Autogenous Tooth Graft Biomaterial in Guided Bone Regeneration: A Comprehensive Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Strategy
2.2. Eligibility Criteria
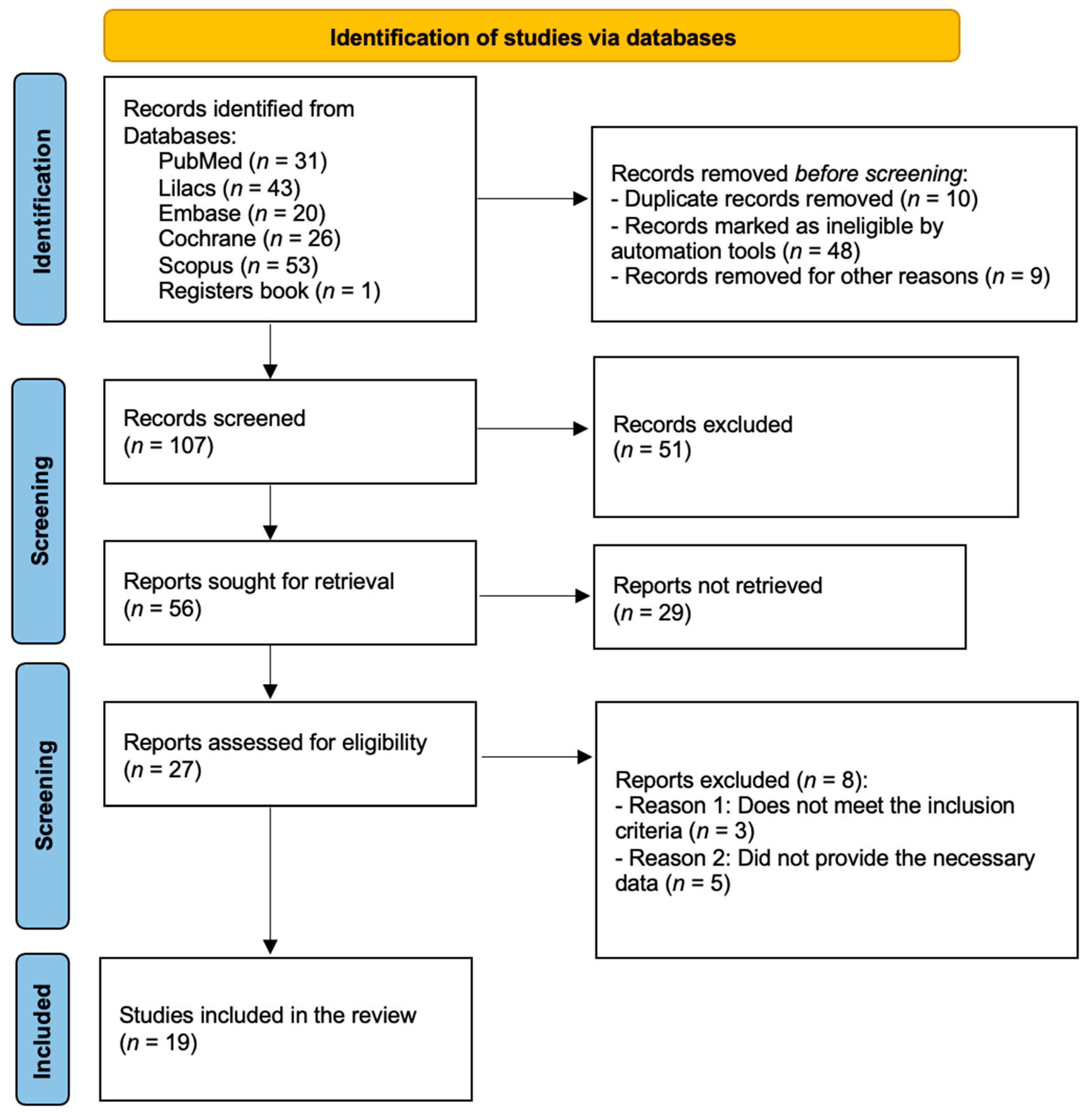
2.2.1. Screening and Selection of Studies
2.2.2. Study Data Collection
2.3. Risk of Bias/Quality Assessment
3. Results
3.1. Quality Assessment of the Included Studies
3.2. Descriptive Summarization of the Included Studies
3.2.1. Case Series
3.2.2. Comparative Analysis
3.2.3. Retrospective Study
3.2.4. Pilot Study
3.2.5. Prospective Clinical Studies and Randomized Controlled Trials
4. Discussion
4.1. Evaluating Tooth Biomaterial Performance
4.2. Comparing Tooth Graft with Other Biomaterials or Blood Clots (Negative Control)
4.3. Maxilla versus Mandible
4.4. Membranes and Clinical Complication
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baskaran, P.; Prakash, P.S.G.; Appukuttan, D.; Mugri, M.H.; Sayed, M.; Subramanian, S.; Al Wadei, M.H.D.; Ahmed, Z.H.; Dewan, H.; Porwal, A.; et al. Clinical and radiological outcomes for guided-implant placement in sites preserved with bioactive glass bone graft after tooth extraction: A controlled clinical trial. Biomimetics 2022, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lou, Y.; Sun, J.; Xie, C.; Wu, J.; Yu, H. Accuracy of the novel digital non-cross-arch surgical guides with integration of tooth undercut retention and screw-bone support for implant placement in mandibular free-end. BMC Oral Health 2024, 24, 550. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Han, H.-S.; Ghanaati, S.; Zadeh, H.H.; Kim, S.; Cho, Y.-D. Alveolar Ridge Preservation Using a Collagenated Xenograft: A Randomized Clinical Trial. Int. Dent. J. 2024; in press. [Google Scholar] [CrossRef]
- Cavdar, F.H.; Keceli, H.G.; Hatipoglu, H.; Demiralp, B.; Caglayan, F. Evaluation of Extraction Site Dimensions and Density Using Computed Tomography Treated with Different Graft Materials: A Preliminary Study. Implant Dent. 2017, 26, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, E.; Castro, F.; Curado, D.; Martelete, A.; Heboyan, A.; Saleh, M.H.; Fernandes, J.C.H.; Fernandes, G.V.O. Minimally invasive periodontal regeneration with the buccal approach: A systematic review and meta-analysis of clinical studies. Evid.-Based Dent. 2024, 25, 54. [Google Scholar] [CrossRef]
- Rondone, E.; Leitão-Almeida, B.; Pereira, M.S.; Fernandes, G.V.O.; Borges, T. The use of tissue grafts associated with immediate implant placement to achieve better peri-implant stability and efficacy: A systematic review and meta-analysis. J. Clin. Med. 2024, 13, 821. [Google Scholar] [CrossRef]
- Martins, S.C.R.; Marques, M.C.; Vidal, M.G.; Moreira, P.H.; Tolentino, P.; Dinelli, R.G.; Fernandes, G.V.O.; Shibli, J.A. Is the facial bone wall critical to achieving esthetic outcomes in immediate implant placement with immediate restoration? A systematic review. Adv. Clin. Exp. Med. 2024, 33. [Google Scholar] [CrossRef]
- Li, P.; Zhu, H.C.; Huang, D.H. Autogenous DDM versus Bio-Oss granules in GBR for immediate implantation in periodontal postextraction sites: A prospective clinical study. Clin. Implant Dent. Relat. Res. 2018, 20, 923–928. [Google Scholar] [CrossRef]
