High Fusion Rates with Structured Titanium TLIF Cages: A Retrospective 1-Year Study with and Without Adjacent Level Dynamic Stabilization
Abstract
1. Introduction
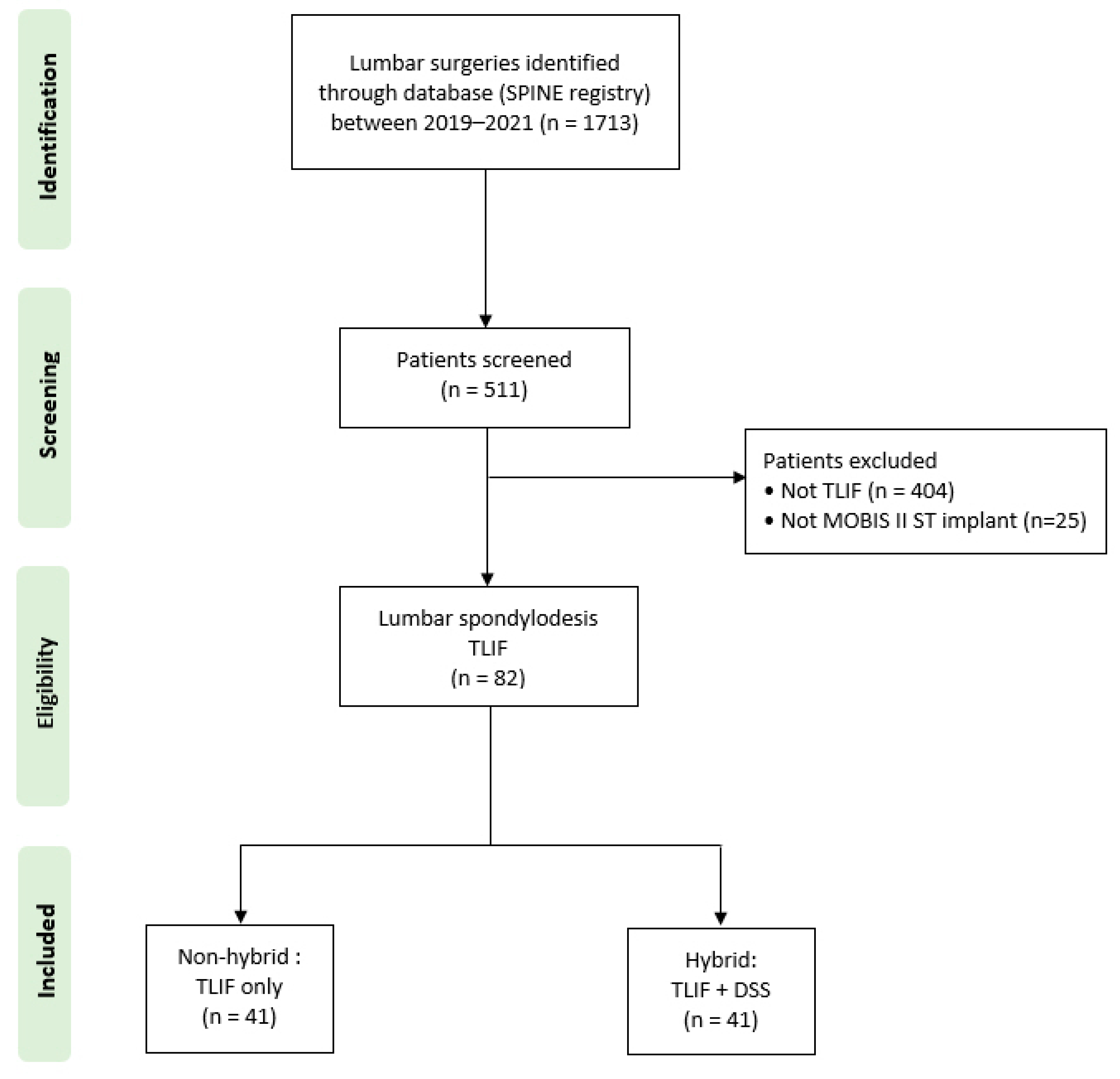
2. Materials and Methods
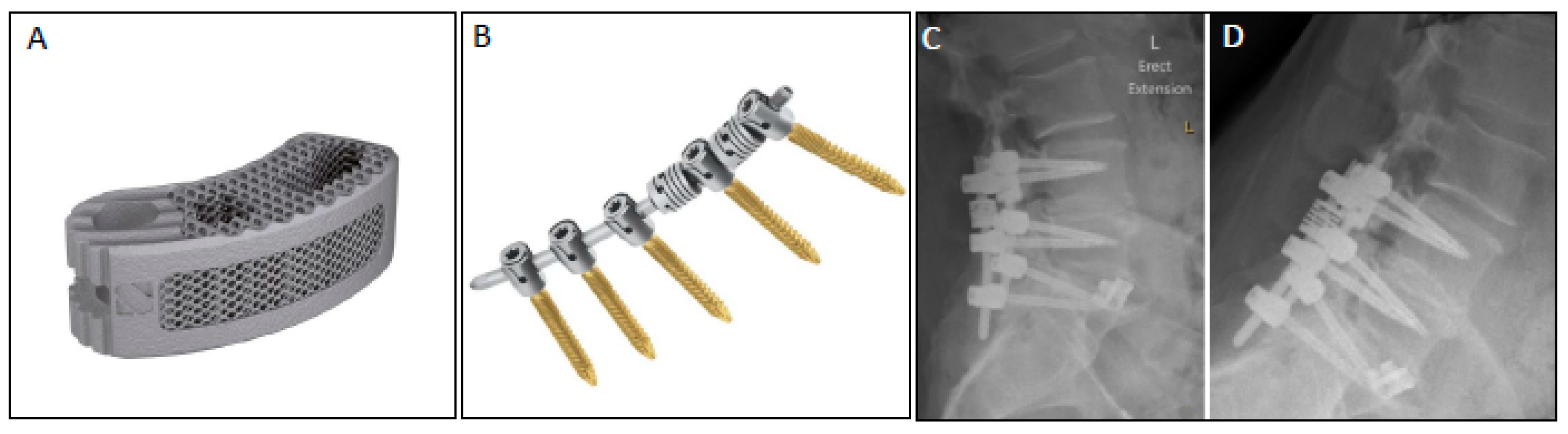
2.1. Surgical Technique
2.2. Data Collection
2.3. Radiographic Outcomes:
2.4. Statistical Analyses
3. Results
3.1. Surgical Characteristics and Complications in Non-Hybrid Versus Hybrid Stabilization
