Carotid Body Tumor Excision with and without Carotid Artery Reconstruction: Equivalency of 30-Day Outcomes over 12 Years in the American College of Surgery National Surgical Quality Improvement Program (ACS-NSQIP) Database
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamblin, W.R.; ReMine, W.H.; Sheps, S.G.; Harrison, E.G., Jr. Carotid body tumor (chemodectoma): Clinicopathologic analysis of ninety cases. Am. J. Surg. 1971, 122, 732–739. [Google Scholar] [CrossRef] [PubMed]
- García, M.A.S.; Pendás, J.L.L.; Tapia, J.P.R.; Rostán, G.G.; Fente, V.S.; Pelaz, A.C.; Nieto, C.S. Head and neck paragangliomas: Revision of 89 cases in 73 patients. Acta Otorrinolaringol. Esp. 2007, 58, 94–100. [Google Scholar]
- Tang, H.; Jiang, X.; Xue, S.; Fu, W.; Tang, X.; Guo, D. Long-Term Surgical Outcomes of Carotid Body Tumors With Pathological Fibrosis: A Cohort Study. Front Oncol. 2021, 11, 684600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ingraham, A.M.; Cohen, M.E.; Ko, C.Y.; Hall, B.L. A current profile and assessment of north american cholecystectomy: Results from the american college of surgeons national surgical quality improvement program. J. Am. Coll. Surg. 2010, 211, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.J.; Passman, M.A.; Dattilo, J.B.; Guzman, R.J.; Naslund, T.C.; Netterville, J.L. Carotid body tumor resection: Does the need for vascular reconstruction worsen outcome. Ann. Vasc. Surg. 2006, 20, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Luna-Ortiz, K.; Rascon-Ortiz, M.; Villavicencio-Valencia, V.; Herrera-Gomez, A. Does Shamblin’s classification predict postoperative morbidity in carotid body tumors? A proposal to modify Shamblin’s classification. Eur. Arch. Otorhinolaryngol. 2006, 263, 171–175, Erratum in Eur. Arch Otorhinolaryngol. 2006, 263, 1161. [Google Scholar] [CrossRef] [PubMed]
- van der Bogt, K.E.; Vrancken Peeters, M.P.; van Baalen, J.M.; Hamming, J.F. Resection of carotid body tumors: Results of an evolving surgical technique. Ann. Surg. 2008, 247, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Kim, J.; Kim, S.H.; Lee, S.; Lim, Y.C.; Kim, J.W.; Choi, E.C. Surgical treatment of carotid body paragangliomas: Outcomes and complications according to the shamblin classification. Clin. Exp. Otorhinolaryngol. 