Scholar] [CrossRef] [PubMed]
- Zannis, V.J.; Aliano, K.M. The evolving practice pattern of the breast surgeon with disappearance of open biopsy for nonpalpable lesions. Am. J. Surg. 1998, 176, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Oedayrajsingh-Varma, M.J.; van Ham, S.M.; Knippenberg, M.; Helder, M.N.; Klein-Nulend, J.; Schouten, T.E.; Ritt, M.J.; van Milligen, F.J. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 2006, 8, 166–177. [Google Scholar] [CrossRef]
- Alderete, T.L.; Sattler, F.R.; Sheng, X.; Tucci, J.; Mittelman, S.D.; Grant, E.G.; Goran, M.I. A novel biopsy method to increase yield of subcutaneous abdominal adipose tissue. Int. J. Obes. 2015, 39, 183–186. [Google Scholar] [CrossRef]
- Bruening, W.; Fontanarosa, J.; Tipton, K.; Treadwell, J.R.; Launders, J.; Schoelles, K. Systematic review: Comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann. Intern. Med. 2010, 152, 238–246. [Google Scholar] [CrossRef]
- Bozzini, A.; Cassano, E.; Raciti, D.; Disalvatore, D.; Pala, O.; Vingiani, A.; Renne, G. Analysis of Efficacy and Accuracy of 2 Vacuum-Assisted Breast Biopsy Devices: Mammotome and Elite. Clin. Breast Cancer 2018, 18, e1277–e1282. [Google Scholar] [CrossRef]
- Mohr, Z.; Hirche, C.; Klein, T.; Kneif, S.; Hunerbein, M. Vacuum-assisted minimally invasive biopsy of soft-tissue tumors. J. Bone Jt. Surg. Am. 2012, 94, 103–109. [Google Scholar] [CrossRef]
- Hagman, D.K.; Kuzma, J.N.; Larson, I.; Foster-Schubert, K.E.; Kuan, L.Y.; Cignarella, A.; Geamanu, E.; Makar, K.W.; Gottlieb, J.R.; Kratz, M. Characterizing and quantifying leukocyte populations in human adipose tissue: Impact of enzymatic tissue processing. J. Immunol. Methods 2012, 386, 50–59. [Google Scholar] [CrossRef]
- Teke, M.; Rich, B.S.; Walther, A.; Schwartz, D.; McDuffie, L.A.; Butera, G.; Roach, J.P.; Rothstein, D.H.; Lal, D.R.; Riehle, K.; et al. A Comparison of Commonly Utilized Diagnostic Biopsy Techniques for Pediatric Patients With Cancer: A Systematic Review by the APSA Cancer Committee. J. Pediatr. Surg. 2025, 60, 161893. [Google Scholar] [CrossRef] [PubMed]
- Henegar, C.; Tordjman, J.; Achard, V.; Lacasa, D.; Cremer, I.; Guerre-Millo, M.; Poitou, C.; Basdevant, A.; Stich, V.; Viguerie, N.; et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008, 9, R14. [Google Scholar] [CrossRef]
