Postoperative Multimodal Approach to Pain Control in Anterior Cruciate Ligament Autograft Surgery: A Single-Center Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Ethics Committee
2.2. Detailed Methodology, Randomization, and Allocation
2.3. Method Overview
- Group 1 (Fentanyl Only): Received a pre-anesthetic bolus of 10 mL 0.9% NaCl, followed by placebo infusions mimicking the timing of active drug administrations in the other groups. Intravenous fentanyl boluses of 100 µg were administered as needed for hemodynamic stability.
- Group 2 (Fentanyl + Lidocaine): Began with an intravenous lidocaine bolus of up to 1.5 mg/kg (max 150 mg), then a continuous infusion at 2 mg/kg/h (max 200 mg/h), alongside placebo infusions mimicking the magnesium sulfate rate in Group 3.
- Group 3 (Fentanyl + Lidocaine + Magnesium Sulfate): Received identical lidocaine dosing as Group FL and a continuous magnesium sulfate infusion at 70 mg/kg/h. Both medications were administered intravenously using separate syringe pumps.
2.3.1. Common Anesthesia Technique across Groups
2.3.2. Surgical and Postoperative Care
2.4. Data Analysis Overview
2.5. Descriptive Statistics
2.6. Comparative Analysis
2.7. Assessment of Postoperative Pain Control
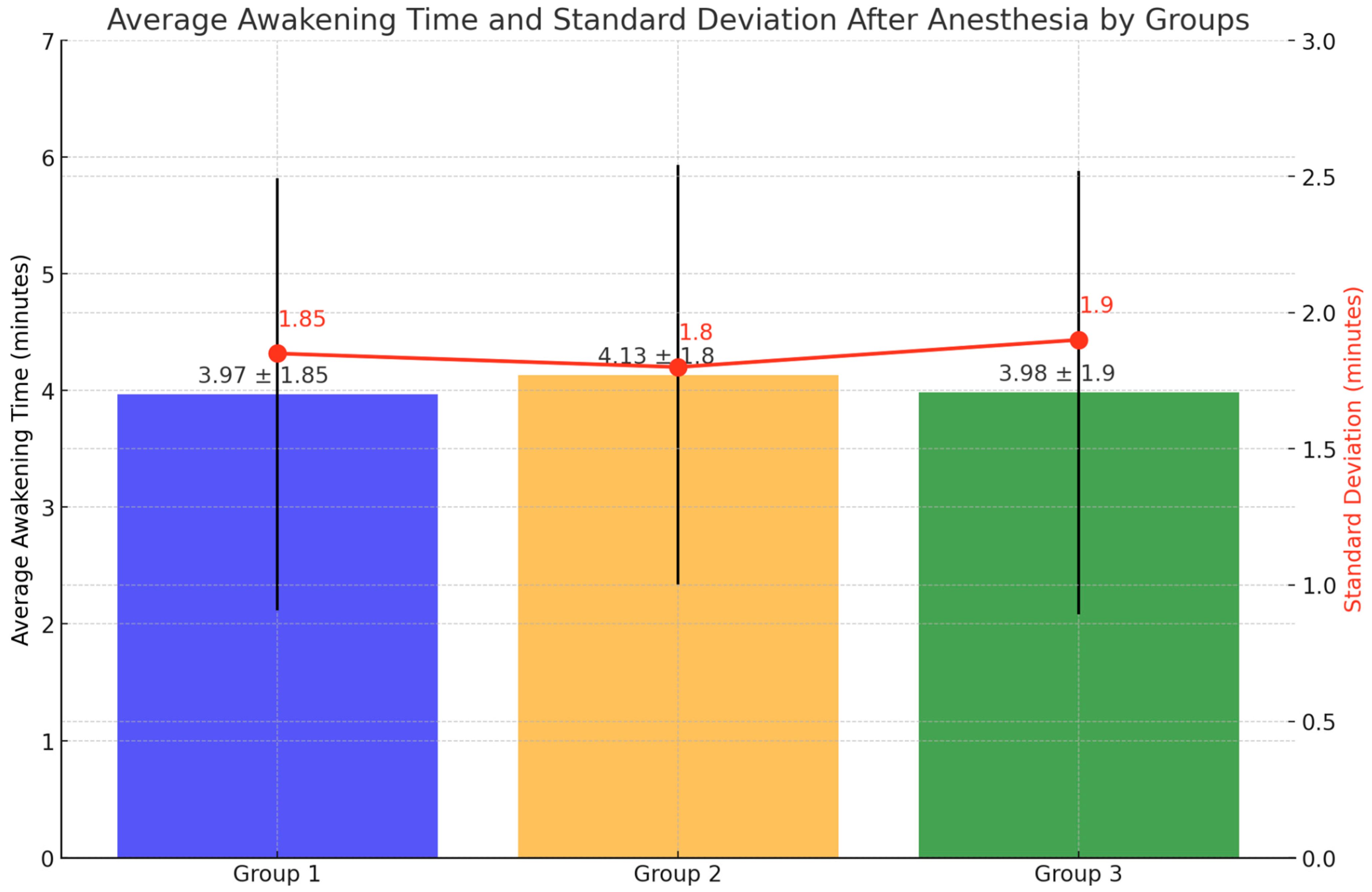
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaremuk, A.M.; Lisitsyn, M.P.; Atlukhanov, R.Y. Surgery of anterior cruciate ligament in knee joint. Comparative analysis of plastic reconstruction methods regarding anterior cruciate ligament in knee joint (BTB and STGT). Endosc. Surg. 2015, 21, 34–38. (In Russian) [Google Scholar] [CrossRef]
- Goncharov, E.N.; Koval, O.A.; Bezuglov, E.N.; Vetoshkin, A.A.; Goncharov, N.G.; Ramirez, M.E.; Nurmukhametov, R.; Montemurro, N. Outcome of Primary Anterior Cruciate Ligament Reconstruction with Peroneus Longus and Bone–Patellar Tendon–Bone Autografts: A Clinical Comparative Study. Surgeries 2023, 4, 434–445. [Google Scholar] [CrossRef]
- Goncharov, E.N.; Koval, O.A.; Dubrov, V.E.; Bezuglov, E.N.; Alekhin, A.A.; Goncharov, N.G. Mid-Term Results of Simultaneous Reconstruction of Anterior Cruciate and Anterolateral Ligaments in Athletes. Traumatol. Orthop. Russ. 2020, 26, 62–71. [Google Scholar] [CrossRef]
- Joseph, A.M.; Collins, C.L.; Henke, N.M.; Yard, E.E.; Fields, S.K.; Comstock, R.D. A Multisport Epidemiologic Comparison of Anterior Cruciate Ligament Injuries in High School Athletics. J. Athl. Train. 2013, 48, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Korolev, A.V.; Afanasyev, A.P.; Il’in, D.O.; Gerasimov, D.O.; Ryazantsev, M.S.; Kadantsev, P.M.; Zaripov, A.R. Damage of the knee posterior cruciate ligament: Biomechanics, basic diagnostics, treatment and secondary osteoarthritis prevention directions. Pirogov Russ. J. Surg. Khirurgiya. Zurnal Im. N.I. Pirogova. 2020, 9, 130–136. (In Russian) [Google Scholar] [CrossRef]
