Abstract
Background: Low-dose computed tomography-based lung cancer screening (LCS) has demonstrated efficacy in reducing lung cancer mortality. However, concerns about overdiagnosis and overtreatment hinder global LCS implementation. Methods: Here, we report the unique case of a slow-growing 1 cm pure ground-glass opacity (GGO) of the lung, known for 15 years, which unexpectedly developed into a 5 cm mixed GGO within 1 year, with an increased solid component and FDG-PET uptake. Results: The patient, asymptomatic, underwent right upper lobectomy and lymphadenectomy, even complicated with postoperative chylothorax, later revealing to be affected by only an unchanged adenocarcinoma in situ (AIS). Conclusions: This case serves as a reminder of the potential behavior of pre-invasive lesions, which can mimic invasive neoplasia and may lead to overtreatment, and underscores the challenge of distinguishing indolent lesions from potentially aggressive malignancies in LCS, highlighting the need for the ongoing refinement of LCS protocols to mitigate this risk.
1. Introduction
Lung cancer remains a leading cause of cancer-related mortality worldwide, accounting for more deaths annually than breast, colon, and prostate cancers combined [1]. Early detection through screening is a crucial strategy in reducing lung cancer (LC) mortality, as it enables the identification and treatment of cancers at an earlier stage. Low-dose computed tomography (LDCT) has emerged as a powerful tool in this endeavor, allowing for the early detection of LCs. LDCT-based lung cancer screening (LCS) programs have been shown to successfully reduce LC mortality in selected patients [2]. Nonetheless, the related risks of overdiagnosis and overtreatment still prevent the worldwide implementation of LCS [3]: overdiagnosis refers to the detection of lung cancers that would not have become clinically significant within the patient’s lifetime, leading to unnecessary treatments, and exposing patients to the risks and side effects of surgical and other therapeutic interventions without a clear benefit, namely overtreatment. Indeed, evidence that indolent LC exists is rising, and a non-negligible proportion of LCS-detected LCs may be harmless [4]. One area of particular concern is the detection of ground-glass opacities (GGOs) on LDCT scans: GGOs can represent a spectrum of lung lesions, from benign or pre-malignant conditions to more aggressive forms of adenocarcinoma [5].
Adenocarcinoma in situ (AIS)—that is a pre-malignancy—may fall into the category of indolent disease, since it is characterized by excellent long-term prognosis [6,7]. In the context of LCS, strategies to mitigate the risks of overdiagnosis and overtreatment are crucial. It has been previously shown that it is feasible to reduce the rate of overdiagnosis by combining clever radiologic active surveillance through careful volumetric and solid component assessment, together with the accurate use of FDG-PET scans [8]. Here, we report the impressive case of a slow-growing GGO of the lung, known since 2005 and under active surveillance since 2015, which unexpectedly developed into a 5 cm GGO with a consistent solid component and consequently underwent resection, revealing to hide just an AIS surrounded by benign parenchymal changes, thus eluding our protocols aimed at minimizing overdiagnosis. This report presents a compelling case that underscores the complexities and challenges of managing GGOs within LCS program.
2. Case Presentation
A 71-year-old male patient, a great former smoker without any noteworthy comorbidity, was enrolled in the BioMILD LCS program in 2015. The BioMILD was a prospective trial investigating the combination of LDCT and plasma microRNAs to enhance the effectiveness of LCS through tailored screening intervals and a personalized risk assessment [9]. At the baseline LDCT scan, less than 1 cm of pure GGO of the right upper lobe of the lung was noted. Previous CT scans of the patient have been retrieved and retrospectively reviewed, revealing the presence of the identified GGO in 2005, with minimal dimensional differences; therefore, the lesion was placed under surveillance. Over the years, the GGO was substantially stable, without significant dimensional or densitometric variations (Table 1). In 2020, the solid component seemed to increase, and an FDG-PET was performed, without pathologic tracer uptake. Therefore, surveillance was resumed. The next year, the result of the LDCT was astonishing: the known GGO developed into a 5 cm mixed GGO, with a consistent solid component (1 cm) and fissure shrinkage (Figure 1), presenting a volume doubling time (VDT) of 233 days. An FDG-PET was performed, showing an increased and diffuse tracer uptake within the pulmonary lesion (Figure 2). The patient was completely asymptomatic, without any sign of current or past infection, and was offered VATS surgical excision of the lesion, driven by the concern that the diagnosis might have been delayed too much. During the procedure, the GGO was not detectable; therefore, upfront right upper lobectomy and hylo-mediastinal lymphadenectomy were performed. The postoperative course was complicated by the occurrence of chylothorax on the first postoperative day (POD), which was conservatively managed through fasting, parenteral nutrition, and intravenous somatostatin, leading to progressive resolution. The chest drain was removed on the 15th POD, and the patient was discharged the next day in good clinical condition. The histologic examination revealed that the excised lesion consisted of a non-mucinous AIS of 1.2 cm, within a 4 cm area of fibrotic and congested lung parenchyma at stage pTis pN0 (0/25). Three years later, eighteen years after the first occurrence of the GGO, the patient is alive without evidence of disease.

