Danger Zone for Paramedian Forehead Flap Elevation: Maximizing Flap Length and Viability
Abstract
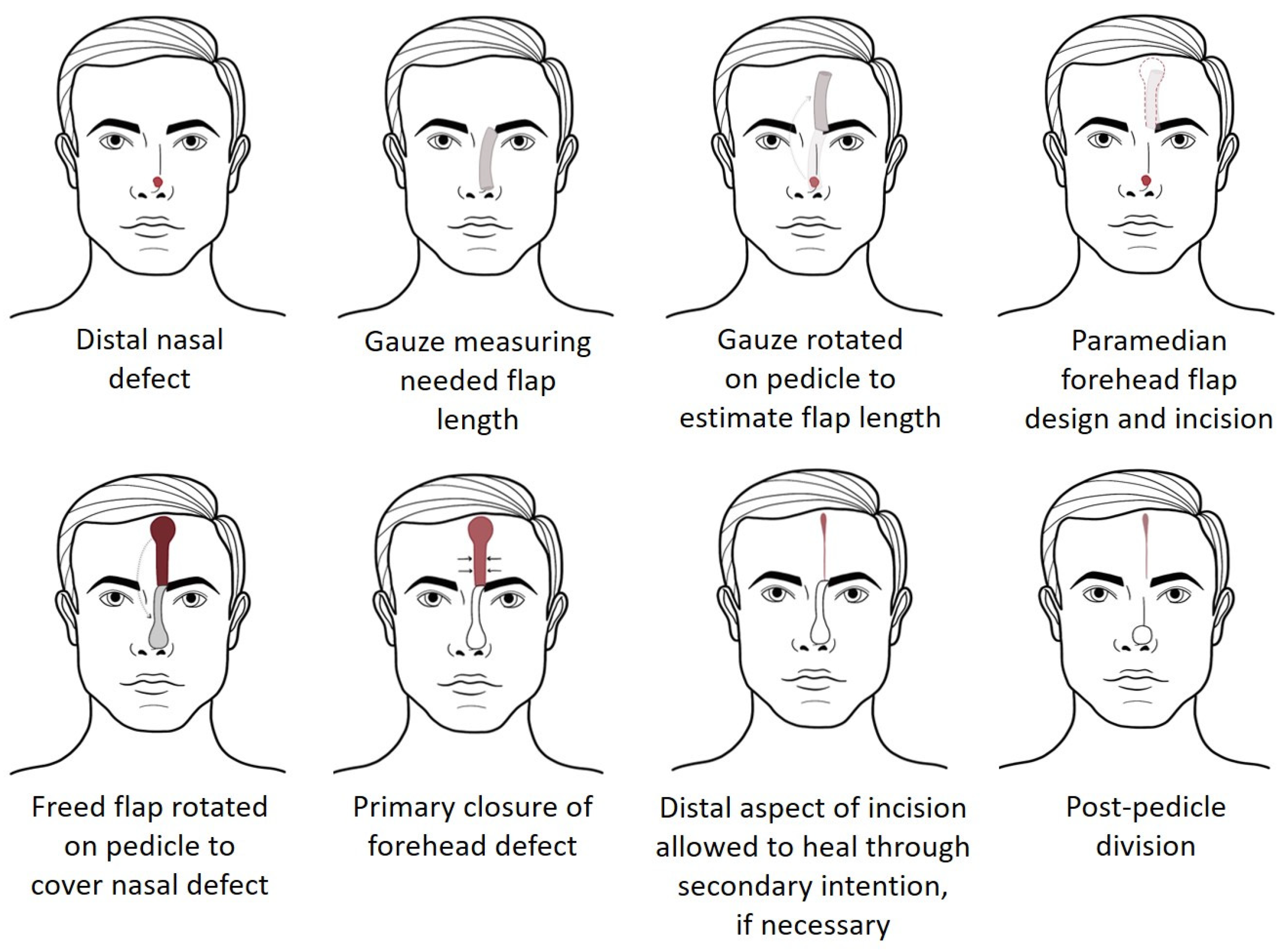