- Skvortsov, D.; Kaurkin, S.; Goncharov, E.; Akhpashev, A. Knee joint function and walking biomechanics in patients in acute phase anterior cruciate ligament tear. Int. Orthop. 2020, 44, 885–891. [Google Scholar] [CrossRef]
- Goncharov, E.N.; Koval, O.A.; Dubrov, V.E.; Bezuglov, E.N.; Filimonova, A.M.; Goncharov, N.G. Clinical experience with combined reconstruction of the anterior cruciate and anterolateral ligaments of the knee in sportsmen. Int. Orthop. 2019, 43, 2781–2788. [Google Scholar] [CrossRef]
- Xu, S.; Wang, S.; Hu, S.; Ju, X.; Li, Q.; Li, Y. Effects of lidocaine, dexmedetomidine, and their combination infusion on postoperative nausea and vomiting following laparoscopic hysterectomy: A randomized controlled trial. BMC Anesthesiol. 2021, 21, 199. [Google Scholar] [CrossRef]
- McIsaac, D.I.; Ladha, K.S. Postoperative Opioid Prescribing: Finding the Balance. Anesthesiology 2022, 137, 131–133. [Google Scholar] [CrossRef]
- Elmallah, R.K.; Chughtai, M.; Khlopas, A.; Newman, J.M.; Stearns, K.L.; Roche, M.; Kelly, M.A.; Harwin, S.F.; Mont, M.A. Pain Control in Total Knee Arthroplasty. J. Knee Surg. 2018, 31, 504–513. [Google Scholar] [CrossRef]
- Elmallah, R.K.; Cherian, J.J.; Pierce, T.P.; Jauregui, J.J.; Harwin, S.F.; Mont, M.A. New and Common Perioperative Pain Management Techniques in Total Knee Arthroplasty. J. Knee Surg. 2016, 29, 169–178. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, H.; Zou, X. The effect of a combination of lidocaine and magnesium sulphate on postoperative pain. Eur. J. Anaesthesiol. 2021, 38, 95. [Google Scholar] [CrossRef]
- Na, H.S.; Ryu, J.H.; Do, S.H. The Role of Magnesium in Pain. In Magnesium in the Central Nervous System; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; Volume 1, p. 1. [Google Scholar]
- Shin, H.J.; Na, H.S.; Do, S.H. Magnesium and Pain. Nutrients 2020, 12, 2184. [Google Scholar] [CrossRef]
- Srebro, D.; Vuckovic, S.; Milovanovic, A.; Kosutic, J.; Vujovic, K.S.; Prostran, M. Magnesium in Pain Research: State of the Art. Curr. Med. Chem. 2017, 24, 424–434. [Google Scholar]
- Toleska, M.; Dimitrovski, A.; Dimitrovska, N.T. Postoperative Nausea and Vomiting in Opioid-Free Anesthesia vs. Opioid Based Anesthesia in Laparoscopic Cholecystectomy. Prilozi 2022, 43, 101–108. [Google Scholar] [CrossRef]
- Beyuo, T.K.; Lawrence, E.R.; Kobernik, E.K.; Oppong, S.A. A novel 12-hour versus 24-hour magnesium sulfate regimen in the management of eclampsia and preeclampsia in Ghana (MOPEP Study): A randomized controlled trial. Int. J. Gynaecol. Obstet. 2022, 159, 495–504. [Google Scholar] [CrossRef]