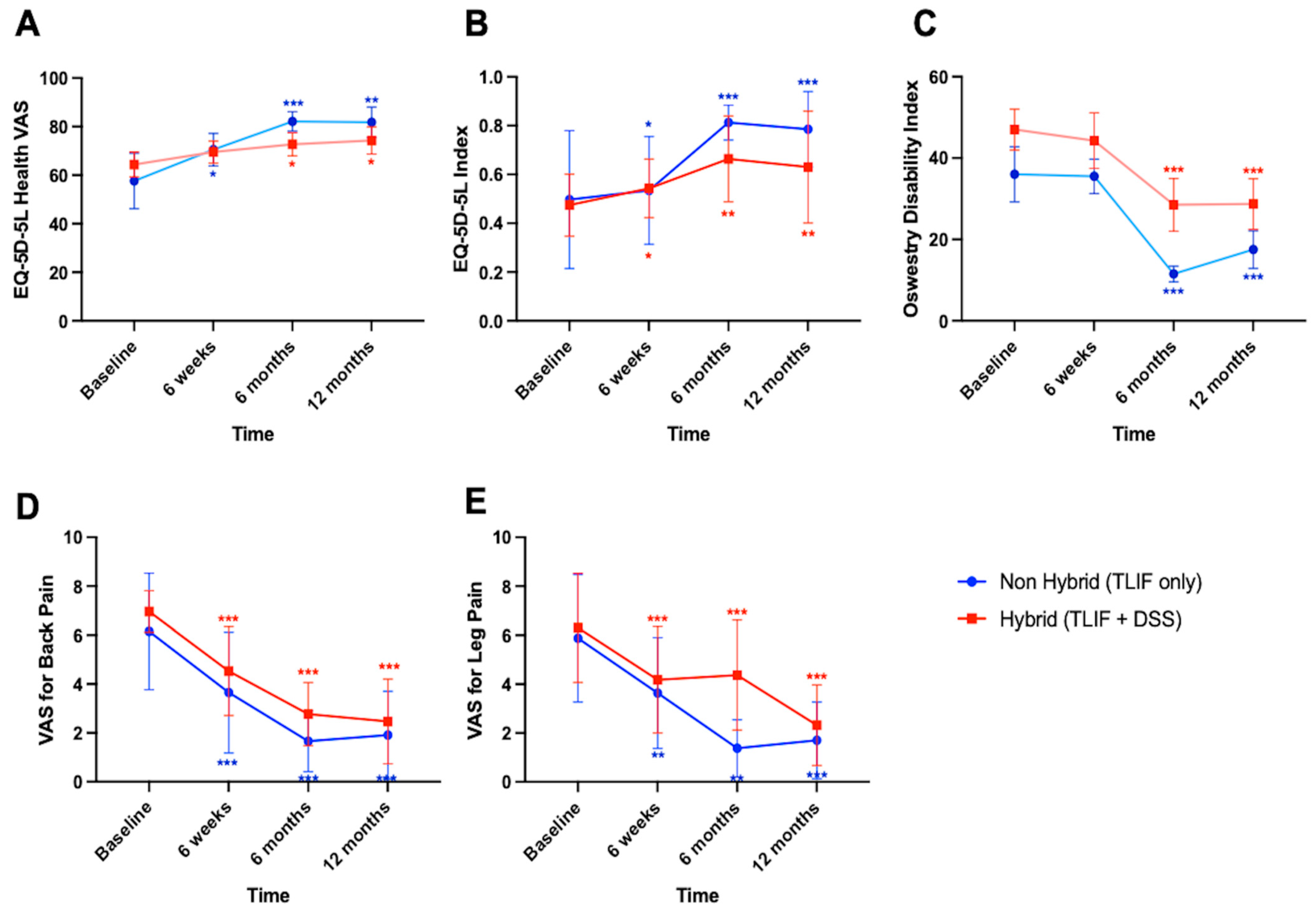
3.2. Significant Clinical Improvements over Time, Independent of Stabilization Technique
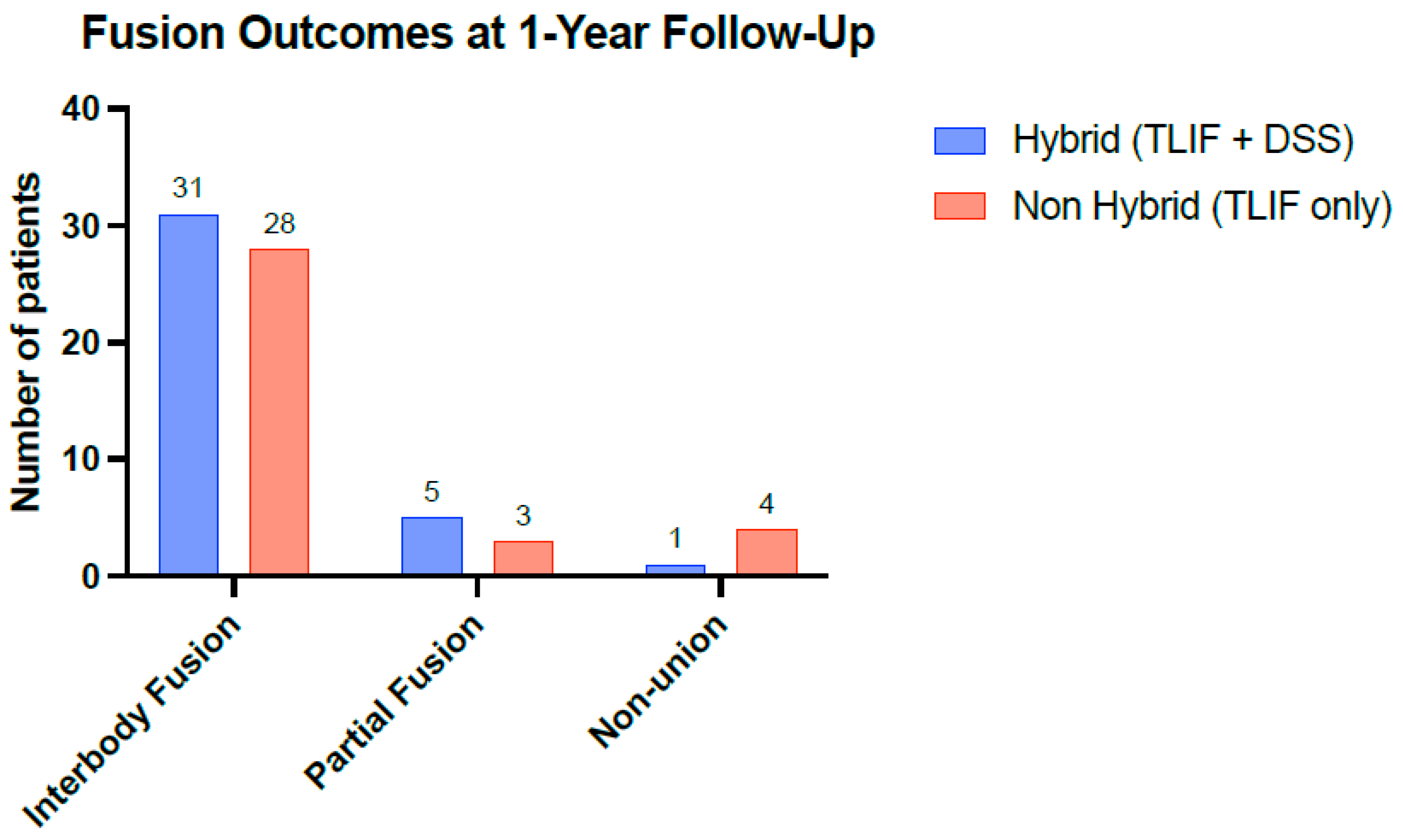
3.3. No Differences in Fusion Rates Between Stabilization Techniques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Adjacent Segment Disease |
| BMI | Body Mass Index |
| CT | Computed Tomography |
| DSS | Dynamic Stabilization System |
| HPS | Hybrid Performance System |
| HRQOL | Health-Related Quality of Life |
| IQR | Interquartile Range |
| MDPI | Multidisciplinary Digital Publishing Institute |
| ODI | Oswestry Disability Index |
| PLIF | Posterior Lumbar Interbody Fusion |
| PROMs | Patient-Reported Outcome Measures |
| PEEK | Polyetheretherketone |
| ST cage | Structured Titanium Cage |
| TLIF | Transforaminal Lumbar Interbody Fusion |
| VAS | Visual Analogue Scale |
References
- Reisener, M.J.; Pumberger, M.; Shue, J.; Girardi, F.P.; Hughes, A.P. Trends in lumbar spinal fusion—A literature review. J. Spine Surg. 2020, 6, 752. [Google Scholar] [CrossRef] [PubMed]
- Malvin Brent, W. Posterolateral Fusion of the Lumbar and Lumbosacral Spine. J. Bone Jt. Surg. 1953, 35, 1014–1018. Available online: https://journals.lww.com/jbjsjournal/Citation/1953/35040/POSTEROLATERAL_FUSION_OF_THE_LUMBAR_AND.24.aspx (accessed on 27 November 2022).
