Association of Preoperative Parameters on Intraoperative Indicators in Myocardial Revascularization Surgery: Insights from a Targeted Complex Network Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
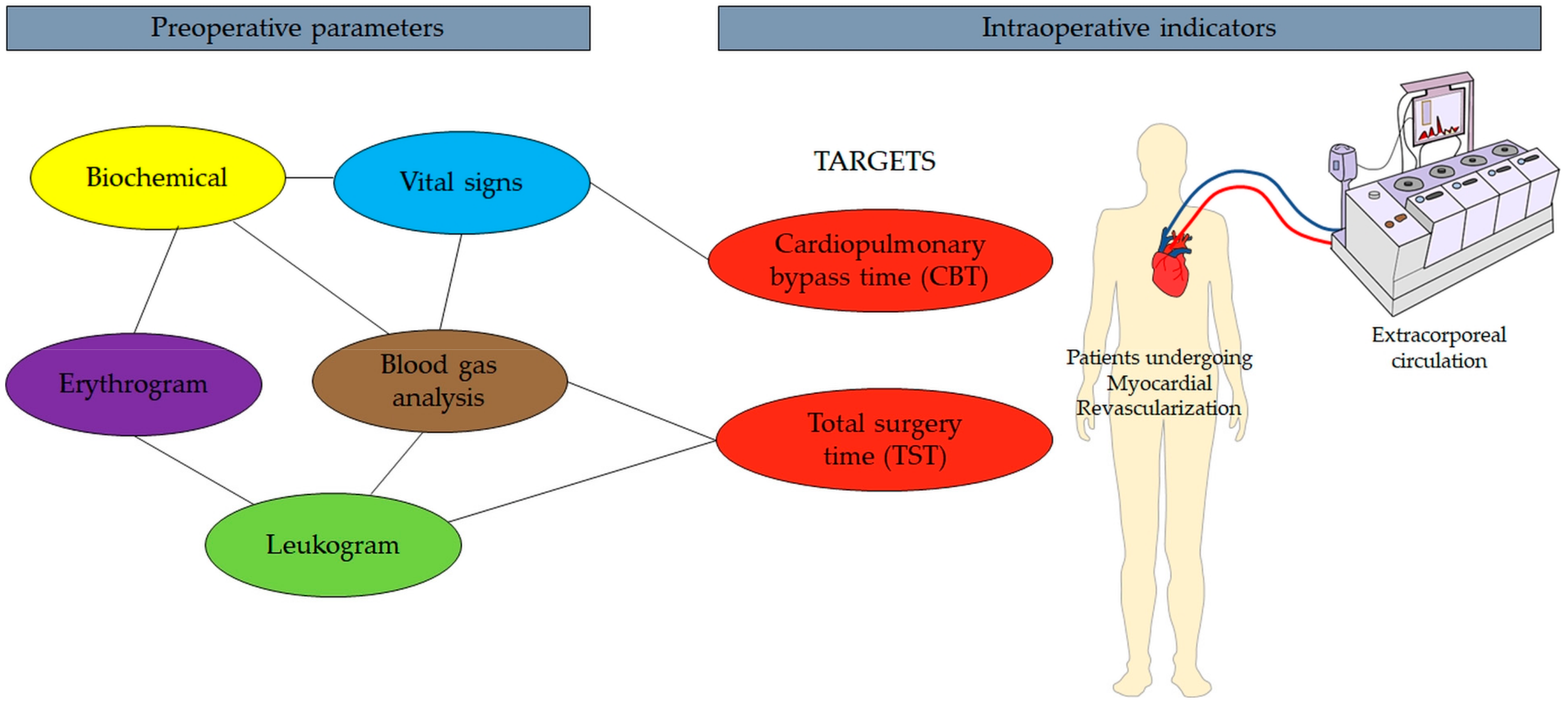
2.2. Data Collection for Mathematical Model Development
2.3. Complex Network Model
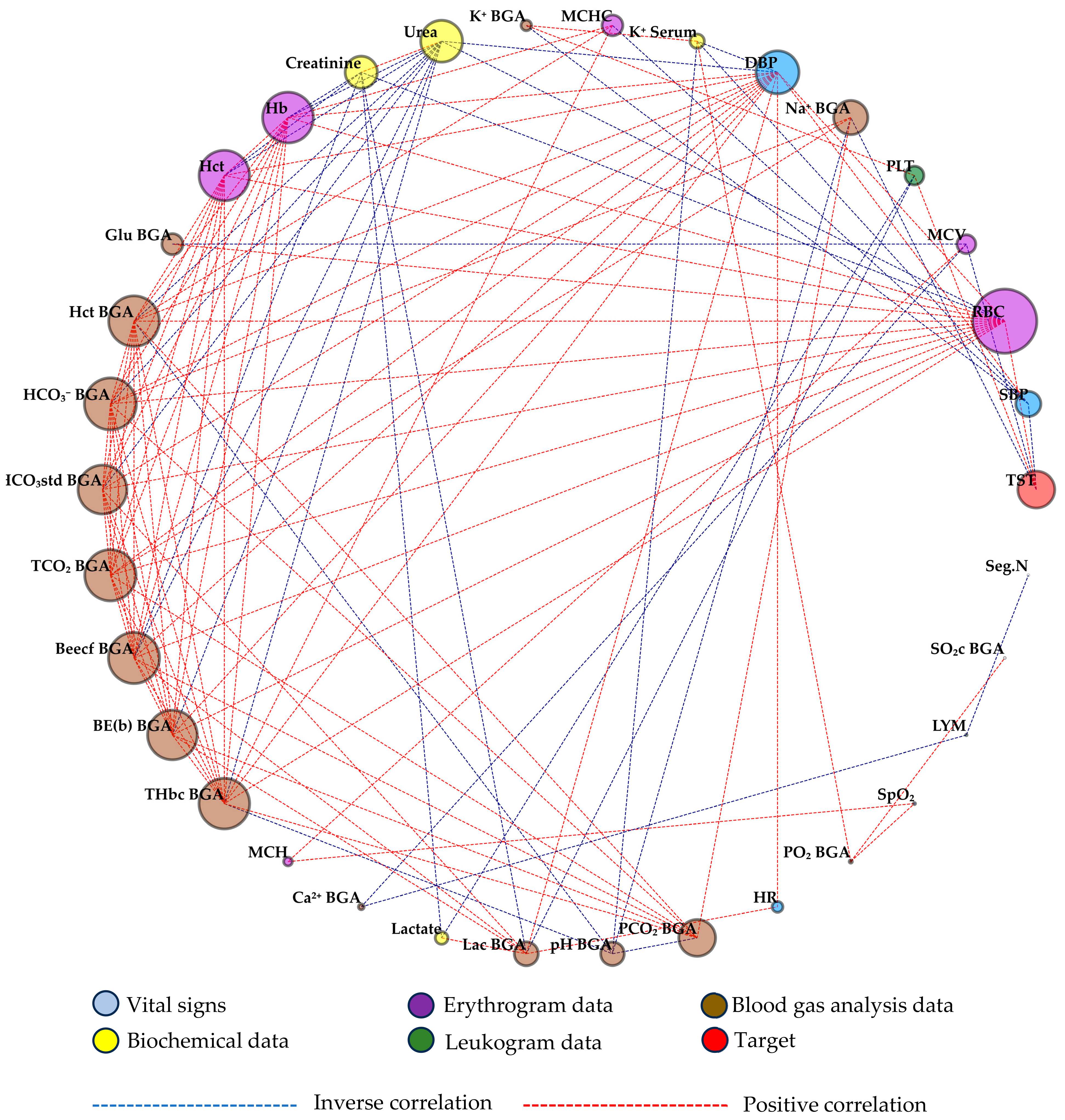
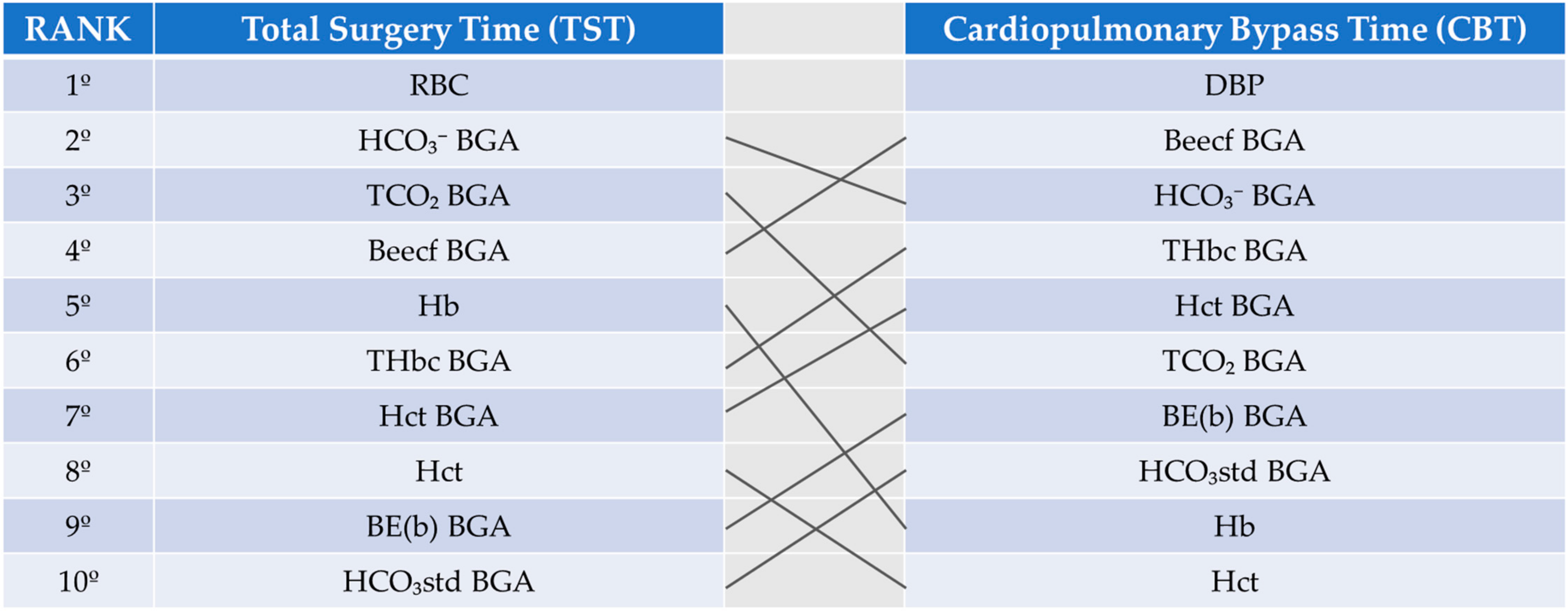
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barabasi, A.L. Network medicine—From obesity to the “diseasome”. N. Engl. J. Med. 2007, 357, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Silverman, E.K.; Schmidt, H.H.; Anastasiadou, E.; Altucci, L.; Angelini, M.; Badimon, L.; Balligand, J.L.; Benincasa, G.; Capasso, G.; Conte, F.; et al. Molecular networks in Network Medicine: Development and applications. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1489. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, A.R.; Weiss, S.T.; Glass, K.; Sharma, A. Network Medicine in the Age of Biomedical Big Data. Front. Genet. 2019, 10, 294. [Google Scholar] [CrossRef]
- Barabási, A.L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef]
- Buphamalai, P.; Kokotovic, T.; Nagy, V.; Menche, J. Network analysis reveals rare disease signatures across multiple levels of biological organization. Nat. Commun. 2021, 12, 6306. [Google Scholar] [CrossRef]
- Li, M.; Wu, J.; Wang, D.; Zhou, T.; Di, Z.; Fan, Y. Evolving model of weighted networks inspired by scientific collaboration networks. Phys. A Stat. Mech. Its Appl. 2017, 375, 355–364. [Google Scholar] [CrossRef]
- Park, K.; Lai, Y.C.; Ye, N. Characterization of weighted complex networks. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2004, 70 Pt 2, 026109. [Google Scholar] [CrossRef]