- Shi, S.; Fan, W.; Tao, R.; Xu, H.; Lu, Y.; Han, F.; Yang, S.; Zhou, X.; Zhou, Z.; Wan, F. Natural Biomineralization-Inspired Magnesium Silicate Composite Coating Upregulates Osteogenesis, Enabling Strong Anterior Cruciate Ligament Graft-Bone Healing In Vivo. ACS Biomater. Sci. Eng. 2021, 7, 133–143. [Google Scholar] [CrossRef]
- Di Benedetto, P.; Di Benedetto, E.; Fiocchi, A.; Beltrame, A.; Causero, A. Causes of failure of anterior cruciate ligament reconstruction and revision surgical strategies. Knee Surg. Relat. Res. 2016, 28, 319–324. [Google Scholar] [CrossRef]
- Moutzouros, V.; Jildeh, T.R.; Khalil, L.S.; Schwartz, K.; Hasan, L.; Matar, R.N.; Okoroha, K.R. A Multimodal protocol to diminish pain following common orthopedic sports procedures: Can we eliminate postoperative opioids? Arthroscopy 2020, 36, 2249–2257. [Google Scholar] [CrossRef]
- Safavi, M.; Honarmand, A.; Sahaf, A.S.; Sahaf, S.M.; Attari, M.; Payandeh, M.; Iazdani, A.; Norian, N. Magnesium sulfate versus Lidocaine pretreatment for prevention of pain on etomidate injection: A randomized, double-blinded placebo controlled trial. J. Res. Pharm. Pract. 2015, 4, 4–8. [Google Scholar]
- Soleimanpour, H.; Imani, F.; Dolati, S.; Soleimanpour, M.; Shahsavarinia, K. Management of pain using magnesium sulphate: A narrative review. Postgrad Med. 2022, 134, 260–266. [Google Scholar] [CrossRef]
- Owusu Obeng, A.; Hamadeh, I.; Smith, M. Review of Opioid Pharmacogenetics and Considerations for Pain Management. Pharmacotherapy 2017, 37, 1105–1121. [Google Scholar] [CrossRef]
- Ren, Z.Y.; Xu, X.Q.; Bao, Y.P.; He, J.; Shi, L.; Deng, J.-H.; Gao, X.-J.; Tang, H.-L.; Wang, Y.-M.; Lu, L. The impact of genetic variation on sensitivity to opioid analgesics in patients with postoperative pain: A systematic review and meta-analysis. Pain Physician 2015, 18, 131–152. [Google Scholar]
- Yung, E.M.; Brull, R.; Albrecht, E.; Joshi, G.P.; Abdallah, F.W. Evidence Basis for regional anesthesia in ambulatory anterior cruciate ligament reconstruction: Part III: Local instillation analgesia—A systematic review and meta-analysis. Anesth. Analg. 2019, 128, 426–437. [Google Scholar] [CrossRef]
- Muthiah, T.; Arora, M.K.; Trikha, A.; Sunder, R.A.; Prasad, G.; Singh, P.M. Efficacy of magnesium as an adjuvant to bupivacaine in 3-in-1 nerve block for arthroscopic anterior cruciate ligament repair. Indian J. Anaesth. 2016, 60, 491–495. [Google Scholar]
- Gupta, R.; Kapoor, D.; Kapoor, L.; Malhotra, A.; Masih, G.D.; Kapoor, A.; Joshi, S. Immediate post-operative pain in anterior cruciate ligament reconstruction surgery with bone patellar tendon bone graft versus hamstring graft. J. Orthop. Surg. Res. 2016, 11, 1–6. [Google Scholar] [CrossRef]
- Ekmekci, P.; Bengisun, Z.K.; Akan, B.; Kazbek, B.K.; Ozkan, K.S.; Suer, A.H. The effect of magnesium added to levobupivacaine for femoral nerve block on postoperative analgesia in patients undergoing ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1119–1124. [Google Scholar] [CrossRef]