- Kim, E.S. Autogenous fresh demineralized tooth graft prepared at chairside for dental implant. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 8. [Google Scholar] [CrossRef][Green Version]
- Maffei, S.H.; Fernandes, G.V.O.; Fernandes, J.C.H.; Orth, C.; Joly, J.C. Clinical and histomorphometric soft tissue assessment comparing free gingival graft (FGG) and a collagen matrix (MS) as alveolar-sealer materials: A randomized controlled pilot clinical trial. Quintessence Int. 2023, 54, 756–769. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.; Kim, Y.K. Comparative analysis of guided bone regeneration using autogenous tooth bone graft material with and without resorbable membrane. J. Dent. Sci. 2013, 8, 281–286. [Google Scholar] [CrossRef]
- Yang, F.; Ruan, Y.; Bai, X.; Li, Q.; Tang, X.; Chen, J.; Chen, Y.; Wang, L. Alveolar ridge preservation in sockets with severe periodontal destruction using autogenous partially demineralized dentin matrix: A randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 2023, 25, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, F.; Wang, R.; Shang, X.; Yang, P.; Zhu, Y.; Tsoi, J.K.H.; Chan, K.; Wang, S. Guided Bone Regeneration in a Periodontally Compromised Individual with Autogenous Tooth Bone Graft: A Radiomics Analysis. J. Funct. Biomater. 2023, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Elfana, A.; El-Kholy, S.; Saleh, H.A.; El-Sayed, K.F. Alveolar ridge preservation using autogenous whole-tooth versus demineralized dentin grafts: A randomized controlled clinical trial. Clin. Oral Implant. Res. 2021, 32, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.P.; Resck, T.; Kirsch, D.; Sperandio, M.; Napimoga, M.H.; Joly, J.C.; Fernandes, G.V.O.; Peruzzo, D.C. Evaluation of the periodontal ligament and tooth-derived substrates effect on osteogenesis: An in vitro study. J. Maxillofac. Oral Surg. 2024. [Google Scholar] [CrossRef]
- Santos, A.; Botelho, J.; Machado, V.; Borrecho, G.; Proença, L.; Mendes, J.J.; Mascarenhas, P.; Alcoforado, G. Autogenous Mineralized Dentin versus Xenograft granules in Ridge Preservation for Delayed Implantation in Post-extraction Sites: A Randomized controlled clinical trial with an 18 month follow-up. Clin. Oral Implant. Res. 2021, 32, 905–915. [Google Scholar] [CrossRef]
- Koga, T.; Minamizato, T.; Kawai, Y.; Miura, K.I.; Takashi, I.; Nakatani, Y.; Sumita, Y.; Asahina, I. Bone regeneration using dentin matrix depends on the degree of demineralization and particle size. PLoS ONE 2016, 11, e0147235. [Google Scholar] [CrossRef]
- Minetti, E.; Dipalma, G.; Palermo, A.; Patano, A.; Inchingolo, A.D.; Inchingolo, A.M.; Inchingolo, F. Biomolecular Mechanisms and Case Series Study of Socket Preservation with Tooth Grafts. J. Clin. Med. 2023, 12, 5611. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, J.H.; Um, I.W.; Cho, W.J. Guided Bone Regeneration Using Demineralized Dentin Matrix: Long-Term Follow-Up. J. Oral Maxillofac. Surg. 2016, 74, 515.e1–515.e9. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, S.G.; Um, I.W.; Kim, K.W. Bone grafts using autogenous tooth blocks: A case series. Implant Dent. 2013, 22, 584–589. [Google Scholar] [CrossRef]