- Sousa, R.A.; Lula-Rocha, V.N.A.; Toutain, T.; Rosário, R.S.; Cambui, E.C.B.; Miranda, J.G.V. Preferential interaction networks: A dynamic model for brain synchronization networks. Phys. A: Stat. Mech. Its Appl. 2020, 554, 124259. [Google Scholar] [CrossRef]
- Kraemer, M.B.; Garbuio, A.L.P.; Kaneko, L.O.; Gobatto, C.A.; Manchado-Gobatto, F.B.; Dos Reis, I.G.M.; Messias, L.H.D. Associations among sleep, hematologic profile, and aerobic and anerobic capacity of young swimmers: A complex network approach. Front. Physiol. 2022, 13, 948422. [Google Scholar] [CrossRef]
- Mendes, F.M.M.; Sanches, P.H.G.; Silva, Á.A.R.; Reis, I.G.M.D.; Carvalho, P.D.O.; Porcari, A.M.; Messias, L.H.D. Plasma Amino Acids and Acylcarnitines Are Associated with the Female but Not Male Adolescent Swimmer’s Performance: An Integration between Mass Spectrometry and Complex Network Approaches. Biology 2022, 11, 1734. [Google Scholar] [CrossRef]
- Silva, Á.A.R.; Bertolucci, V.; Scariot, P.P.M.; da Cruz, J.P.; Mendes, F.M.M.; de Oliveira, D.C.; Plumari, C.D.; Dos Reis, I.G.M.; Porcari, A.M.; Messias, L.H.D. Glycerophospholipids in Red Blood Cells Are Associated with Aerobic Performance in Young Swimmers. Nutrients 2024, 16, 765. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, A.A.; Schult, D.A.; Swart, P.J. Exploring network structure, dynamics, and function using NetworkX. In Proceedings of the 7th Python in Science Conference, Pasadena, CA, USA, 19–24 August 2008; pp. 11–15. [Google Scholar]
- Newman, M.E.J. Networks: An. Introduction; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Garcia-Miguel, F.J.; Serrano-Aguilar, P.G.; Lopez-Bastida, J. Preoperative assessment. Lancet 2003, 362, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Columbo, J.A.; Stone, D.H.; Creager, M.A.; Henkin, S. Preoperative evaluation and perioperative management of patients undergoing major vascular surgery. Vasc. Med. 2022, 27, 496–512. [Google Scholar] [CrossRef] [PubMed]