- Katsuta, L.; Fujioka, T.; Kubota, K.; Mori, M.; Yamaga, E.; Yashima, Y.; Sato, A.; Adachi, M.; Ishiba, T.; Oda, G.; et al. Comparison of state-of-the-art biopsy systems for ultrasound-guided breast biopsy using a chicken breast phantom. J. Med. Ultrason. 2024, 51, 627–633. [Google Scholar] [CrossRef]
- Nakano, S.; Imawari, Y.; Mibu, A.; Otsuka, M.; Oinuma, T. Differentiating vacuum-assisted breast biopsy from core needle biopsy: Is it necessary? Br. J. Radiol. 2018, 91, 20180250. [Google Scholar] [CrossRef] [PubMed]
- Hoorntje, L.E.; Peeters, P.H.; Mali, W.P.; Borel Rinkes, I.H. Vacuum-assisted breast biopsy: A critical review. Eur. J. Cancer 2003, 39, 1676–1683. [Google Scholar] [CrossRef]
- Berg, W.A.; Krebs, T.L.; Campassi, C.; Magder, L.S.; Sun, C.C. Evaluation of 14- and 11-gauge directional, vacuum-assisted biopsy probes and 14-gauge biopsy guns in a breast parenchymal model. Radiology 1997, 205, 203–208. [Google Scholar] [CrossRef]
- DeStephanis, D.; Long, M.R.; Williams, A.G.; Santiago, M.; Tonkin, J.; Stevens, C.M.; Davis, M.A.; Ruggiero, A.D.; Henstridge, D.C.; Premilovac, D.; et al. Metabolically unhealthy adipose tissue is characterized by reductions in mitochondrial size and function. Obesity 2025, 33, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Pawde, A.M.; Kumar, R.; Kalaiselvan, E.; Kinjavdekar, P.; Dhama, K.; Pal, A. Standardization and characterization of adipose-derived stromal vascular fraction from New Zealand white rabbits for bone tissue engineering. Vet. World 2021, 14, 508–514. [Google Scholar] [CrossRef]
- Jimenez-Gomez, Y.; Mattison, J.A.; Pearson, K.J.; Martin-Montalvo, A.; Palacios, H.H.; Sossong, A.M.; Ward, T.M.; Younts, C.M.; Lewis, K.; Allard, J.S.; et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013, 18, 533–545. [Google Scholar] [CrossRef]
- Sharples, F.; Anestidou, L.; Beil, K.; Fletcher, C.; Haycraft, R.; Zurlo, J. Guide for the Use and Care of Laboratory Animals. In National Research Council (US) Committee or the Update of the Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Graham, M.L.; Rieke, E.F.; Mutch, L.A.; Zolondek, E.K.; Faig, A.W.; DuFour, T.A.; Munson, J.W.; Kittredge, J.A.; Schuurman, H.J. Successful implementation of cooperative handling eliminates the need for restraint in a complex non-human primate disease model. J. Med. Primatol. 2012, 41, 89–106. [Google Scholar] [CrossRef]
