Post-Surgical Outcomes of Kidney-Sparing Surgery vs. Radical Nephroureterectomy for Upper-Tract Urothelial Cancer in a Propensity-Weighted Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort and Ethics
2.2. Baseline Data
2.3. Outcomes and Follow-Up
2.4. Endpoints
2.5. Risk Score
2.6. Statistics
3. Results
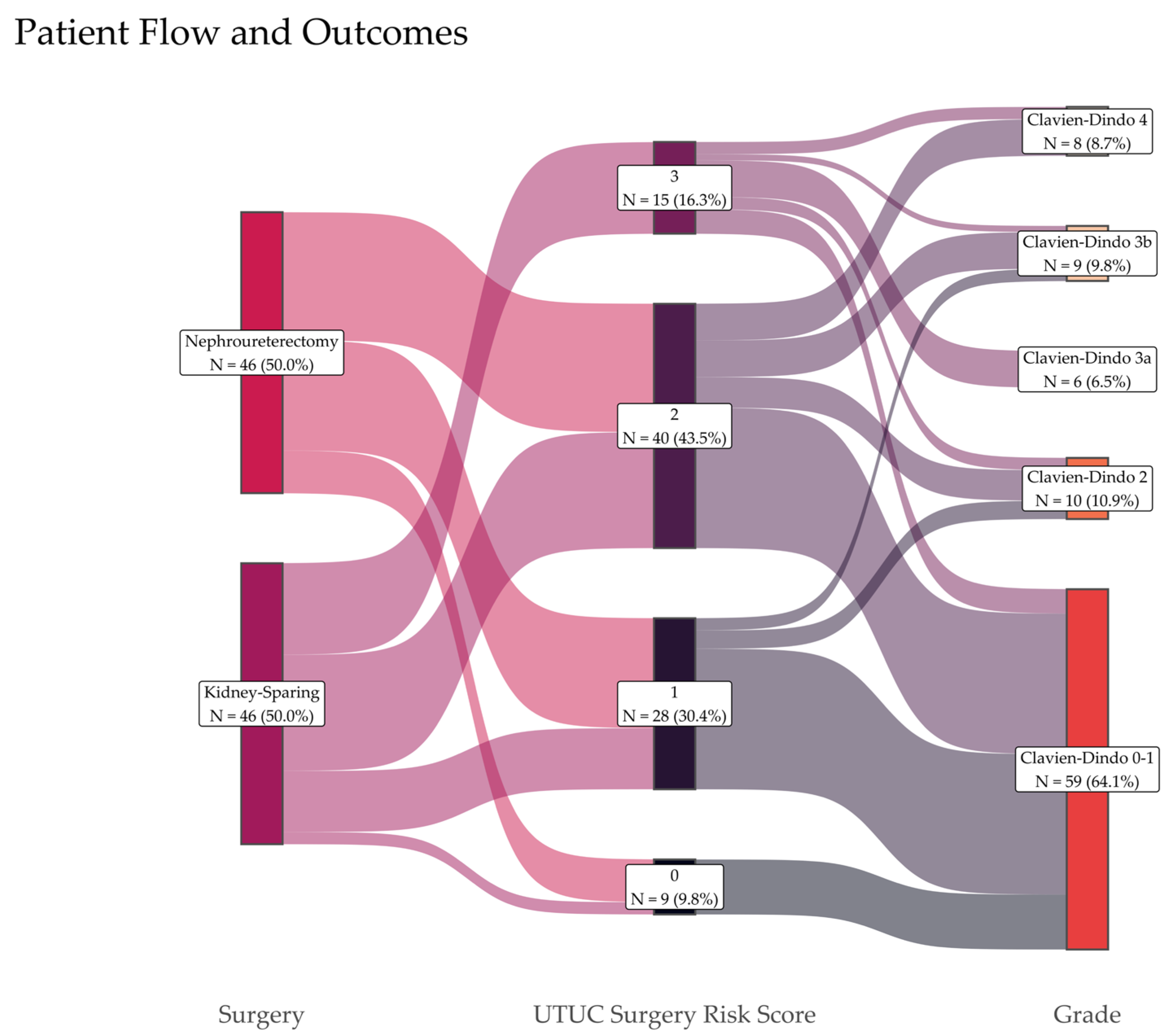
3.1. Surgical Complications and Risk Score
3.2. Kidney Function
3.3. Survival Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists Physical Status Classification System |
| AUA | American Urological Association |
| CI | Confidence Interval |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| CSS | Cancer-Specific Survival |
| CT | Computed Tomography |
| DAOH30 | Days Alive and Out of the Hospital within 30 days |
| DFS | Disease-Free Survival |
| EAU | European Association of Urology |
| eGFR | Estimated Glomerular Filtration Rate |
| GLM | Generalized Linear Model |
| KSS | Kidney-Sparing Surgery |
| NR | Not Reached |
| RCC | Renal Cell Carcinoma |
| RNU | Radical Nephroureterectomy |
| UTUC | Upper-Tract Urothelial Cancer |
References
- Collà Ruvolo, C.; Nocera, L.; Stolzenbach, L.F.; Wenzel, M.; Cucchiara, V.; Tian, Z.; Shariat, S.F.; Saad, F.; Longo, N.; Montorsi, F.; et al. Incidence and Survival Rates of Contemporary Patients with Invasive Upper Tract Urothelial Carcinoma. Eur. Urol. Oncol. 2021, 4, 792–801. [Google Scholar] [CrossRef]
- Soria, F.; Shariat, S.F.; Lerner, S.P.; Fritsche, H.-M.; Rink, M.; Kassouf, W.; Spiess, P.E.; Lotan, Y.; Ye, D.; Fernández, M.I.; et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 379–387. [Google Scholar] [CrossRef]
- Rouprêt, M.; Babjuk, M.; Compérat, E.; Zigeuner, R.; Sylvester, R.J.; Burger, M.; Cowan, N.C.; Gontero, P.; Van Rhijn, B.W.G.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur. Urol. 2018, 73, 111–122. [Google Scholar] [CrossRef]
- Schuil, H.W.; Figaroa, O.J.A.; Hendriks, N.; Schout, B.M.A.; Beerlage, H.P.; van Jamaludin, F.S.; Henderickx, M.M.E.L.; van Moorselaar, R.J.A.; Kamphuis, G.M.; Baard, J. Navigating the Aftermath: A Comprehensive Scoping Review on Follow-up Strategies After Kidney-sparing Surgery for Upper Tract Urothelial Carcinoma. Eur. Urol. Open Sci. 2024, 66, 82–92. [Google Scholar] [CrossRef]
- Coleman, J.A.; Clark, P.E.; Bixler, B.R.; Buckley, D.I.; Chang, S.S.; Chou, R.; Hoffman-Censits, J.; Kulkarni, G.S.; Matin, S.F.; Pierorazio, P.M.; et al. Diagnosis and Management of Non-Metastatic Upper Tract Urothelial Carcinoma: AUA/SUO Guideline. J. Urol. 2023, 209, 1071–1081. [Google Scholar] [CrossRef]