Table 1.
Dimensional and densitometric evolution of the right upper lobe lesion throughout the years.
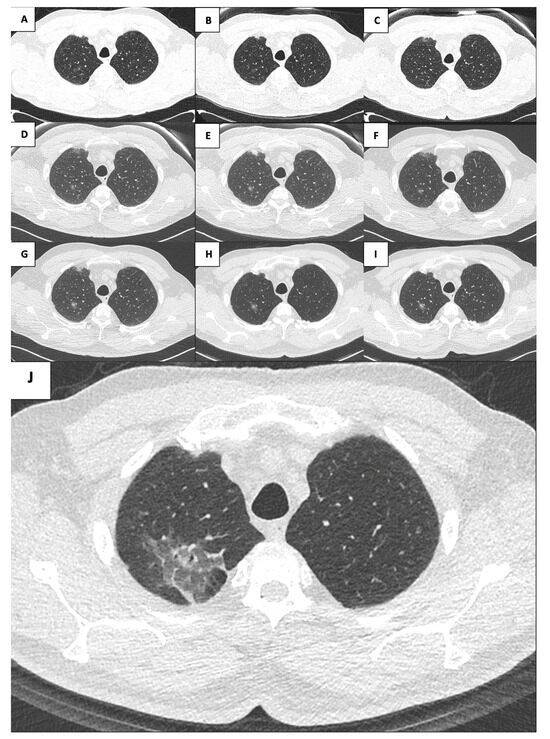
Figure 1.
Evolution of the right upper lobe lesion throughout the years ((A): 2005; (B): 2009; (C): 2011; (D): 2015; (E): 2016; (F): 2017; (G): 2018; (H): 2019; (I): 2020; (J): 2021).
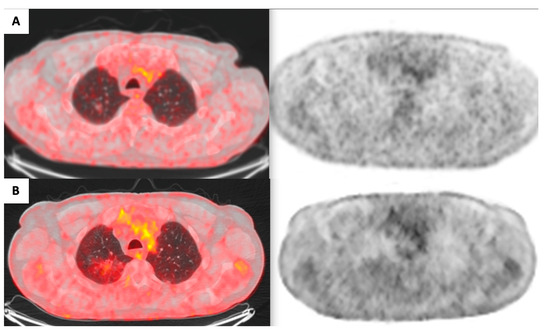
Figure 2.
Evolution of FDG uptake within the right upper lobe lesion ((A): 2020; (B): 2021).
3. Discussion
We have presented the unique case of a pure GGO of the lung that after almost 15 years abruptly developed into a 5 cm mixed GGO, leading to a right upper lobectomy even complicated with postoperative chylothorax, albeit being later pathologically diagnosed as only an unchanged AIS with benign surrounding parenchymal changes masquerading as progressive lepidic growth adenocarcinoma.
LCS programs aim to detect LCs at their earliest stage, allowing for radical and curative resection. Nevertheless, in such a setting, the risk of identifying indolent disease is significant. Pure and mixed GGOs may harbor pre-invasive, minimally invasive, or invasive malignancies: discriminating between them based on radiological appearance is anything but simple. Usually, the natural course consists of a small pure GGO, representing an atypical adenomatous hyperplasia or an AIS, which slowly develops into a mixed GGO with initial consolidation, representing a minimally invasive adenocarcinoma, until the consolidation part grows, giving rise to an invasive adenocarcinoma [10]. So, all these categories represent different stages of the same disease, which should be identified and treated at the appropriate time.