- Minetti, E.; Celko, M.; Contessi, M.; Carini, F.; Gambardella, U.; Giacometti, E.; Santillana, J.; Campoy, T.B.; Schmitz, J.H.; Libertucci, M.; et al. Implants survival rate in regenerated sites with innovative graft biomaterials: 1 year follow-up. Materials 2021, 14, 5292. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, S.G.; Byeon, J.H.; Lee, H.J.; Um, I.U.; Lim, S.C.; Kim, S.Y. Development of a novel bone grafting material using autogenous teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 109, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Berardini, M.; Trisi, P. A New Tooth Processing Apparatus Allowing to Obtain Dentin Grafts for Bone Augmentation: The Tooth Transformer. Open Dent. J. 2019, 13, 6–14. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, S.G.; Bae, J.H.; Um, I.W.; Oh, J.S.; Jeong, K.I. Guided bone regeneration using autogenous tooth bone graft in implant therapy: Case series. Implant Dent. 2014, 23, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, Y.-K.; Yi, Y.-J.; Choi, J.-H. Clinical evaluation of ridge augmentation using autogenous tooth bone graft material: Case series study. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 156. [Google Scholar] [CrossRef] [PubMed]
- Hee-Yung, C.; Taek-Ka, K.; Nunn, M.E.; Miyamoto, T.; Kwang-Won, L.; Young-Kyun, K.; Hyo-Jung, L. Feasibility Analysis of Autogenous Tooth-based Bone Graft Material after Guided Bone Regeneration Technique. J. Case Rep. Stud. 2014, 2, 304. [Google Scholar] [CrossRef]
- Um, I.W.; Ku, J.K.; Kim, Y.M.; Yun, P.Y.; Chang, N.H.; Kim, Y.K.; Choi, Y. Allogeneic demineralized dentin matrix graft for guided bone regeneration in dental implants. Appl. Sci. 2020, 10, 4661. [Google Scholar] [CrossRef]
- Sah, A.; Baliga, S.D. Clinical application of autogenous tooth as bone graft material in extraction socket—A prospective study. Clin. Epidemiol. Glob. Health 2022, 16, 101063. [Google Scholar] [CrossRef]
- Pang, K.M.; Um, I.W.; Kim, Y.K.; Woo, J.M.; Kim, S.M.; Lee, J.H. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: A prospective randomized clinical trial in comparison with anorganic bovine bone. Clin. Oral Implant. Res. 2017, 28, 809–815. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Száva, D.-T.; Ormenișan, A.; Markovics, E.; Bögözi, B.; Mártha, K. Alveolar Bone Resorption Evaluation Around Single-piece Designed Bicortical Implants, Using Immediate Loading Protocol, Based on Orthopantomographs. J. Interdiscip. Med. 2017, 2, 328–331. [Google Scholar] [CrossRef]
- Mahesh, L.; Castro, A.B.; Bhas, M.T. The Survival Rate of Posterior Immediate Implants in the Maxilla and Mandible: An Observational Retrospective Study of 158 Dental Implants. Cureus 2023, 15, e45579. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, G.; Grunder, U.; Goene, R.; Hatano, N.; Henry, P.; Jackson, W.J.; Kawamura, K.; Renouard, F.; Rosenberg, R.; Triplett, G.; et al. Immediate and delayed implant placement into extraction sockets: A 5-year report. Clin. Implant Dent. Relat. Res. 2000, 2, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wagenberg, B.; Froum, S.J. A retrospective study of 1925 consecutively placed immediate implants from 1988 to 2004. Int. J. Oral Maxillofac. Implant. 2006, 21, 71–80. [Google Scholar]