- Cutress, M.L.; Stewart, G.D.; Zakikhani, P.; Phipps, S.; Thomas, B.G.; Tolley, D.A. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): Systematic review. BJU Int. 2012, 110, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Giulioni, C.; Brocca, C.; Tramanzoli, P.; Stramucci, S.; Mantovan, M.; Perpepaj, L.; Cicconofri, A.; Gauhar, V.; Merseburger, A.S.; Galosi, A.B.; et al. Endoscopic intervention versus radical nephroureterectomy for the management of localized upper urinary tract urothelial carcinoma: A systematic review and meta-analysis of comparative studies. World J. Urol. 2024, 42, 318. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, N.; Baard, J.; Beerlage, H.P.; Schout, B.M.A.; Doherty, K.S.G.; Pelger, R.C.M.; Kamphuis, G.M. Survival and Long-term Effects of Kidney-sparing Surgery Versus Radical Nephroureterectomy on Kidney Function in Patients with Upper Urinary Tract Urothelial Carcinoma. Eur. Urol. Open Sci. 2022, 40, 104–111. [Google Scholar] [CrossRef]
- Seisen, T.; Peyronnet, B.; Dominguez-Escrig, J.L.; Bruins, H.M.; Yuan, C.Y.; Babjuk, M.; Böhle, A.; Burger, M.; Compérat, E.M.; Cowan, N.C.; et al. Oncologic Outcomes of Kidney-sparing Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-muscle Invasive Bladder Cancer Guidelines Panel. Eur. Urol. 2016, 70, 1052–1068. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Peng, Y.; Ding, S.; Zheng, Y.; He, Y.; Liu, J. Kidney-sparing surgery for distal high-risk ureteral carcinoma: Clinical efficacy and preliminary experiences in 22 patients. Cancer Med. 2023, 12, 7835–7843. [Google Scholar] [CrossRef]
- Kim, D.; You, D.; Jeong, I.G.; Hong, J.H.; Ahn, H.; Hong, B. Kidney sparing surgery in upper tract urothelial carcinoma: Paradigm change in surgical treatment for ureter cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 13717–13725. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, H.; Ng, C.F.; Teoh, J.Y.C.; Laguna, P.; de la Rosette, J. Kidney-Sparing Surgery Has Equivalent Oncological Outcomes to Radical Nephroureterectomy for Ureteral Urothelial Carcinoma. J. Endourol. 2024, 38, 921–928. [Google Scholar] [CrossRef]
- Ślusarczyk, A.; Zapała, P.; Zapała, Ł.; Rajwa, P.; Moschini, M.; Laukhtina, E.; Radziszewski, P. Oncologic outcomes of patients treated with kidney-sparing surgery or radical nephroureterectomy for upper urinary tract urothelial cancer: A population-based study. Urol. Oncol. 2024, 42, 22.e1–22.e11. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Myles, P.S.; Shulman, M.A.; Heritier, S.; Wallace, S.; McIlroy, D.R.; McCluskey, S.; Sillar, I.; Forbes, A. Validation of days at home as an outcome measure after surgery: A prospective cohort study in Australia. BMJ Open 2017, 7, e015828. [Google Scholar] [CrossRef]
- Dalpiaz, O.; Ehrlich, G.; Quehenberger, F.; Pummer, K.; Zigeuner, R. Distal ureterectomy is a safe surgical option in patients with urothelial carcinoma of the distal ureter. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 34.e1–34.e8. [Google Scholar] [CrossRef] [PubMed]