Over a lifetime, only a small subset of GGOs will develop from pre-invasive to invasive neoplasia, usually characterized by increasing size and density, as well as developing a solid component [11,12]. Pleural tag and spiculation have been described as features of invasiveness [13]. Our case presented all these warning features, with sufficient likelihood to warrant excision; nevertheless, the pathologic examination showed a pre-invasive neoplasm, which perhaps could have been surveilled for other years before being removed.
AIS has been defined as a localized and small (<3 cm) adenocarcinoma, whose growth is confined along a pre-existing alveolar structure without vascular, stromal, or pleural invasion, presenting lepidic growth [14]. Typically, AIS manifests radiologically as pure GGO, categorized by the former Noguchi type A, whereas occasionally it may occur as a part-solid GGO, classified by the former Noguchi type B [15], due to alveolar collapse, benign scars, or aggregates of histocytes or lymphocytes within alveolar spaces or walls, as illustrated by our case.
In the literature, AIS is often described as a GGO with an approximate diameter of 1 cm [16]. However, there have been reports of larger AIS lesions, with the largest documented size being up to 2.4 cm [17]. Our case showed a significantly larger lesion, prompting us to adopt a proactive approach.
Furthermore, another element misleading us was the accelerated VDT. An average VDT of 457 days has been described for mixed GGOs [18]. According to the NELSON trial [19], a VDT of 400 days is the optimal cut-off to distinguish indolent from malignant pulmonary lesions, whereas our lesion has shown a clearly lower VDT.
Considering the circumstances, surgery was unavoidable. A preoperative needle biopsy was taken into account, but after a multidisciplinary discussion, it was deemed unnecessary. On the one hand, some could argue that a potentially lethal neoplasia had been eradicated in the early stage, providing a definitive cure. Indeed, the key feature of AIS is the 100% disease-specific survival when the lesion is fully resected [20,21]. On the other hand, wedge resection has recently proven to be curative for AIS [22], suggesting that extensive surgical procedures may not be necessary in such cases. Our patient, although being finally satisfied from the treatment, faced significant postoperative complications, likely associated with the magnitude of the surgical intervention, and which could have probably been avoided if a less extensive approach was adopted. Nevertheless, in our case, less extensive resection was likely unfeasible due to the extent of the GGO on the preoperative CT scan. It could be disputed that a less extensive lymphadenectomy should have been performed to avoid chylothorax, but in surgical oncology, we strongly believe that radical lymphadenectomy should be a surgical tenet to be followed.
Overtreatment is a significant consequence of overdiagnosis [23]. When GGOs are detected, it often causes fear and anxiety among patients. This emotional response drives them to seek aggressive diagnostic and therapeutic interventions to prevent the progression of GGOs. Meanwhile, many healthcare professionals do not proactively assess whether these nodules will necessarily impact a patient’s quality of life. Instead, they tend to act based on patient requests, which can lead to overtreatment.
The standard treatment for lung cancer is surgical removal. However, regardless of advancements in surgical techniques, organ damage from surgery is inevitable. This can result in unnecessary physical and psychological harm, especially in cases where lung cancer would have not caused symptoms or death within the patient’s lifespan if left untreated.
Overdiagnosis and overtreatment in a patient population can lower disease-specific mortality rates, but they do not lead to a decrease in overall mortality from all causes [24]. No medical procedure is exempt from complications, even more so if invasive.
The primary goal remains to achieve the best possible outcomes and quality of life while minimizing the risks and side effects associated with treatment. In the management of lung cancer, it is crucial to practice personalized medicine, selecting treatments based on factors such as the tumor’s location and size, the patient’s overall health, and the potential for complications. Moving forward, the focus should shift from advocating for the most aggressive treatment to promoting the least invasive yet effective options. Furthermore, encouraging shared decision-making between patients and physicians could provide new strategies to address the issue of surgical overtreatment.