- Schiefermueller, J.; Myerson, S.; Handa, A.I. Preoperative assessment and perioperative management of cardiovascular risk. Angiology 2013, 64, 146–150. [Google Scholar] [CrossRef]
- Mihalj, M.; Carrel, T.; Urman, R.D.; Stueber, F.; Luedi, M.M. Recommendations for Preoperative Assessment and Shared Decision-Making in Cardiac Surgery. Curr. Anesthesiol. Rep. 2020, 10, 185–195. [Google Scholar] [CrossRef]
- Lazzeroni, D.; Moderato, L.; Marazzi, P.L.; Pellegrino, C.; Musiari, E.; Castiglioni, P.; Camaiora, U.; Bini, M.; Geroldi, S.; Brambilla, L.; et al. Red blood cell distribution width as a novel prognostic marker after myocardial revascularization or cardiac valve surgery. Sci. Rep. 2021, 11, 7889. [Google Scholar] [CrossRef]
- Wu, T.T.; Zheng, Y.Y.; Hou, X.G.; Yang, Y.; Ma, X.; Ma, Y.T.; Xie, X. Red blood cell distribution width as long-term prognostic markers in patients with coronary artery disease undergoing percutaneous coronary intervention. Lipids Health Dis. 2019, 18, 140. [Google Scholar] [CrossRef]
- Bax, B.E. Erythrocytes as Carriers of Therapeutic Enzymes. Pharmaceutics 2020, 12, 435. [Google Scholar] [CrossRef]
- Koleva, L.; Bovt, E.; Ataullakhanov, F.; Sinauridze, E. Erythrocytes as Carriers: From Drug Delivery to Biosensors. Pharmaceutics 2020, 12, 276. [Google Scholar] [CrossRef]
- Mao, Y.; Zou, C.; Jiang, Y.; Fu, D. Erythrocyte-derived drug delivery systems in cancer therapy. Chin. Chem. Lett. 2021, 32, 990–998. [Google Scholar] [CrossRef]
- Massaccesi, L.; Galliera, E.; Romanelli, M.M.C. Erythrocytes as markers of oxidative stress related pathologies. Mech. Ageing Dev. 2020, 191, 111333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, P.; Yan, Z.; Liu, Z.; Ma, Q.; Zhang, Z.; Wang, Y.; Su, Y. The Relationship between Erythrocytes and Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 6656062. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, B.; Mallett, S.V.; Klein, A.A.; Richards, T. Patient blood management to reduce surgical risk. Br. J. Surg. 2015, 102, 1325–1337, discussion 1324. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, L.T.; Shander, A. Patient blood management. Anesthesiology 2012, 116, 1367–1376. [Google Scholar] [CrossRef]
- Kloeser, R.; Buser, A.; Bolliger, D. Treatment Strategies in Anemic Patients Before Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2023, 37, 266–275. [Google Scholar] [CrossRef]
- Shander, A.; Van Aken, H.; Colomina, M.J.; Gombotz, H.; Hofmann, A.; Krauspe, R.; Lasocki, S.; Richards, T.; Slappendel, R.; Spahn, D.R. Patient blood management in Europe. Br. J. Anaesth. 2012, 109, 55–68. [Google Scholar] [CrossRef]
- Lonjaret, L.; Lairez, O.; Minville, V.; Geeraerts, T. Optimal perioperative management of arterial blood pressure. Integr. Blood Press. Control 2014, 7, 49–59. [Google Scholar] [CrossRef]
- Meng, L.; Yu, W.; Wang, T.; Zhang, L.; Heerdt, P.M.; Gelb, A.W. Blood Pressure Targets in Perioperative Care. Hypertension 2018, 72, 806–817. [Google Scholar] [CrossRef]
- Saugel, B.; Sessler, D.I. Perioperative Blood Pressure Management. Anesthesiology 2021, 134, 250–261. [Google Scholar] [CrossRef]

| N = 30 | Mean ± SD or Frequency |
|---|---|
| Age (years) | 66 ± 8 |
| Sex and Age (years) | Female = 26% (67 ± 8)/Male = 74% (65 ± 8) |
| Conditions or Diseases | |
| Hypertension | 87% (Female = 8; Male = 18) |
| Type 2 Diabetes Mellitus | 33% (Female = 3; Male = 7) |
| Acute Myocardial Infarction | 30% (Female = 3; Male = 6) |
| Dyslipidemia | 37% (Female = 1; Male = 10) |
| Human Immunodeficiency Virus | 3% (Female = 0; Male = 1) |
| Chronic Obstructive Pulmonary Disease | 10% (Female = 2; Male = 1) |
| Depression | 3% (Female = 1; Male = 0) |