- Ramalingam, S.; Al-Hindi, M.; Al-Eid, R.A.; Nooh, N. Clinical evaluation of implant survival based on size and site of placement: A retrospective study of immediate implants at single rooted teeth sites. Saudi Dent. J. 2015, 27, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Martins, D.; Sá, J.; Mendes, J.M. Clinical evaluation of the implant survival rate in patients subjected to immediate implant loading protocols. Dent. Med. Probl. 2021, 58, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodazadeh, M.; Amid, R.; Moscowchi, A.; Khoshkam, V. Clinical and radiographic evaluation of jumping distance management using a collagen matrix in flapless immediate implant placement. Dent. Med. Probl. 2021, 58, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.V.O.; Castro, F.; Pereira, R.M.; Teixeira, W.; Gehrke, S.; Joly, J.C.; Blanco-Carrion, J.; Fernandes, J.C.H. Critical-size defects reconstruction with four different bone grafts associated with e-PTFE membrane: A histomorphometric experimental in vivo study. Clin. Oral Implant. Res. 2024, 35, 167–178. [Google Scholar] [CrossRef]
- Flynn, R.; Foschi, F.; Maloney, B.; Creavin, G.; Duncan, H.F. The impact of bone grafting with/without barrier membrane placement on the outcome of apical surgery: A systematic review and meta-analysis. Int. Endod. J. 2024, 57, 1006–1020. [Google Scholar] [CrossRef]
| Author | Study Type | Sample (F/M) | Average Age—Years | Technique and Material Used | Site of the Implants | n of Dental Implants | Inflammatory Signs and Complications | |
|---|---|---|---|---|---|---|---|---|
| 1 | Kim et al., 2016 [19] | Case series | 3F/2M | 41.6 | Demineralized Auto-BT®; GBR; dental implants | 1 mandibular implant 4 maxillary implants | 5 | No |
| 2 | Kim et al., 2013 [20] | Case series | 4F/8M | NR | Demineralized Auto-BT® Block; GBR; implants; collagen membrane (5 patients) or without collagen membrane (7 patients) | 19 maxilla 10 mandible | 29 | 2 |
| 3 | Kim et al., 2015 [9] | Case series | 9F/29M | 49.8 | Auto-FDT®; GBR; implants; resorbable collagen membrane (29 patients) or a titanium mesh (9 patients) (CTi-memTM®, Neobiotech, Seoul, Republic of Korea) | 32 maxilla 26 mandible | 58 | No |
| 4 | Minetti et al., 2023 [18] | Case series | 8F/12M | 57.33 ± 11.09 | AutoBT®; GBR; resorbable osseoguard membrane (Zimmer [Warsaw, Indiana, USA]); implants | NR | 20 | No |
| 5 | Minetti et al., 2021 [21] | Case series | 269F/235M | 54.09 | AutoBT®; GBR; dental implants; resorbable collagen membrane | 278 maxilla 205 mandible | 483 | 27 |
| 6 | Kim et al., 2010 [22] | Case series | 3F/3M | 44.83 | AutoBT®; GBR; dental implants | 6 maxilla 1 mandible | 7 | No |
| 7 | Minetti et al., 2019 [23] | Case series | 8F/7M | 43 | Auto-BT®; GBR (11 patients) or maxillary sinus elevation (4 patients); dental implants; resorbable porcine pericardial membrane® (BEGO Implant Systems GmbH & Co., KG, Bremen, Germany) | NR | 12 | 1 |
| 8 | Kim et al., 2014 [24] | Case series | 7F/8M | 49.9 | Auto BT® block (1 patient) or particulate (14 patients); GBR; dental implants; resorbable collagen membrane (8 patients) | 5 maxilla; 18 mandible | 23 | 3 |