- Palmer, S.; Oppler, S.H.; Graham, M.L. Behavioral management as a coping strategy for managing stressors in primates: The influence of temperament and species. Biology 2022, 11, 423. [Google Scholar] [CrossRef]
- Busebee, B.; Ghusn, W.; Cifuentes, L.; Acosta, A. Obesity: A Review of Pathophysiology and Classification. Mayo Clin. Proc. 2023, 98, 1842–1857. [Google Scholar] [CrossRef]
- Kumanyika, S.; Dietz, W.H. Solving Population-wide Obesity—Progress and Future Prospects. N. Engl. J. Med. 2020, 383, 2197–2200. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Shastri, N. The Role of T Cells in Obesity-Associated Inflammation and Metabolic Disease. Immune Netw. 2022, 22, e13. [Google Scholar] [CrossRef] [PubMed]
- Vijay, J.; Gauthier, M.F.; Biswell, R.L.; Louiselle, D.A.; Johnston, J.J.; Cheung, W.A.; Belden, B.; Pramatarova, A.; Biertho, L.; Gibson, M.; et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat. Metab. 2020, 2, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Makar, K.W.; Kratz, M.; Foster-Schubert, K.E.; McTiernan, A.; Ulrich, C.M. A pilot study of sampling subcutaneous adipose tissue to examine biomarkers of cancer risk. Cancer Prev. Res. 2009, 2, 37–42. [Google Scholar] [CrossRef]
- Chung, Y.; Chang, J.Y.; Soedono, S.; Julietta, V.; Joo, E.J.; Kwon, S.H.; Choi, S.I.; Kim, Y.J.; Cho, K.W. Distinct T Cell Subset Profiles and T-Cell Receptor Signatures in Metabolically Unhealthy Obesity. Int. J. Mol. Sci. 2025, 26, 3372. [Google Scholar] [CrossRef] [PubMed]
- Havel, P.J.; Kievit, P.; Comuzzie, A.G.; Bremer, A.A. Use and Importance of Nonhuman Primates in Metabolic Disease Research: Current State of the Field. ILAR J. 2017, 58, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.L.; Prescott, M.J. The multifactorial role of the 3Rs in shifting the harm-benefit analysis in animal models of disease. Eur. J. Pharmacol. 2015, 759, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Oppler, S.H.; Palmer, S.D.; Phu, S.N.; Graham, M.L. The Role of Behavioral Management in Enhancing Clinical Care and Efficiency, Minimizing Social Disruption, and Promoting Welfare in Captive Primates. Vet. Sci. 2024, 11, 401. [Google Scholar] [CrossRef]