- Sedighinejad, A.; Haghighi, M.; Naderi Nabi, B.; Rahimzadeh, P.; Mirbolook, A.; Mardani-Kivi, M.; Nekufard, M.; Biazar, G. Magnesium sulfate and sufentanil for patient-controlled analgesia in orthopedic surgery. Anesth. Pain Med. 2014, 4, e11334. [Google Scholar] [CrossRef]
- Peng, Y.N.; Sung, F.C.; Huang, M.L.; Lin, C.L.; Kao, C.H. The use of intravenous magnesium sulfate on postoperative analgesia in orthopedic surgery: A systematic review of randomized controlled trials. Medicine 2018, 97, e13583. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, E.M.; Lavoie-Gagne, O.; Lu, Y.; Cohn, M.R.; Chang, E.; Yanke, A.B.; Cole, B.J.; Verma, N.N.; Forsythe, B. Preoperative Opioid use predicts prolonged postoperative opioid use and inferior patient outcomes following anterior cruciate ligament reconstruction. Arthroscopy 2020, 36, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Anthony, C.A.; Westermann, R.W.; Bedard, N.; Glass, N.; Bollier, M.; Hettrich, C.M.; Wolf, B.R. Opioid demand before and after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2017, 45, 3098–3103. [Google Scholar] [CrossRef]
- Li, M.M.J.; Ocay, D.D.; Larche, C.L.; Vickers, K.; Saran, N.; Ouellet, J.A.; Gélinas, C.; Ferland, C.E. Validation of the Critical-Care Pain Observation Tool (CPOT) in pediatric patients undergoing orthopedic surgery. Can. J. Pain 2023, 7, 2156332. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.P.; Ogunnaike, B.O. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesth. Clin. North Am. 2005, 23, 21–36. [Google Scholar] [CrossRef]
- Bolia, I.K.; Haratian, A.; Bell, J.A.; Hasan, L.K.; Saboori, N.; Palmer, R.; Petrigliano, F.A.; Weber, A.E. Managing Perioperative Pain After Anterior Cruciate Ligament (ACL) Reconstruction: Perspectives from a Sports Medicine Surgeon. Open Access J. Sports Med. 2021, 12, 129–138. [Google Scholar] [CrossRef]
- Koh, I.J.; Chang, C.B.; Seo, E.S.; Kim, S.J.; Seong, S.C.; Kim, T.K. Pain management by periarticular multimodal drug injection after anterior cruciate ligament reconstruction: A randomized, controlled study. Arthroscopy 2012, 28, 649–657. [Google Scholar] [CrossRef]
- Montemurro, N.; Ortenzi, V.; Naccarato, G.A.; Perrini, P. Angioleiomyoma of the knee: An uncommon cause of leg pain. A systematic review of the literature. Interdiscipl. Neurosurg. 2020, 22, 100877. [Google Scholar] [CrossRef]
- Pershad, J.; Todd, K.; Waters, T. Cost-effectiveness analysis of sedation and analgesia regimens during fracture manipulation in the pediatric emergency department. Pediatr. Emerg. Care 2006, 22, 729–736. [Google Scholar] [CrossRef]