- Abrate, A.; Sessa, F.; Sessa, M.; Campi, R.; Sebastianelli, A.; Varca, V.; Pavone, C.; Vella, M.; Bartoletti, R.; Ficarra, V.; et al. Segmental Ureterectomy Versus Radical Nephroureterectomy in Older Patients Treated for Upper Tract Urothelial Carcinoma. Clin. Genitourin. Cancer 2022, 20, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Campi, R.; Cotte, J.; Sessa, F.; Seisen, T.; Tellini, R.; Amparore, D.; Mormile, N.; Gobert, A.; Mari, A.; Porpiglia, F.; et al. Robotic radical nephroureterectomy and segmental ureterectomy for upper tract urothelial carcinoma: A multi-institutional experience. World J. Urol. 2019, 37, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Candela, L.; Ventimiglia, E.; Solano, C.; Chicaud, M.; Kutchukian, S.; Panthier, F.; Corrales, M.; Villa, L.; Briganti, A.; Montorsi, F.; et al. Endoscopic Conservative Treatment of Upper Urinary Tract Urothelial Carcinoma with a Thulium Laser: A Systematic Review. J. Clin. Med. 2023, 12, 4907. [Google Scholar] [CrossRef]
- Kawada, T.; Laukhtina, E.; Quhal, F.; Yanagisawa, T.; Rajwa, P.; Pallauf, M.; von Deimling, M.; Bianchi, A.; Pradere, B.; Fajkovic, H.; et al. Oncologic and Safety Outcomes for Endoscopic Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: An Updated Systematic Review and Meta-analysis. Eur. Urol. Focus 2023, 9, 236–240. [Google Scholar] [CrossRef]
- Saini, S.; Deveshwar, S.P.; Hemal, A.K. Narrative review of nephron-sparing surgical management of upper tract urothelial carcinoma: Is there a role for distal ureterectomy, segmental ureterectomy, and partial nephrectomy. Transl. Androl. Urol. 2024, 13, 156–164. [Google Scholar] [CrossRef]
- Bizzarri, F.P.; Campetella, M.; Russo, P.; Marino, F.; Gavi, F.; Rossi, F.; Foschi, N.; Sacco, E. Risk factors for benign uretero-enteric anastomotic strictures after open radical cystectomy and ileal conduit. Urol. J. 2025, 92, 224–230. [Google Scholar] [CrossRef]
- Kutikov, A.; Uzzo, R.G. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Jerath, A.; Austin, P.C.; Wijeysundera, D.N. Days Alive and Out of Hospital: Validation of a Patient-centered Outcome for Perioperative Medicine. Anesthesiology 2019, 131, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Shim, J.-K.; Kim, E.H.; Song, J.W.; Soh, S.; Kwak, Y.-L. Association between comprehensive geriatric assessment and Days Alive and Out of Hospital at 30 Days After Cardiac Surgery in Older Patients. J. Nutr. Health Aging 2025, 29, 100490. [Google Scholar] [CrossRef] [PubMed]
- Moonesinghe, S.R.; Jackson, A.I.R.; Boney, O.; Stevenson, N.; Chan, M.T.V.; Cook, T.M.; Lane-Fall, M.; Kalkman, C.; Neuman, M.D.; Nilsson, U.; et al. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine initiative: Patient-centred outcomes. Br. J. Anaesth. 2019, 123, 664–670. [Google Scholar] [CrossRef]
- Fang, D.; Seisen, T.; Yang, K.; Liu, P.; Fan, X.; Singla, N.; Xiong, G.; Zhang, L.; Li, X.; Zhou, L. A systematic review and meta-analysis of oncological and renal function outcomes obtained after segmental ureterectomy versus radical nephroureterectomy for upper tract urothelial carcinoma. Eur. J. Surg. Oncol. 2016, 42, 1625–1635. [Google Scholar] [CrossRef]