| 9 | Lee et al., 2013 [25] | Case series | 2F/7M | 49.88 ± 12.98 | Auto BT® block (2 Areas) or particulate (13 areas) or both (11 Areas); GBR; dental implants; titanium mesh or resorbable membrane (BioGide®; Osteohealth/Ossix; OraPharma, Warminster, PA, USA) or non-resorbable membrane (TR Goretex®; WL Gore & Associates, Flagstaff, AZ, USA) | 24 maxilla 2 mandible | 26 | NR |
| 10 | Lee et al., 2013 [11] | Comparative analysis | Test group: 1F/8M; Control group: 4F/8M | Test group: 49.8 Control group: 57 | Test group: With resorbable membrane (Bio-Arm®, ACE Surgical. Supply Company, Inc., West Columbia, SC, USA); Demineralized Auto-BT®; GBR; dental implants Control group: Without resorbable membrane; Demineralized Auto-BT®; GBR; dental implants | Test group: 3 maxilla 13 mandible Control group: 6 maxilla 8 mandible | NR | 1 case for each group |
| 11 | Chang et al., 2014 [26] | Retrospective study | 6F/4M | 55.4 | Demineralized Auto-BT®; GBR; implants; resorbable membrane (Bio-Gide®, Geistlich Pharma AG®, Wolhusen, Switzerland) and non-resorbable membrane (Gore-tex®, WL Gore & Associates®, Flagstaff, AZ, USA) | 4 maxilla 7 mandible | 11 | No |
| 12 | Um et al., 2020 [27] | Pilot study | 37F/59M | 57.13 | Test group (44 patients): Auto-DDM®; GBR; dental implants Control group (52 patients): Allo-DDM®; GBR; dental implants | 54 maxilla 42 mandible | 96 | No |
| 13 | Li et al., 2018 [8] | Prospective clinical study | 16F/24M | 35.81 | Test group (20 patients): Autologous DDM granules from the extracted tooth; GBR; immediate implants; BioGide membrane® (Osteohealth, Wolhusen, Switzerland) Control group (20 patients): Bio-Oss® granules (Geistlich Pharma AG, Wolhusen, Switzerland); GBR; immediate implants; BioGide membrane® (Osteohealth, Wolhusen, Switzerland) | Test group: 21 mandible Control group: 21 mandible | 45 | 2 |
| 14 | Sah and Baliga, 2022 [28] | Prospective study | 8F/12M | 27 | Test group: AutoBT® with PRF membrane Control group: PRF membrane (PRF) | NR | NR | No |
| 15 | Pang et al., 2017 [29] | Prospective randomized clinical study | Test group: 11F/10M Control group: 6F/6M | Test group: 58.53 Control group: 60.56 | Test group (21 patients): Auto-BT®; GBR; dental implants Control group (12 patients): Bio-Oss®; GBR; dental implants | NR | 15 | No |
| 16 | Yang et al., 2023 [12] | Randomized controlled clinical study | Test group: 9F/7M Control group: 6F/10M | Test group: 48.56 ± 13.46 Control group: 58.94 ± 16.09 | Test group: Dentin matrix, partially demineralized autologous; GBR; implants; collagen sponge® (Wuxi BIOT Biologics Engineering Co., Ltd., Wuxi, China) Control group: Spontaneous healing (SH) | Test group: 7 maxilla 9 mandible Control group: 4 maxilla 12 mandible | 11 jaws; 21 mandibular | No |
| 17 | Elfana et al., 2021 [14] | Randomized controlled clinical study | Test group: 7F/3M Control group: 9F/1M | Test group: 33.5 ± 7.37 Control group: 31.2 ± 6.44 | Test group: Autologous whole tooth; GBR; implants; bioabsorbable collagen membrane® (Hypro-Sorb®, Bioimplon GmbH, Munich, Germany) Control group: Demineralized dentin graft, autologous; GBR; implants; bioabsorbable collagen membrane® (Hypro-Sorb®, Bioimplon GmbH, Munich, Germany) | Test group: 7 maxilla 3 mandible Control group: 6 maxilla 4 mandible | Test group: 10 Control group:10 | No |