- Modir, H.; Jafarirismani, R.; Almasi-Hashiani, A.; Shamaii, K. Efficacy appraisal of four regimens (granisetron, ketamine, dexmedetomidine, and lidocaine combined with fentanyl) for cystoscopy-associated sedation and analgesia and catheter-related bladder tolerance: A randomized clinical trial. Med. Gas Res. 2023, 13, 181–186. [Google Scholar] [CrossRef]
- Yari, M.; Saeb, M.; Golfam, P.; Makhloogh, Z. Analgesic efficacy of intra-articular morphine after arthroscopic knee surgery in sport injury patients. J. Inj. Violence Res. 2013, 5, 84–88. [Google Scholar] [CrossRef]
- Wang, X.; Jia, D.; Chen, X.; Xu, Y. Comparison of intra-articular low-dose sufentanil, ropivacaine, and combined sufentanil and ropivacaine on post-operative analgesia of isolated anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Graff, V.; Gabutti, L.; Treglia, G.; Pascale, M.; Anselmi, L.; Cafarotti, S.; La Regina, D.; Mongelli, F.; Saporito, A. Perioperative costs of local or regional anesthesia versus general anesthesia in the outpatient setting: A systematic review of recent literature. Braz. J. Anesthesiol. 2023, 73, 316–339. [Google Scholar] [CrossRef] [PubMed]
- Juncker, R.B.; Mirza, F.M.; Gagnier, J.J. Reduction in opioid use with perioperative non-pharmacologic analgesia in total knee arthroplasty and ACL reconstruction: A systematic review. SICOT J. 2021, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Murrone, D.; Romanelli, B.; Ierardi, A. Postoperative Textiloma Mimicking Intracranial Rebleeding in a Patient with Spontaneous Hemorrhage: Case Report and Review of the Literature. Case Rep Neurol. 2020, 12, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Pierozzi, E.; Inchingolo, A.M.; Pahwa, B.; De Carlo, A.; Palermo, A.; Scarola, R.; Dipalma, G.; Corsalini, M.; Inchingolo, A.D.; et al. New biograft solution, growth factors and bone regenerative approaches in neurosurgery, dentistry, and orthopedics: A review. Eur Rev Med Pharmacol Sci. 2023, 27, 7653–7664. [Google Scholar] [PubMed]
- Saka, T. Principles of postoperative anterior cruciate ligament rehabilitation. World J. Orthop. 2014, 5, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Sampognaro, G.; Harrell, R. Multimodal Postoperative Pain Control After Orthopaedic Surgery; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kruse, L.M.; Gray, B.; Wright, R.W. Rehabilitation after anterior cruciate ligament reconstruction: A systematic review. J. Bone Jt. Surg. 2012, 94, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Filbay, S.R.; Roemer, F.W.; Lohmander, L.S.; Turkiewicz, A.; Roos, E.M.; Frobell, R.; Englund, M. Evidence of ACL healing on MRI following ACL rupture treated with rehabilitation alone may be associated with better patient-reported outcomes: A secondary analysis from the KANON trial. Br. J. Sports Med. 2023, 57, 91–98. [Google Scholar] [CrossRef]