| Habits | |
| Smoking | 7% (Female = 0; Male = 2) |
| Former smoker | 27% (Female = 1; Male = 6) |
| Alcohol | 3% (Female = 0; Male = 1) |
| Former drinker | 3% (Female = 0; Male = 1) |
| Parameters | Mean ± SD | Confidence Interval |
|---|---|---|
| Targets | ||
| TST (min) | 227.2 ± 41.3 | 212.4–242.0 |
| CBT (min) | 74.7 ± 28.6 | 64.5–85.0 |
| Vital signs | ||
| HR (bpm) | 64.7 ± 11.4 | 60.6–68.8 |
| SpO2 (%) | 97.7 ± 1.5 | 97.2–98.3 |
| SBP (mmHg) | 148.0 ± 21.6 | 140.2–155.7 |
| DBP (mmHg) | 77.3 ± 12.4 | 72.8–81.7 |
| Biochemical data | ||
| Na+ Serum (mEq/L) | 137.9 ± 2.3 | 137.0–138.7 |
| K+ Serum (mEq/L) | 4.3 ± 0.5 | 4.1–4.5 |
| Urea (mg/dL) | 42.3 ± 22.4 | 34.3–50.3 |
| Creatinine (mg/dL) | 1.0 ± 0.4 | 0.8–1.1 |
| Lactate (mg/dL) | 12.7 ± 5.6 | 10.6–14.7 |
| Erythrogram | ||
| RBC (106/ul) | 4.4 ± 0.6 | 4.1–4.62 |
| Hb (g/dL) | 13.2 ± 1.8 | 12.5–13.91 |
| Hct (%) | 39.2 ± 5.2 | 37.3–41.11 |
| MCV (fl) | 90.4 ± 5.0 | 88.6–92.25 |
| MCH (pg) | 30.1 ± 1.8 | 29.4–30.76 |
| MCHC (mg/dL) | 33.4 ± 0.9 | 33.1–33.77 |
| RDW (%) | 13.7 ± 1.3 | 13.2–14.26 |
| Leukogram | ||
| WBC (109/ul) | 7810.3 ± 2747.1 | 6827.3–8793.36 |
| Seg.N (109/L) | 53.1 ± 14.7 | 47.8–58.40 |
| BAS (109/L) | 0.1 ± 0.3 | 0.0–0.26 |
| LYM (109/L) | 34.3 ± 12.0 | 30.0–38.60 |
| PLT (109/L) | 219.137 ± 50.047 | 201.220–237.040 |
| BGA | ||
| pH BGA | 7.4 ± 0.0 | 7.3–7.4 |
| PCO2 BGA (mmHg) | 38.7 ± 5.8 | 36.7–40.8 |
| PO2 BGA (mmHg) | 137.1 ± 43.8 | 121.4–152.8 |
| Na+ BGA (mEq/L) | 136.5 ± 6.6 | 134.1–138.9 |
| K+ BGA (mEq/L) | 3.8 ± 0.3 | 3.7–3.9 |
| Ca2+ BGA (mEq/L) | 0.9 ± 0.1 | 0.9–1.0 |
| Glu BGA (mg/dL) | 115.2 ± 41.7 | 100.2–130.1 |
| Lac BGA (mg/dL) | 9.9 ± 4.1 | 8.4–11.4 |
| Hct BGA (%) | 35.7 ± 6.2 | 33.5–38.0 |
| HCO3− BGA (mEq/L) | 24.2 ± 2.2 | 23.4–25.0 |
| HCO3std BGA (mEq/L) | 24.6 ± 1.4 | 24.1–25.2 |