| 18 | Santos et al., 2021 [16] | Randomized controlled study | Test group: 15F/11M Control group: 16F/10M | Test group: 56.8 ± 12.3 Control group: 61.5 ± 13.1 | Test group: Dentin matrix mineralized autologous; GBR; implants; absorbable barrier membrane (Bio-Gide®, Geistlich, Wolhusen, Switzerland) Control group: Granules of xenograft (Bio-Oss®, Geistlich, Switzerland); GBR; implants; resorbable barrier membrane (Bio-Gide®, Geistlich, Wolhusen, Switzerland) | NR | 66 | No |
| 19 | Li et al., 2023 [13] | Radiomics analysis | 14F/11M | 46 | AutoBT®; Bio-Gide® collagen membranes; GBR; implants | First premolar (n = 3) Second premolar (n = 5) First molar (n = 14) Second molar (n = 14) | 36 | No |
| Author | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | % (Yes) | Risk Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim et al. [19] | N.A. | U | Yes | Yes | Yes | Yes | Yes | Yes | N.A. | No | No | No | 60% | Medium quality/Moderate risk |
| Lee et al. [25] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | 100% | High quality/Low risk |
| Chang et al. [26] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | 100% | High quality/Low risk |
| Um et al. [27] | U | U | Yes | Yes | Yes | Yes | Yes | Yes | U | Yes | No | No | 70% | Medium quality/Moderate risk |
| Kim et al. [10] | N.A. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | 90% | High quality/Low risk |
| Li et al. [13] | U | Yes | Yes | U | U | Yes | Yes | Yes | Yes | N.A. | Yes | No | 64% | Medium quality/Moderate risk |
| Yang et al. [12] | Yes | Yes | Yes | N.A. | N.A. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 83% | High quality/Low risk |
| Elfana et al. [14] | Yes | Yes | Yes | Yes | N.A. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 92% | High quality/Low risk |
| Santos et al. [16] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 100% | High quality/Low risk |
| Kim et al. [9] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N.A. | Yes | No | No | 90% | High quality/Low risk |
| Li et al. [8] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | 100% | High quality/Low risk |
| Minetti et al. [18] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | U | No | No | 90% | High quality/Low risk |
| Minetti et al. [21] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N.A. | No | No | 90% | High quality/Low risk |
| Sah and Baliga [28] | U | Yes | Yes | Yes | Yes | Yes | Yes | Yes | U | Yes | No | No | 80% | High quality/Low risk |
| Kim et al. [22] | N.A. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | U | No | No | 80% | High quality/Low risk |
| Minetti et al. [23] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N.A. | No | No | 90% | High quality/Low risk |
| Kim et al. [24] | N.A. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | U | No | No | 80% | High quality/Low risk |
| Pang et al. [29] | Yes | Yes | Yes | N.A. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 92% | High quality/Low risk |
| Lee et al. [11] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | U | No | No | 90% | High quality/Low risk |
| Study | Author | Primary ISQ Average | p-Value | Secondary ISQ Average | p-Value |
|---|---|---|---|---|---|
| 3 | Kim et al. [9] | 68 | – | 81 | – |
| 8 | Kim et al. [24] | 72 | – | 81 | – |
| 9 | Lee et al. [25] | 62 | – | 72 | – |