- Ruiz Ibán, M.A.; Maculé, F.; Torner, P.; Gil Garay, E.; Oteo-Álvaro, A.; López Millán, J.M.; Díaz Heredia, J.; Loza, E. SECOT-GEDOS consensus on pre-surgical pain management in knee and hip arthrosis. Rev. Esp. Cir. Ortop. Traumatol. 2015, 59, 186–199, In English, Spanish. [Google Scholar] [CrossRef] [PubMed]
- Filbay, S.R.; Ackerman, I.N.; Russell, T.G.; Crossley, K.M. Return to sport matters-longer-term quality of life after ACL reconstruction in people with knee difficulties. Scand. J. Med. Sci. Sports 2017, 27, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, E.N.; Koval, O.A.; Nikolaevich Bezuglov, E.; Encarnacion Ramirez, M.J.; Engelgard, M.; Igorevich, E.I.; Saporiti, A.; Valentinovich Kotenko, K.; Montemurro, N. Stromal Vascular Fraction Therapy for Knee Osteoarthritis: A Systematic Review. Medicina 2023, 59, 2090. [Google Scholar] [CrossRef]
- Montemurro, N. Telemedicine: Could it represent a new problem for spine surgeons to solve? Glob. Spine J. 2022, 12, 1306–1307. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, L.; Angelico, R.; Angrisani, M.; Zampogna, B.; Materazzo, M.; Sorge, R.; Giordano, L.; Meniconi, R.; Coppola, A.; SPIGC Survey Collaborative Group. How future surgery will benefit from SARS-CoV-2-related measures: A SPIGC survey conveying the perspective of Italian surgeons. Updates Surg. 2023, 75, 1711–1727. [Google Scholar] [CrossRef] [PubMed]
- Millenson, M.L. Mobile Health Applications. Health Aff. 2017, 36, 1144. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, E.N.; Koval, O.A.; Nikolaevich Bezuglov, E.; Aleksandrovich Vetoshkin, A.; Gavriilovich Goncharov, N.; Encarnación Ramirez, M.J.; Montemurro, N. Conservative Treatment in Avascular Necrosis of the Femoral Head: A Systematic Review. Med. Sci. 2024, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Perrini, P.; Pieri, F.; Montemurro, N.; Tiezzi, G.; Parenti, G.F. Thoracic extradural haematoma after epidural anaesthesia. Neurol. Sci. 2010, 31, 87–88. [Google Scholar] [CrossRef]

| Group | Medication | Initial Dose | Maintenance Dose | Route of Administration |
|---|---|---|---|---|
| Group 1 | Fentanyl | N/A | 100 µg boluses as needed | Intravenous |
| Group 2 | Fentanyl + | N/A | 100 µg boluses as needed | Intravenous |
| Lidocaine | Up to 1.5 mg/kg (max 150 mg) | 2 mg/kg/h (max 200 mg/h) | ||
| Group 3 | Fentanyl + | N/A | 100 µg boluses as needed | Intravenous |
| Lidocaine + | Up to 1.5 mg/kg (max 150 mg) | 2 mg/kg/h (max 200 mg/h) | ||
| Magnesium Sulfate | N/A | 70 mg/kg/h |
| Category | Description | Median/Means ± SD |
|---|---|---|
| Total Patients | Number of participants | 90 |
| Gender Distribution | Percentage male/female | Male: 70%; female: 30% |
| Age Range | Age range and median age | 16–59 years (mean 35 years ± 10 years) |