| Mean ± SEM | Median (Min, Max) | |
|---|---|---|
| Age (years) | 9.9 ± 0.07 | 9.9 (9.3, 10.3) |
| Weight (kg) | 15.4 ± 0.5 | 16.1 (11.3, 18.6) |
| Mean ± SEM | Median (Min, Max) | ||
|---|---|---|---|
| Biopsy Characteristics (n = 18) | |||
| Procedure Time (min) | 11.4 ± 1.1 | 10 (3, 23) | |
| Resolution of Ecchymosis (days) | 7.5 ± 1.3 | 6 (3, 28) | |
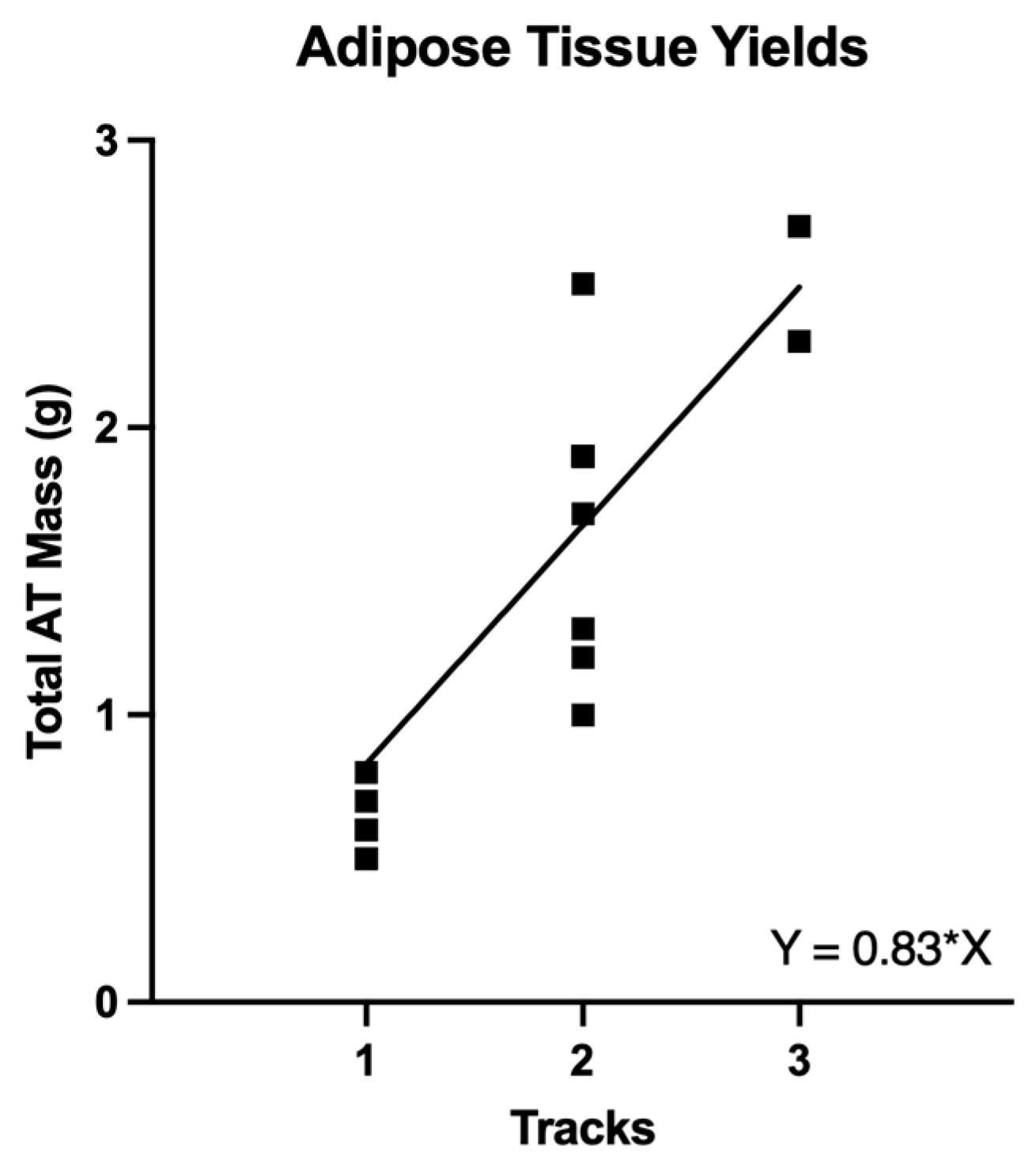
| Separate Tracks per Event | 1.8 ± 0.2 | 2 (1, 3) | |
| Total Mass per Event (g) | 1.44 ± 0.2 | 1.5 (0.5, 2.7) | |
| Adipose Processing of Initial Biopsy Set (n = 6) | |||
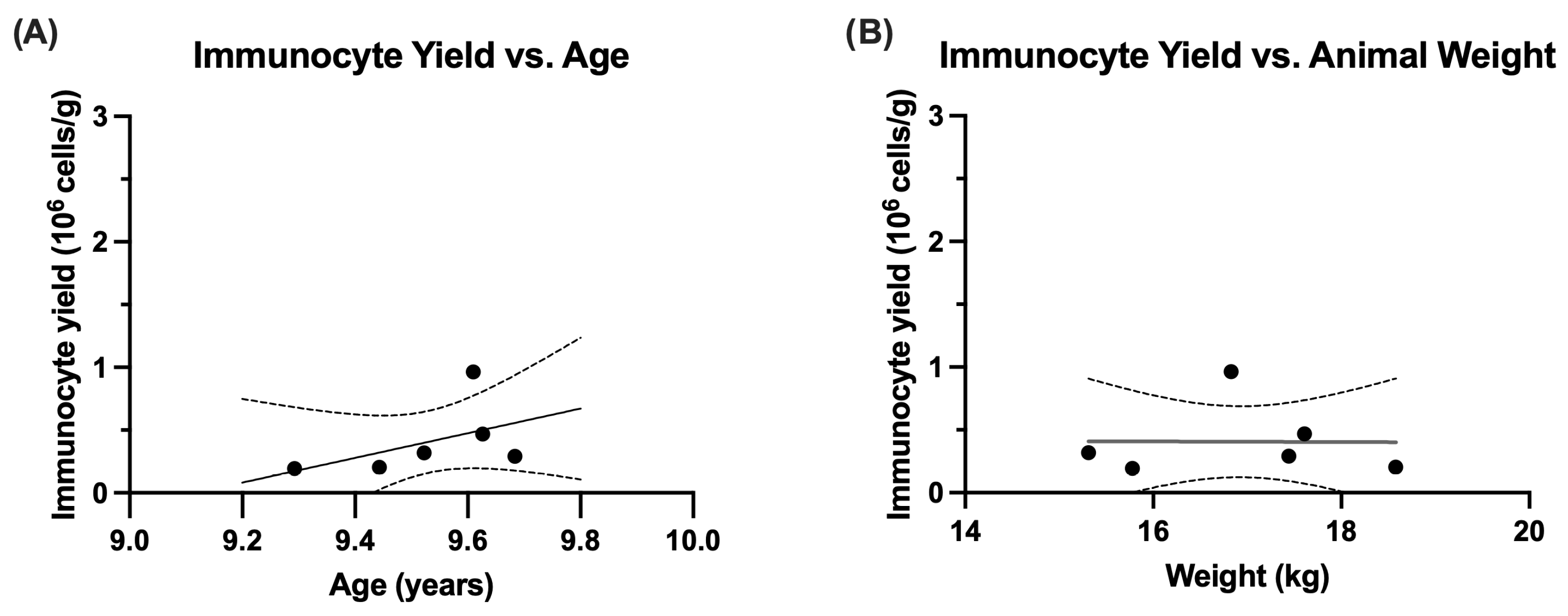
| AT Immunocyte Yield (106 cells/g) | 0.41 ± 0.12 | 0.30 (0.19, 0.96) | |
| Biopsies | Mean ± SEM | Median (Min, Max) | |
|---|---|---|---|
| Single Track | n = 6 | 0.62 ± 0.05 g | 0.6 (0.5, 0.8) |
| Two Tracks | n = 10 | 1.7 ± 0.14 g | 1.9 (1.0, 2.5) |
| Three Tracks | n = 2 | 2.6 ± 0.2 g | 2.5 (2.3, 2.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, C.M.; Myers, C.; Oppler, S.H.; Hocum Stone, L.; Seelig, D.; Rangarajan, P.; Ramachandran, S.; Graham, M.L. Minimally Invasive Subcutaneous Adipose Tissue Biopsy in a Nonhuman Primate Model: Approach and Outcomes. Surgeries 2025, 6, 106. https://doi.org/10.3390/surgeries6040106
Espinoza CM, Myers C, Oppler SH, Hocum Stone L, Seelig D, Rangarajan P, Ramachandran S, Graham ML. Minimally Invasive Subcutaneous Adipose Tissue Biopsy in a Nonhuman Primate Model: Approach and Outcomes. Surgeries. 2025; 6(4):106. https://doi.org/10.3390/surgeries6040106
Chicago/Turabian StyleEspinoza, Cheyenna M., Cole Myers, Scott H. Oppler, Laura Hocum Stone, Davis Seelig, Parthasarathy Rangarajan, Sabarinathan Ramachandran, and Melanie L. Graham. 2025. "Minimally Invasive Subcutaneous Adipose Tissue Biopsy in a Nonhuman Primate Model: Approach and Outcomes" Surgeries 6, no. 4: 106. https://doi.org/10.3390/surgeries6040106
APA StyleEspinoza, C. M., Myers, C., Oppler, S. H., Hocum Stone, L., Seelig, D., Rangarajan, P., Ramachandran, S., & Graham, M. L. (2025). Minimally Invasive Subcutaneous Adipose Tissue Biopsy in a Nonhuman Primate Model: Approach and Outcomes. Surgeries, 6(4), 106. https://doi.org/10.3390/surgeries6040106