| 10 | Lee et al. [11] | Test group: | >0.05 | Test group: | >0.05 |
| 63.64 ± 11.81 | 78.38 ± 6.85 | ||||
| Control group: | Control group: | ||||
| 65.53 ± 8.14 | 76.15 ± 7.08 | ||||
| 13 | Li et al. [8] | 53.6 ± 11.9 | 0.14 | 79.5 ± 6.0 | 0.09 |
| 15 | Pang et al. [29] | 72.80 ± 10.81 | 0.755 | – | – |
| 18 | Santos et al. [16] | 77.1 ± 6.9 | 0.807 | 81.8 ± 5.1 | 0.54 |
| Study | Author | MBLevel T0 (mm): Average | p-Value | MBL PO (mm): Average | p-Value | Bone Height (mm) | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | Kim et al. [19] | 8.02 | - | 6.86 | - | (−) from 0.2 to 3.25 | - |
| 5 | Minetti et al. [21] | - | - | - | - | (−) 0.37 ± 0.68 | >0.568 |
| 8 | Kim et al. [24] | - | - | - | - | (−) 0.47 | - |
| 9 | Lee et al. [25] | - | - | - | - | (−) 0.12 ± 0.19 | - |
| 10 | Lee et al. [11] | Test group: 2.38 ± 0.28 Control group: 2.58 ± 0.34 | >0.05 | Test group: 2.19 ± 0.32 Control group: 2.35 ± 0.40 | >0.05 | Test group: (−) 0.19 ± 0.1 Control group: (−) 0.23 ± 0.11 | >0.05 |
| 11 | Chang et al. [26] | 5.67 | >0.05 | 5.99 | >0.05 | (+) 0.29 | <0.01 |
| 12 | Um et al. [27] | 11.76 ± 1.84 | <0.001 | 10.45 ± 1.77 | <0.001 | (−) 0.69 ± 0.81 | 0.141 |
| 13 | Li et al. [8] | - | - | - | - | (−) 1.9 ± 0.6 | 0.18 |
| 15 | Pang et al. [29] | 12.04 ± 5.50 | 0.777 | 6.08 ± 5.53 | 0.887 | − | - |
| 16 | Yang et al. [12] | 7.24 ± 2.10 | 0.950 | 7.17 ± 1.56 | 0.005 | (−) 0.07 ± 1.56 | 0.005 |
| 17 | Elfana et al. [14] | 8.95 ± 1.6 | 0.71 | 8.23 ± 0.27 | 0.31 | (−) 0.72 ± 0.27 | 0.31 |
| 19 | Li et al. [13] | 6.63 ± 3.75 | <0.001 | 9.82 ± 3.72 | <0.001 | (+) 3.19 ± 0.88 | <0.001 |
| Study | Author | NB (%) | p-Value |
|---|---|---|---|
| 4 | Minetti et al. [18] | 40.39 ± 15.86 | - |
| 5 | Minetti et al. [21] | 32.38 ± 17.15 | - |
| 6 | Kim et al. [22] | 46–87 | - |
| 10 | Lee et al. [11] | Test group: | >0.05 |
| 89.06 ± 27.33 | |||
| Control group: | |||
| 86.92 ± 22.78 | |||
| 15 | Pang et al. [29] | 31.24 ± 13.87 | 0.606 |
| 16 | Yang et al. [12] | 39.67 ± 8.28 | - |
| 17 | Elfana et al. [14] | 48.4 ± 11.56 | - |
| 18 | Santos et al. [16] | 47.3 ± 14.8 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picone, A.; Castro, F.; Falcão, A.; Medina, J.G.; Minetti, E.; Fernandes, J.C.H.; Fernandes, G.V.O. Autogenous Tooth Graft Biomaterial in Guided Bone Regeneration: A Comprehensive Review. Surgeries 2024, 5, 929-947. https://doi.org/10.3390/surgeries5040075
Picone A, Castro F, Falcão A, Medina JG, Minetti E, Fernandes JCH, Fernandes GVO. Autogenous Tooth Graft Biomaterial in Guided Bone Regeneration: A Comprehensive Review. Surgeries. 2024; 5(4):929-947. https://doi.org/10.3390/surgeries5040075
Chicago/Turabian StylePicone, Aurora, Filipe Castro, Artur Falcão, Jesus Glez Medina, Elio Minetti, Juliana Campos Hasse Fernandes, and Gustavo Vicentis Oliveira Fernandes. 2024. "Autogenous Tooth Graft Biomaterial in Guided Bone Regeneration: A Comprehensive Review" Surgeries 5, no. 4: 929-947. https://doi.org/10.3390/surgeries5040075
APA StylePicone, A., Castro, F., Falcão, A., Medina, J. G., Minetti, E., Fernandes, J. C. H., & Fernandes, G. V. O. (2024). Autogenous Tooth Graft Biomaterial in Guided Bone Regeneration: A Comprehensive Review. Surgeries, 5(4), 929-947. https://doi.org/10.3390/surgeries5040075