| Groups | Number of participants for groups | Group 1 (N = 30); Group 2 (N = 30); Group 3 (N = 30) |
| Catheter Sizes | Range and median size | Range: 1–7; 4 ± 1.5 |
| Average VAS Score | Post-surgery score | Median 3.5 ± 1.2 |
| Fentanyl Dosage (mg) | Median dosage used | 0.55 mg ± 0.15 mg |
| Operation Time (min) | Median operation time | 25–90 min (median 60 min ± 20 min) |
| Periods of Measurement | Specified measurement times | 30 min after surgery, morning, evening (N/A) |
| High VAS Instances | Percentage of high VAS scores | 15% |
| % of Absences | Percentage of absences | 2.22% |
| Non-Numerical Data | Presence of non-numerical data | 100% at the beginning |
| Unique Meanings | Variability across categories | Varied across categories |
| Scale Type | Type of data scale used | Nominal and number |
| Group | Indicator | M ± S, Control Point One | M ± S (%), Control Point Two | M ± S (%), Control Point Three | Value p |
|---|---|---|---|---|---|
| 1 Group (Fentanyl) | VAS | 4.03 ± 1.52 | 2.70 ± 1.29 (−33.06%) | 1.97 ± 1.10 (−51.24%) | <0.0001 |
| 2 Group (Fentanyl + Lidocaine) | VAS | 3.73 ± 1.55 | 2.83 ± 1.18 (−24.11%) | 1.87 ± 1.01 (−50.00%) | <0.0001 |
| 3 Group (Fentanyl + Lidocaine + Magnesium Sulfate) | VAS | 2.97 ± 1.90 | 2.50 ± 1.11 (−15.73%) | 1.77 ± 1.07 (−40.45%) | 0.0007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheykin, A.; Goncharov, E.N.; Koval, O.A.; Goncharov, N.; Bezuglov, E.; Vetoshkin, A.; Encarnacion Ramirez, M.D.J.; Montemurro, N. Postoperative Multimodal Approach to Pain Control in Anterior Cruciate Ligament Autograft Surgery: A Single-Center Series. Surgeries 2024, 5, 660-673. https://doi.org/10.3390/surgeries5030052
Cheykin A, Goncharov EN, Koval OA, Goncharov N, Bezuglov E, Vetoshkin A, Encarnacion Ramirez MDJ, Montemurro N. Postoperative Multimodal Approach to Pain Control in Anterior Cruciate Ligament Autograft Surgery: A Single-Center Series. Surgeries. 2024; 5(3):660-673. https://doi.org/10.3390/surgeries5030052
Chicago/Turabian StyleCheykin, Alexey, Evgeniy Nikolaevich Goncharov, Oleg Aleksandrovich Koval, Nikolay Goncharov, Eduard Bezuglov, Aleksandr Vetoshkin, Manuel De Jesus Encarnacion Ramirez, and Nicola Montemurro. 2024. "Postoperative Multimodal Approach to Pain Control in Anterior Cruciate Ligament Autograft Surgery: A Single-Center Series" Surgeries 5, no. 3: 660-673. https://doi.org/10.3390/surgeries5030052
APA StyleCheykin, A., Goncharov, E. N., Koval, O. A., Goncharov, N., Bezuglov, E., Vetoshkin, A., Encarnacion Ramirez, M. D. J., & Montemurro, N. (2024). Postoperative Multimodal Approach to Pain Control in Anterior Cruciate Ligament Autograft Surgery: A Single-Center Series. Surgeries, 5(3), 660-673. https://doi.org/10.3390/surgeries5030052