Several reports on resected GGOs are presented in the literature: GGO hiding the adenoma-to-carcinoma sequence [25], rapidly progressive GGO showing invasive papillary adenocarcinoma [26], fluctuating GGO showing lepidic growth adenocarcinoma [27], slow-growing cavitary lesion harboring a well-differentiated adenocarcinoma followed-up for 9 years [28], pure GGOs nesting invasive adenocarcinomas [29], papillary predominant adenocarcinoma presenting as 1.7 cm mixed GGO which recurred 10 years after resection [30], rapidly growing GGO showing an inflammatory pseudotumor [31], invasive foci of adenocarcinoma in smaller than 1 cm pure GGO [32], adenocarcinoma within a multiloculated cystic GGO [33], and adenocarcinoma in situ hiding within a 6 mm GGO [34]. Anyway, to the best of our knowledge, our report stands out for its singularity: a 1 cm pure GGO which after 15 years of substantial stability developed into a 5 cm mixed GGO, later revealing to hide an unchanged 1 cm AIS with surrounding benign modifications.
In light of our strict active surveillance protocol, the presented case may be a setback; nonetheless, a worse setback would have been to miss the resection of a supposed invasive adenocarcinoma, and we find it crucial to share this experience within the scientific community. In our opinion, to err on the side of caution in this circumstance is not a failure; instead, it is the cost of performing lung cancer surveillance. This serves as a reminder of the potential behavior of pre-invasive lesions, which, despite surveillance efforts, can mimic invasive neoplasia and may lead to overtreatment.
4. Conclusions
GGOs’ behavior still surprises us. LCS holds the potential to revolutionize the prognosis for LC patients. However, the persistent risk of overdiagnosis requires careful consideration. To mitigate this risk, we have instituted an active surveillance protocol, which turned out to be highly effective, still recognizing that complete eradication of the risk is unattainable based on our experience. The case here described represents the exception that proves the rule, underscoring the challenges to identify invasive LCs among indolent lesions and emphasizing the need to personalize screening protocols and to minimize unnecessary interventions.
Author Contributions
R.O.: Conceptualization, Methodology, Writing—Original Draft, Writing—Review and Editing, Visualization; G.M.: Formal Analysis, Writing—Original Draft, Writing—Review and Editing; L.R.: Formal Analysis, Investigation, Resources, Data Curation; U.P.: Conceptualization, Validation, Investigation, Writing—Original Draft, Supervision, Project Administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study by local laws, due to the nature of the report itself (retrospective, no change to usual clinical practice).
Informed Consent Statement
Informed consent was obtained from the subject involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Field, J.K.; Vulkan, D.; Davies, M.P.A.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Gosney, J.; Green, B.A.; Holemans, J.A.; et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg. Health Eur. 2021, 10, 100179. [Google Scholar] [CrossRef] [PubMed]
- Voss, T.; Krag, M.; Martiny, F.; Heleno, B.; Jørgensen, K.J.; Brandt Brodersen, J. Quantification of overdiagnosis in randomised trials of cancer screening: An overview and re-analysis of systematic reviews. Cancer Epidemiol. 2023, 84, 102352. [Google Scholar] [CrossRef] [PubMed]
- Jett, J.R.; Midthun, D.E. Commentary: CT screening for lung cancer—Caveat emptor. Oncologist 2008, 13, 439–444. [Google Scholar] [CrossRef]
- Austin, J.H.; Garg, K.; Aberle, D.; Yankelevitz, D.; Kuriyama, K.; Lee, H.J.; Brambilla, E.; Travis, W.D. Radiologic implications of the 2011 classification of adenocarcinoma of the lung. Radiology 2013, 266, 62–71. [Google Scholar] [CrossRef]