| TCO2 BGA (mEq/L) | 25.4 ± 2.3 | 24.6–26.3 |
| Beecf BGA (mEq/L) | −0.4 ± 2.1 | −1.1–0.3 |
| BE(b) BGA (mEq/L) | −0.3 ± 1.8 | −1.0–0.3 |
| SO2c BGA (%) | 98.0 ± 4.4 | 96.4–99.5 |
| THbc BGA (g/dL) | 11.1 ± 1.9 | 10.4–11.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertolucci, V.; Ninomiya, A.F.; Souza, J.P.; Barbosa, F.F.P.; Nonose, N.; de Carvalho, L.M.; Scariot, P.P.M.; dos Reis, I.G.M.; Messias, L.H.D. Association of Preoperative Parameters on Intraoperative Indicators in Myocardial Revascularization Surgery: Insights from a Targeted Complex Network Model. Surgeries 2025, 6, 1. https://doi.org/10.3390/surgeries6010001
Bertolucci V, Ninomiya AF, Souza JP, Barbosa FFP, Nonose N, de Carvalho LM, Scariot PPM, dos Reis IGM, Messias LHD. Association of Preoperative Parameters on Intraoperative Indicators in Myocardial Revascularization Surgery: Insights from a Targeted Complex Network Model. Surgeries. 2025; 6(1):1. https://doi.org/10.3390/surgeries6010001
Chicago/Turabian StyleBertolucci, Vanessa, André Felipe Ninomiya, João Paulo Souza, Felipe Fernandes Pires Barbosa, Nilson Nonose, Lucas Miguel de Carvalho, Pedro Paulo Menezes Scariot, Ivan Gustavo Masseli dos Reis, and Leonardo Henrique Dalcheco Messias. 2025. "Association of Preoperative Parameters on Intraoperative Indicators in Myocardial Revascularization Surgery: Insights from a Targeted Complex Network Model" Surgeries 6, no. 1: 1. https://doi.org/10.3390/surgeries6010001
APA StyleBertolucci, V., Ninomiya, A. F., Souza, J. P., Barbosa, F. F. P., Nonose, N., de Carvalho, L. M., Scariot, P. P. M., dos Reis, I. G. M., & Messias, L. H. D. (2025). Association of Preoperative Parameters on Intraoperative Indicators in Myocardial Revascularization Surgery: Insights from a Targeted Complex Network Model. Surgeries, 6(1), 1. https://doi.org/10.3390/surgeries6010001