- Harms, J.G.; Jeszenszky, D. Die posteriore; lumbale, interkorporelle Fusion in unilateraler transforaminaler Technik. Oper. Orthop. Traumatol. 1998, 10, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Wasinpongwanich, K.; Nopsopon, T.; Pongpirul, K. Surgical Treatments for Lumbar Spine Diseases (TLIF vs. Other Surgical Techniques): A Systematic Review and Meta-Analysis. Front. Surg. 2022, 9, 829469. [Google Scholar] [CrossRef]
- Levin, J.M.; Tanenbaum, J.E.; Steinmetz, M.P.; Mroz, T.E.; Overley, S.C. Posterolateral fusion (PLF) versus transforaminal lumbar interbody fusion (TLIF) for spondylolisthesis: A systematic review and meta-analysis. Spine J. 2018, 18, 1088–1098. [Google Scholar] [CrossRef]
- Nurmukhametov, R.; Dosanov, M.; Encarnacion, M.D.J.; Barrientos, R.; Matos, Y.; Alyokhin, A.I.; Baez, I.P.; Efe, I.E.; Restrepo, M.; Chavda, V.; et al. Transforaminal Fusion Using Physiologically Integrated Titanium Cages with a Novel Design in Patients with Degenerative Spinal Disorders: A Pilot Study. Surgeries 2022, 3, 175–184. [Google Scholar] [CrossRef]
- Nemoto, O.; Asazuma, T.; Yato, Y.; Imabayashi, H.; Yasuoka, H.; Fujikawa, A. Comparison of fusion rates following transforaminal lumbar interbody fusion using polyetheretherketone cages or titanium cages with transpedicular instrumentation. Eur. Spine J. 2014, 23, 2150–2155. [Google Scholar] [CrossRef]
- Lee, J.J.; Jacome, F.P.; Hiltzik, D.M.; Pagadala, M.S.; Hsu, W.K. Evolution of Titanium Interbody Cages and Current Uses of 3D Printed Titanium in Spine Fusion Surgery. Curr. Rev. Musculoskelet. Med. 2024, 7, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Zhang, Z.; Miao, J. The postoperative clinical effects of utilizing 3D printed (Ti6Al4V) interbody fusion cages in posterior lumbar fusion: A retrospective cohort study. Medicine 2024, 103, e38431. [Google Scholar] [CrossRef]
- Amini, D.A.; Moser, M.; Oezel, L.; Shue, J.; Pumberger, M.; Sama, A.A.; Cammisa, F.P.; Girardi, F.P.; Hughes, A.P. Fusion assessment in standalone lateral lumbar interbody fusion: 3D-printed titanium versus polyetheretherketone (PEEK) cages. J. Spine Surg. 2022, 8, 323–332. [Google Scholar] [CrossRef]
- Chiou, K.; Chiu, Y.-C.; Lee, C.-Y.; Huang, T.-J.; Lai, Y.-C.; Yang, C.-J.; Hsu, J.C.; Wu, M.-H. Comparison of long-term outcomes of spinal fusion surgeries supplemented with “topping-off” implants in lumbar degenerative diseases: A systematic review and network meta-analysis. N. Am. Spine Soc. J. (NASSJ) 2022, 12, 100177. [Google Scholar] [CrossRef] [PubMed]
- Duits, A.A.; van Urk, P.R.; Lehr, A.M.; Nutzinger, D.; Reijnders, M.R.; Weinans, H.; Foppen, W.; Oner, F.C.; van Gaalen, S.M.; Kruyt, M.C. Radiologic Assessment of Interbody Fusion: A Systematic Review on the Use, Reliability, and Accuracy of Current Fusion Criteria. JBJS Rev. 2024, 12, e23.00065. [Google Scholar] [CrossRef]