- Moro-Sibilot, D.; Fievet, F.; Jeanmart, M.; Lantuejoul, S.; Arbib, F.; Laverribre, M.H.; Brambilla, E.; Brambilla, C. Clinical prognostic indicators of high-grade pre-invasive bronchial lesions. Eur. Respir. J. 2004, 24, 24–29. [Google Scholar] [CrossRef]
- Russell, P.A.; Wainer, Z.; Wright, G.M.; Daniels, M.; Conron, M.; Williams, R.A. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J. Thorac. Oncol. 2011, 6, 1496–1504. [Google Scholar] [CrossRef]
- Silva, M.; Prokop, M.; Jacobs, C.; Capretti, G.; Sverzellati, N.; Ciompi, F.; van Ginneken, B.; Schaefer-Prokop, C.M.; Galeone, C.; Marchianò, A.; et al. Long-Term Active Surveillance of Screening Detected Subsolid Nodules is a Safe Strategy to Reduce Overtreatment. J. Thorac. Oncol. 2018, 13, 1454–1463. [Google Scholar] [CrossRef]
- Pastorino, U.; Boeri, M.; Sestini, S.; Sabia, F.; Milanese, G.; Silva, M.; Suatoni, P.; Verri, C.; Cantarutti, A.; Sverzellati, N.; et al. Baseline computed tomography screening and blood microRNA predict lung cancer risk and define adequate intervals in the BioMILD trial. Ann. Oncol. 2022, 33, 395–405. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Bolejack, V.; Crowley, J.; Ball, D.; Kim, J.; Lyons, G.; Rice, T.; Suzuki, K.; Thomas, C.F., Jr.; Travis, W.D.; et al. IASLC Staging and Prognostic Factors Committee, Advisory Boards and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac. Oncol. 2015, 10, 990–1003. [Google Scholar]
- Infante, M.; Berghmans, T.; Heuvelmans, M.A.; Hillerdal, G.; Oudkerk, M. Slow-growing lung cancer as an emerging entity: From screening to clinical management. Eur. Respir. J. 2013, 42, 1706–1722. [Google Scholar] [CrossRef] [PubMed]
- Godoy, M.C.; Sabloff, B.; Naidich, D.P. Subsolid pulmonary nodules: Imaging evaluation and strategic management. Curr. Opin Pulm. Med. 2012, 18, 304–312. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Liu, J. Prediction of the Invasiveness of Ground-Glass Nodules in Lung Adenocarcinoma by Radiomics Analysis Using High-Resolution Computed Tomography Imaging. Cancer Control 2022, 29, 10732748221089408. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; Van Schil, P.E.; et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef]
- Noguchi, M.; Morikawa, A.; Kawasaki, M.; Matsuno, Y.; Yamada, T.; Hirohashi, S.; Kondo, H.; Shimosato, Y. Small adenocarcinoma of the lung. Histol. Charact. Prognosis. Cancer 1995, 75, 2844–2852. [Google Scholar]
- Liu, J.; Yang, X.; Li, Y.; Xu, H.; He, C.; Zhou, P.; Qing, H. Predicting the Invasiveness of Pulmonary Adenocarcinomas in Pure Ground-Glass Nodules Using the Nodule Diameter: A Systematic Review, Meta-Analysis, and Validation in an Independent Cohort. Diagnostics 2024, 14, 147. [Google Scholar] [CrossRef]
- Ishida, H.; Shimizu, Y.; Sakaguchi, H.; Nitanda, H.; Kaneko, K.; Yamazaki, N.; Yanagihara, A.; Taguchi, R.; Sakai, F.; Yasuda, M.; et al. Distinctive clinicopathological features of adenocarcinoma in situ and minimally invasive adenocarcinoma of the lung: A retrospective study. Lung Cancer 2019, 129, 16–21. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sone, S.; Takashima, S.; Li, F.; Yang, Z.G.; Maruyama, Y.; Watanabe, T. Growth rate of small lung cancers detected on mass CT screening. Br. J. Radiol. 2000, 73, 1252–1259. [Google Scholar] [CrossRef]
- Yousaf-Khan, U.; van der Aalst, C.; de Jong, P.A.; Heuvelmans, M.; Scholten, E.; Lammers, J.W.; van Ooijen, P.; Nackaerts, K.; Weenink, C.; Groen, H.; et al. Final screening round of the NELSON lung cancer screening trial: The effect of a 2.5-year screening interval. Thorax 2017, 72, 48–56. [Google Scholar] [CrossRef]