2010, 3, 91–95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Texakalidis, P.; Charisis, N.; Giannopoulos, S.; Xenos, D.; Rangel-Castilla, L.; Tassiopoulos, A.K.; Jabbour, P.; Grossberg, J.A.; Machinis, T. Role of Preoperative Embolization in Carotid Body Tumor Surgery: A Systematic Review and Meta-Analysis. World Neurosurg. 2019, 42, 503–513.e2. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.; Sayed, A.; Elwan, H.; Fouad, F.M.; Kamal Eldin, H.; Khairy, H.; Elhindawy, K.; Taha, A.; Hefnawy, E. Carotid body tumors: A review of 25 years experience in diagnosis and management of 56 tumors. Ann. Vasc. Dis. 2014, 7, 292–299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sajid, M.S.; Hamilton, G.; Baker, D.M.; Joint Vascular Research Group. A multicenter review of carotid body tumour management. Eur. J. Vasc. Endovasc. Surg. 2007, 34, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Valdagni, R.; Amichetti, M. Radiation therapy of carotid body tumors. Am. J. Clin. Oncol. 1990, 13, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.T., Jr.; Gonzalez, J.A.; Rary, J.M.; Rush, D.S. Current concepts for the surgical management of carotid body tumor. Am. J. Surg. 2006, 191, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Liu, L.; Yao, H.; Hu, Y.; Ji, T.; Liu, X.; Zhang, C.; Qiu, W. A retrospective study in management of carotid body tumour. Br. J. Oral. Maxillofac. Surg. 2009, 47, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Kotelis, D.; Rizos, T.; Geisbüsch, P.; Attigah, N.; Ringleb, P.; Hacke, W.; Allenberg, J.-R.; Böckler, D. Late outcome after surgical management of carotid body tumors from a 20-year single-center experience. Langenbecks Arch Surg. 2009, 394, 339–344. [Google Scholar] [CrossRef]
- Kim, G.Y.; Lawrence, P.F.; Moridzadeh, R.S.; Zimmerman, K.; Munoz, A.; Luna-Ortiz, K.; Oderich, G.S.; de Francisco, J.; Ospina, J.; Huertas, S.; et al. New predictors of complications in carotid body tumor resection. J. Vasc. Surg. 2017, 65, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Heesterman, B.L.; de Pont, L.M.H.; Verbist, B.M.; van der Mey, A.G.L.; Corssmit, E.P.M.; Hes, F.J.; van Benthem, P.P.G.; Jansen, J.C. Age and Tumor Volume Predict Growth of Carotid and Vagal Body Paragangliomas. J. Neurol. Surg. B Skull. Base. 2017, 78, 497–505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoang, V.T.; Trinh, C.T.; Lai, T.A.K.; Doan, D.T.; Tran, T.T.T. Carotid body tumor: A case report and literature review. J. Radiol. Case Rep. 2019, 13, 19–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American Society of Interventional and Therapeutic Neuroradiology. Carotid artery balloon test occlusion. AJNR Am. J. Neuroradiol. 2001, 22 (Suppl. S8), S8–S9. [Google Scholar] [PubMed] [PubMed Central]