- Wu, S.-H.; Li, Y.; Zhang, Y.-Q.; Li, X.-K.; Yuan, C.-F.; Hao, Y.-L.; Zhang, Z.-Y.; Guo, Z. Porous titanium-6 aluminum-4 vanadium cage has better osseointegration and less micromotion than a poly-ether-ether-ketone cage in sheep vertebral fusion. Artif. Organs 2013, 37, E191–E201. [Google Scholar] [CrossRef]
- Park, P.J.; Lehman, R.A. Optimizing the Spinal Interbody Implant: Current Advances in Material Modification and Surface Treatment Technologies. Curr. Rev. Musculoskelet. Med. 2020, 13, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.-E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. BioMed Res. Int. 2016, 2016, 2908570. [Google Scholar] [CrossRef]
- Patel, N.A.; O’bRyant, S.; Rogers, C.D.; Boyett, C.K.; Chakravarti, S.; Gendreau, J.; Brown, N.J.; Pennington, Z.A.; Hatcher, N.B.; Kuo, C.; et al. Three-Dimensional-Printed Titanium Versus Polyetheretherketone Cages for Lumbar Interbody Fusion: A Systematic Review of Comparative In Vitro, Animal, and Human Studies. Neurospine 2023, 20, 451–463. [Google Scholar] [CrossRef]
- Khoueir, P.; Kim, K.A.; Wang, M.Y. Classification of posterior dynamic stabilization devices. Neurosurg. Focus 2007, 22, 1–8. [Google Scholar] [CrossRef]
- Wilke, H.-J.; Heuer, F.; Schmidt, H. Prospective design delineation and subsequent in vitro evaluation of a new posterior dynamic stabilization system. Spine 2009, 34, 255–261. [Google Scholar] [CrossRef]
- Mulholland, R.C.; Sengupta, D.K. Rationale, principles and experimental evaluation of the concept of soft stabilization. Eur. Spine J. 2002, 11, S198–S205. [Google Scholar] [CrossRef]
- Sun, X.; Sun, X.; Zhang, T.; Sun, S.; Kong, C.; Ding, J.; Li, X.; Lu, S. Dynamic Stabilization Adjacent to Fusion versus Posterior Lumbar Interbody Fusion for the Treatment of Lumbar Degenerative Disease: A Meta-Analysis. BioMed Res. Int. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Z.; Sun, S.; Wang, W.; Zhang, T.; Kong, C.; Lu, S.; Daamen, W.F. Comparison between topping-off technology and posterior lumbar interbody fusion in the treatment of chronic low back pain: A meta-analysis. Medicine 2020, 99, E18885. [Google Scholar] [CrossRef] [PubMed]
- Fuster, S.; Martínez-Anda, J.J.; Castillo-Rivera, S.A.; Vargas-Reverón, C.; Tornero, E. Dynamic Fixation Techniques for the Prevention of Adjacent Segment Disease: A Retrospective Controlled Study. Asian Spine J. 2022, 16, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Sears, W.R.; Solterbeck, A.C.; Kos, J.A. Risk of adjacent segment disease after “topping-off” multi-level lumbar fusions with posterior dynamic stabilisers: An observational cohort study. Eur. Spine J. 2021, 30, 181–190. [Google Scholar] [CrossRef]
- Donnally, C.J.; Patel, P.D.; Canseco, J.A.; Divi, S.N.; Goz, V.; Sherman, M.B.; Shenoy, K.; Markowitz, M.; Rihn, J.A.; Vaccaro, A.R. Current incidence of adjacent segment pathology following lumbar fusion versus motion-preserving procedures: A systematic review and meta-analysis of recent projections. Spine J. 2020, 20, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total n = 82 | Non-Hybrid (TLIF Only) n = 41 | Hybrid (TLIF + DSS) n = 41 | p (Fisher’s Exact) |