- Kadota, K.; Villena-Vargas, J.; Yoshizawa, A.; Motoi, N.; Sima, C.S.; Riely, G.J.; Rusch, V.W.; Adusumilli, P.S.; Travis, W.D. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am. J. Surg. Pathol. 2014, 38, 448–460. [Google Scholar] [CrossRef]
- Yotsukura, M.; Asamura, H.; Motoi, N.; Kashima, J.; Yoshida, Y.; Nakagawa, K.; Shiraishi, K.; Kohno, T.; Yatabe, Y.; Watanabe, S.I. Long-Term Prognosis of Patients with Resected Adenocarcinoma In Situ and Minimally Invasive Adenocarcinoma of the Lung. J. Thorac. Oncol. 2021, 16, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Deng, C.; Wang, S.; Li, Y.; Zhang, Y.; Chen, H. Ten-year follow-up of lung cancer patients with resected adenocarcinoma in situ or minimally invasive adenocarcinoma: Wedge resection is curative. J. Thorac. Cardiovasc. Surg. 2022, 164, 1614–1622.e1. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, C.; Ye, X. Overdiagnosis and overtreatment of ground-glass nodule-like lung cancer. Asia Pac. J. Clin. Oncol. 2024, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ledda, R.E.; Funk, G.C.; Sverzellati, N. The pros and cons of lung cancer screening. Eur. Radiol. 2024, 1–9. [Google Scholar] [CrossRef]
- Nakamura, H.; Hirata, T.; Taguchi, M.; Kitamura, H. Ground-glass opacities showing an adenoma-to-carcinoma sequence in the lung. Gen. Thorac. Cardiovasc. Surg. 2008, 56, 421–423. [Google Scholar] [CrossRef]
- Kaneda, H.; Sakaida, N.; Saito, T.; Maniwa, T.; Uemura, Y.; Saito, Y. Appearance of bronchioloalveolar carcinoma and the rapid progression into invasive papillary adenocarcinoma. Gen. Thorac. Cardiovasc. Surg. 2009, 57, 224–227. [Google Scholar] [CrossRef]
- Sadohara, J.; Fujimoto, K.; Terasaki, H.; Nonoshita, M.; Hayabuchi, N. Bronchioloalveolar carcinoma with fluctuating extent of consolidation on chest radiography. J. Thorac. Imaging 2004, 19, 63–66. [Google Scholar] [CrossRef]
- Shaw, J.P.; Bejarano, P.A.; Thurer, R.J. Pseudocavitating bronchioloalveolar carcinoma followed over a decade. Ann. Thorac. Surg. 2008, 85, 1432–1434. [Google Scholar] [CrossRef]
- Ichinose, J.; Kohno, T.; Fujimori, S.; Harano, T.; Suzuki, S.; Fujii, T. Invasiveness and malignant potential of pulmonary lesions presenting as pure ground-glass opacities. Ann. Thorac. Cardiovasc. Surg. 2014, 20, 347–352. [Google Scholar] [CrossRef]
- Sekihara, K.; Yoshida, J.; Oda, M.; Oki, T.; Ueda, T.; Ito, T.; Miyoshi, T.; Aokage, K.; Tane, K.; Tsuboi, M. Delayed cut-end recurrence after wedge resection for pulmonary ground-glass opacity adenocarcinoma despite negative surgical margin. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 644–648. [Google Scholar] [CrossRef]
- Yano, Y.; Mori, M.; Kagami, S.; Fushitani, K.; Sugano, T.; Namba, Y.; Yoneda, T.; Yokota, S.; Maeda, H.; Ueda, K. Inflammatory pseudotumor of the lung with rapid growth. Intern Med. 2009, 48, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Heidinger, B.H.; Anderson, K.R.; Nemec, U.; Costa, D.B.; Gangadharan, S.P.; VanderLaan, P.A.; Bankier, A.A. Lung Adenocarcinoma Manifesting as Pure Ground-Glass Nodules: Correlating CT Size, Volume, Density, and Roundness with Histopathologic Invasion and Size. J. Thorac. Oncol. 2017, 12, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Harada, T.; Fuke, S.; Konishi, J.; Yamazaki, K.; Kaji, M.; Morikawa, T.; Ota, S.; Itoh, T.; Dosaka-Akita, H.; et al. Lung adenocarcinoma presenting with enlarged and multiloculated cystic lesions over 2 years. Respir. Care 2004, 49, 1522–1524. [Google Scholar] [PubMed]
- Nagata, M.; Otani, S.; Kanai, Y.; Yamamoto, S.; Tsubochi, H.; Endo, S. Laser-Trélat sign improved after the resection of a tiny ground-grass nodule: A case report. J. Surg. Case Rep. 2021, 2021, rjab275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).