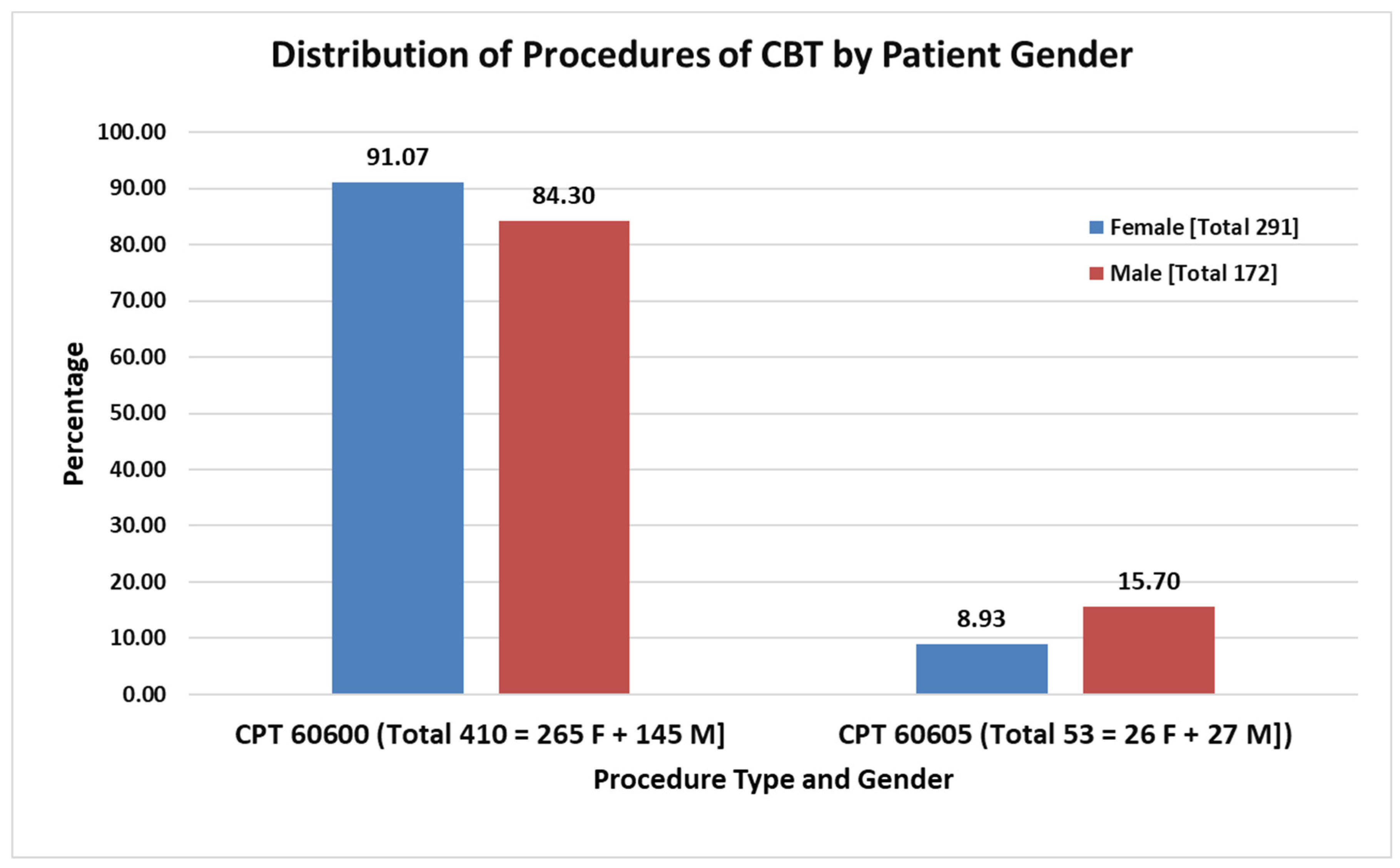
| Group A [CPT Code = 60600] [n = 410] | Group B [Code = 60605] [n = 53] | Total [N = 463] | p-Value | |
|---|---|---|---|---|
| Female Sex, n (%) | 265 (64.6) | 26 (49.1) | 291 (62.9) | 0.028 |
| Age, years, mean (SD) | 58.1 (16.0) | 53.1 (18.0) | 57.5 (16.2) | 0.035 |
| BMI, KG/M2, mean (SD) | 30.0 (7.2) | 28.0 (6.3) | 29.8 (7.1) | 0.05 |
| Medical Comorbidities, n (%) | ||||
| DM | 74 (18.0) | 9 (17.0) | 83 (17.9) | 0.855 |
| Smoking | 75 (18.3) | 9 (17.0) | 84 (18.1) | 0.854 |
| Dyspnea | 27 (6.6) | 3 (5.7) | 30 (6.5) | 1.00 |
| Functional Status: Partially or totally dependent | 5 (1.2) | 0 (0.0) | 5 (1.1) | 0.644 |
| Ventilator-dependent | 1 (0.2) | 1 (1.9) | 1 (0.4) | 0.216 |
| History of COPD | 10 (2.4) | 0 (0.0) | 10 (2.2) | 0.388 |
| History of CHF | 1(0.2) | 1 (1.9) | 2 (0.4) | 0.216 |
| HTN requiring Medication | 215 (52.4) | 22 (41.5) | 237 (51.2) | 0.144 |
| Acute Renal Failure | 1 (0.2) | 0 (0.0) | 1 (0.2) | 1.000 |
| Currently on dialysis | 2 (0.5) | 0 (0) | 2 (0.4) | 1.000 |
| Disseminated cancer | 1 (0.2) | 2 (3.8) | 3 (0.6) | 0.036 |
| Chronic wound | 2 (0.5) | 0 (0) | 2 (0.4) | 1.000 |
| Chronic Steroid Use | 10 (2.4) | 1 (1.9) | 11 (2.4) | 1.000 |
| Bleeding Disorders | 12 (2.9) | 2 (3.8) | 14 (3.0) | 1.000 |
| Present with sepsis | 1 (0.2) | 0 (0) | 1 (0.2) | 1.000 |
| Group A [CPT Code = 60600] [n = 410] | Group B [Code = 60605] [n = 53] | Total [N = 463] | p-Value | |
|---|---|---|---|---|
| Mortality (30-day) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 1.000 |
| Morbidity * | ||||
| ▪ Minor | 10 (2.4) | 1 (1.9) | 11 (2.4) | 1.000 |
| ▪ Serious | 16 (3.9) | 3 (5.7) | 19 (4.1) | 0.711 |
| ▪ Overall | 24 (5.9) | 4 (7.5) | 28 (6.0) | 0.758 |
| Return to operating room | 10 (2.4) | 2 (3.8) | 12 (2.6) | 0.636 |
| Readmission within 30 days | 8 (2.0) | 0 (0.0) | 8 (1.7) | 0.396 |
| Operative Time, minutes, mean (SD) | 138 (66) | 187 (107) | 144 (74) | <0.001 |
| Total Hospital LOS, days, median (IQR), | 2 (1, 3) | 2 (1, 4) | 2 (1, 4) | 0.134 |