- Kates, M.; Badalato, G.M.; Pitman, M.; McKiernan, J.M. Increased risk of overall and cardiovascular mortality after radical nephrectomy for renal cell carcinoma 2 cm or less. J. Urol. 2011, 186, 1247–1253. [Google Scholar] [CrossRef]

| Parameter | RNU n = 46 | KSS n = 46 | Overall n = 92 | p-Value 1 |
|---|---|---|---|---|
| Age (years) | 0.867 | |||
| Median [Range] | 69.2 [43.8, 87.9] | 69.8 [40.6, 93.2] | 69.5 [40.6, 93.2] | |
| Sex (n, %) | 1.000 | |||
| Female | 15 (32.6%) | 15 (32.6%) | 30 (32.6%) | |
| Male | 31 (67.4%) | 31 (67.4%) | 62 (67.4%) | |
| Localization (n, %) | 1.000 | |||
| Renal Pelvis | 15 (32.6%) | 14 (30.4%) | 29 (31.5%) | |
| Ureter | 31 (67.4%) | 32 (69.6%) | 63 (68.5%) | |
| Side (n, %) | 1.000 | |||
| Right | 24 (52%) | 23 (50%) | 47 (51%) | |
| Left | 22 (48%) | 23 (50%) | 45 (49%) | |
| EAU Risk Group (n, %) | 0.479 | |||
| High Risk | 10 (22%) | 14 (30%) | 24 (26%) | |
| Low Risk | 36 (78%) | 32 (70%) | 68 (74%) | |
| ASA Score (n, %) | 0.197 | |||
| 1 | 3 (7%) | 0 (0%) | 3 (3%) | |
| 2 | 20 (43%) | 25 (54%) | 45 (49%) | |
| 3 | 23 (50%) | 21 (46%) | 44 (48%) | |
| eGFR (mL/min) | 0.991 | |||
| Median [Range] | 56 [10, 103] | 58 [14, 109] | 57 [10, 109] | |
| Type of Surgery | NA | |||
| Radical Nephroureterectomy | 46 (100%) | -- | 46 (50%) | |
| Segmental Ureterectomy | -- | 26 (56%) | 26 (28%) | |
| Partial Nephrectomy/Pyeloplasty | -- | 10 (212%) | 10 (11%) | |
| Ureterorenoscopy | -- | 10 (22%) | 10 (11%) |
| Outcome | RNU n = 46 | KSS n = 46 | Overall n = 92 | p-Value 1 |
|---|---|---|---|---|
| Clavien–Dindo grade (n, %) | 0.005 ** | |||
| 0–1 | 34 (74%) | 25 (54%) | 59 (64%) | |
| 2 | 6 (13%) | 4 (9%) | 10 (11%) | |
| 3a | 0 (0%) | 6 (13%) | 6 (7%) | |
| 3b | 1 (2%) | 8 (17%) | 9 (9%) | |
| 4a | 5 (11%) | 3 (7%) | 8 (9%) | |
| DAOH30 (days) | 0.011 * | |||
| Median [range] | 23 [0–28] | 16 [0–20] | 20 [0–29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büttner, T.; Pooyeh, A.; Ritter, M.; Hauser, S. Post-Surgical Outcomes of Kidney-Sparing Surgery vs. Radical Nephroureterectomy for Upper-Tract Urothelial Cancer in a Propensity-Weighted Cohort. Surgeries 2025, 6, 71. https://doi.org/10.3390/surgeries6030071
Büttner T, Pooyeh A, Ritter M, Hauser S. Post-Surgical Outcomes of Kidney-Sparing Surgery vs. Radical Nephroureterectomy for Upper-Tract Urothelial Cancer in a Propensity-Weighted Cohort. Surgeries. 2025; 6(3):71. https://doi.org/10.3390/surgeries6030071
Chicago/Turabian StyleBüttner, Thomas, Armin Pooyeh, Manuel Ritter, and Stefan Hauser. 2025. "Post-Surgical Outcomes of Kidney-Sparing Surgery vs. Radical Nephroureterectomy for Upper-Tract Urothelial Cancer in a Propensity-Weighted Cohort" Surgeries 6, no. 3: 71. https://doi.org/10.3390/surgeries6030071
APA StyleBüttner, T., Pooyeh, A., Ritter, M., & Hauser, S. (2025). Post-Surgical Outcomes of Kidney-Sparing Surgery vs. Radical Nephroureterectomy for Upper-Tract Urothelial Cancer in a Propensity-Weighted Cohort. Surgeries, 6(3), 71. https://doi.org/10.3390/surgeries6030071