|---|---|---|---|---|
| Age (years), median (IQR) | 62.1 (17.6) | 58.2 (18.9) | 65.2 (10.5) | 0.009 a |
| Body mass index a, median (IQR) | 29.4 (7.5) | 28.7 (9.2) | 30.4 (6.2) | 0.14 a |
| Female, n (%) | 47 (57) | 21 (51) | 26 (63) | 0.37 |
| Current smoker, n (%) b | 12 (15) | 7 (17) | 5 (12) | 0.55 |
| Radicular deficit, n (%) | 28 (34) | 17 (41) | 11 (27) | 0.24 |
| Surgical Indications, n (%) | 0.04 | |||
| Radiculopathy | 49 (60) | 30 (73) | 19 (46) | |
| Claudication | 21 (26) | 6 (15) | 15 (37) | |
| Low Back pain | 9 (11) | 3 (7) | 6 (15) | |
| Spondylolisthesis | 3 (4) | 2 (5) | 1 (2) | |
| Surgery type, n (%) | 0.04 | |||
| Primary | 67 (82) | 30 (71) | 37 (90) | |
| Re-operation (same level, decompression or nucleotomy) | 15 (17) | 11 (27) | 3 (7) | |
| Surgery duration (min), median (IQR) | 180 (90) | 120 (60) | 180 (60) | <0.0001 a |
| Estimated blood loss (mL), median (IQR) | 500 (300) | 400 (200) | 500 (400) | 0.08 a |
| Post-operative complications, n (%) | 30 (37) | 13 (32) | 17 (41) | 0.49 |
| Superficial infection | 5 (6) | 2 (5) | 3 (7) | |
| Complicated superficial infection | 10 (12) | 3 (7) | 7 (17) | |
| Infection requiring surgical debridement | 6 (7) | 5 (12) | 1 (2) | |
| Extruded graft requiring revision | 2 (2) | 0 | 2 (5) | |
| Repair Cerebrospinal Fluid leak | 1 (1) | 1 (2) | 0 | |
| Screw loosening | 1 (1) | 0 | 1 (2) | |
| Screw malpositioning requiring revision | 1 (1) | 0 | 1 (2) | |
| Adjacent level surgery | 1 (1) | 1 (2) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Häckel, S.; Gaff, J.; Celenza, A.; Cunningham, G.; Kern, M.; Taylor, P.; Miles, A. High Fusion Rates with Structured Titanium TLIF Cages: A Retrospective 1-Year Study with and Without Adjacent Level Dynamic Stabilization. Surgeries 2025, 6, 52. https://doi.org/10.3390/surgeries6030052
Häckel S, Gaff J, Celenza A, Cunningham G, Kern M, Taylor P, Miles A. High Fusion Rates with Structured Titanium TLIF Cages: A Retrospective 1-Year Study with and Without Adjacent Level Dynamic Stabilization. Surgeries. 2025; 6(3):52. https://doi.org/10.3390/surgeries6030052
Chicago/Turabian StyleHäckel, Sonja, Jessica Gaff, Alana Celenza, Gregory Cunningham, Michael Kern, Paul Taylor, and Andrew Miles. 2025. "High Fusion Rates with Structured Titanium TLIF Cages: A Retrospective 1-Year Study with and Without Adjacent Level Dynamic Stabilization" Surgeries 6, no. 3: 52. https://doi.org/10.3390/surgeries6030052
APA StyleHäckel, S., Gaff, J., Celenza, A., Cunningham, G., Kern, M., Taylor, P., & Miles, A. (2025). High Fusion Rates with Structured Titanium TLIF Cages: A Retrospective 1-Year Study with and Without Adjacent Level Dynamic Stabilization. Surgeries, 6(3), 52. https://doi.org/10.3390/surgeries6030052