| Group A [CPT Code = 60600] [n = 410] | Group B [Code = 60605] [n = 53] | Total [N = 463] | p-Value | |
|---|---|---|---|---|
| Superficial SSI | 2 (0.5) | 0 (0) | 2 (0.4) | 1.000 |
| Organ-Space SSI | 1 (0.2) | 0 (0) | 1 (0.2) | 1.000 |
| Pneumonia | 4 (1.0) | 1 (1.9) | 5 (1.1) | 1.000 |
| Unplanned Re-intubation | 2 (0.5) | 0 (0) | 2 (0.4) | 1.000 |
| Pulmonary Embolism | 1 (0.2) | 0 (0.0) | 1 (0.2) | 1.000 |
| Failure to wean of ventilation (>48 h) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 1.000 |
| Renal Failure | 1 (0.2) | 0 (0) | 1 (0.2) | 1.000 |
| Urinary tract infection (UTI) | 1 (0.2) | 0 (0) | 0 (0) | 1.000 |
| Cerebrovascular accident (CVA) | 5 (1.2) | 0 (0.0) | 5 (1.1) | 0.646 |
| Myocardial Infarction | 1 (0.2) | 0 (0.0) | 1 (0.2) | 1.000 |
| Bleeding (intra-op/post op) | 10 (2.4) | 3 (5.7) | 13 (2.8) | 0.177 |
| Deep venous thrombosis (DVT) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaney, M.; Ko, A.; Coster, S.; Shebrain, S.; Ryan, J. Carotid Body Tumor Excision with and without Carotid Artery Reconstruction: Equivalency of 30-Day Outcomes over 12 Years in the American College of Surgery National Surgical Quality Improvement Program (ACS-NSQIP) Database. Surgeries 2024, 5, 342-349. https://doi.org/10.3390/surgeries5020028
Chaney M, Ko A, Coster S, Shebrain S, Ryan J. Carotid Body Tumor Excision with and without Carotid Artery Reconstruction: Equivalency of 30-Day Outcomes over 12 Years in the American College of Surgery National Surgical Quality Improvement Program (ACS-NSQIP) Database. Surgeries. 2024; 5(2):342-349. https://doi.org/10.3390/surgeries5020028
Chicago/Turabian StyleChaney, Michael, Alexander Ko, Samuel Coster, Saad Shebrain, and Jason Ryan. 2024. "Carotid Body Tumor Excision with and without Carotid Artery Reconstruction: Equivalency of 30-Day Outcomes over 12 Years in the American College of Surgery National Surgical Quality Improvement Program (ACS-NSQIP) Database" Surgeries 5, no. 2: 342-349. https://doi.org/10.3390/surgeries5020028
APA StyleChaney, M., Ko, A., Coster, S., Shebrain, S., & Ryan, J. (2024). Carotid Body Tumor Excision with and without Carotid Artery Reconstruction: Equivalency of 30-Day Outcomes over 12 Years in the American College of Surgery National Surgical Quality Improvement Program (ACS-NSQIP) Database. Surgeries, 5(2), 342-349. https://doi.org/10.3390/surgeries5020028