1. Introduction
2. Materials and Methods
2.1. Cadaveric Donors
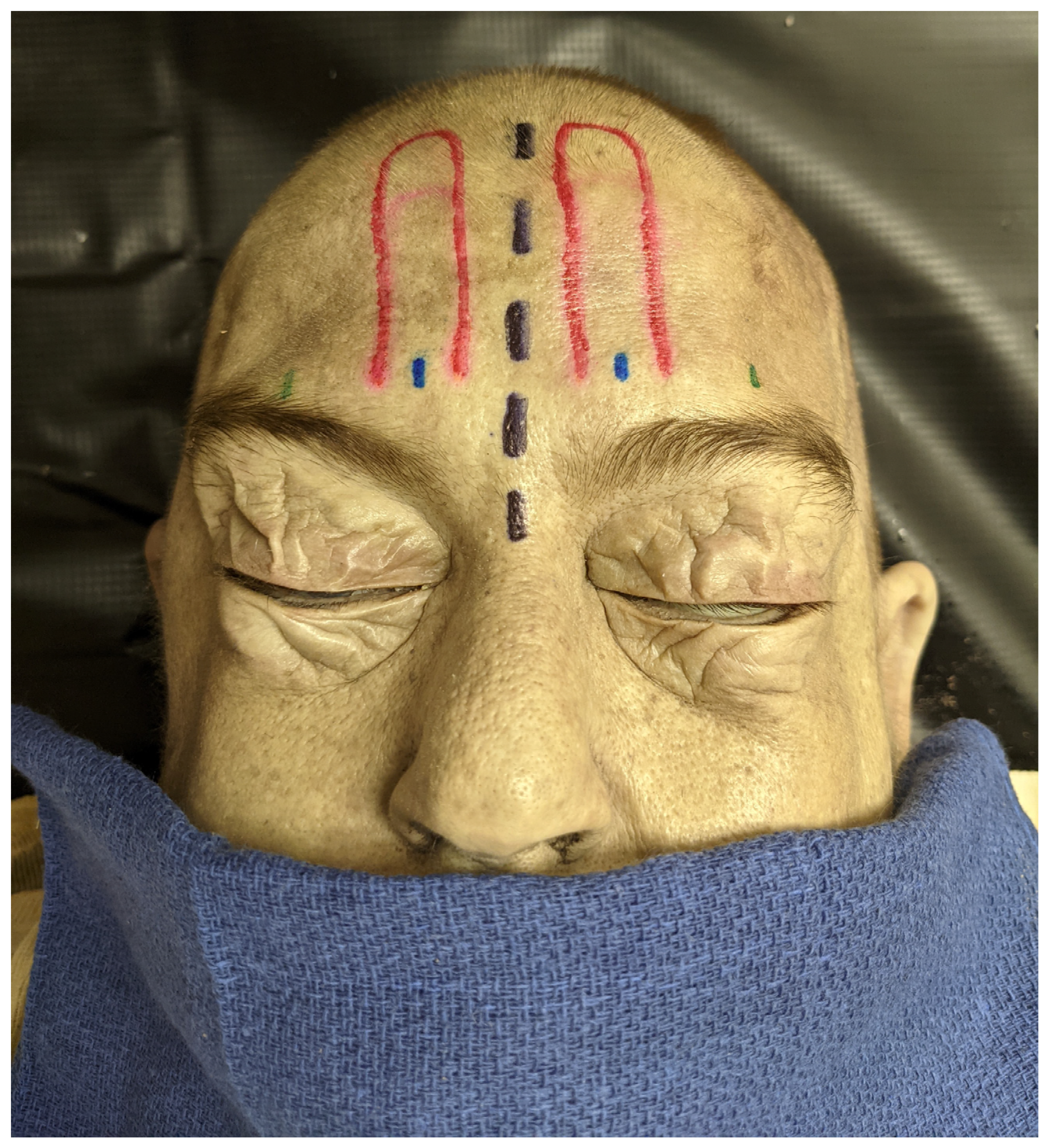
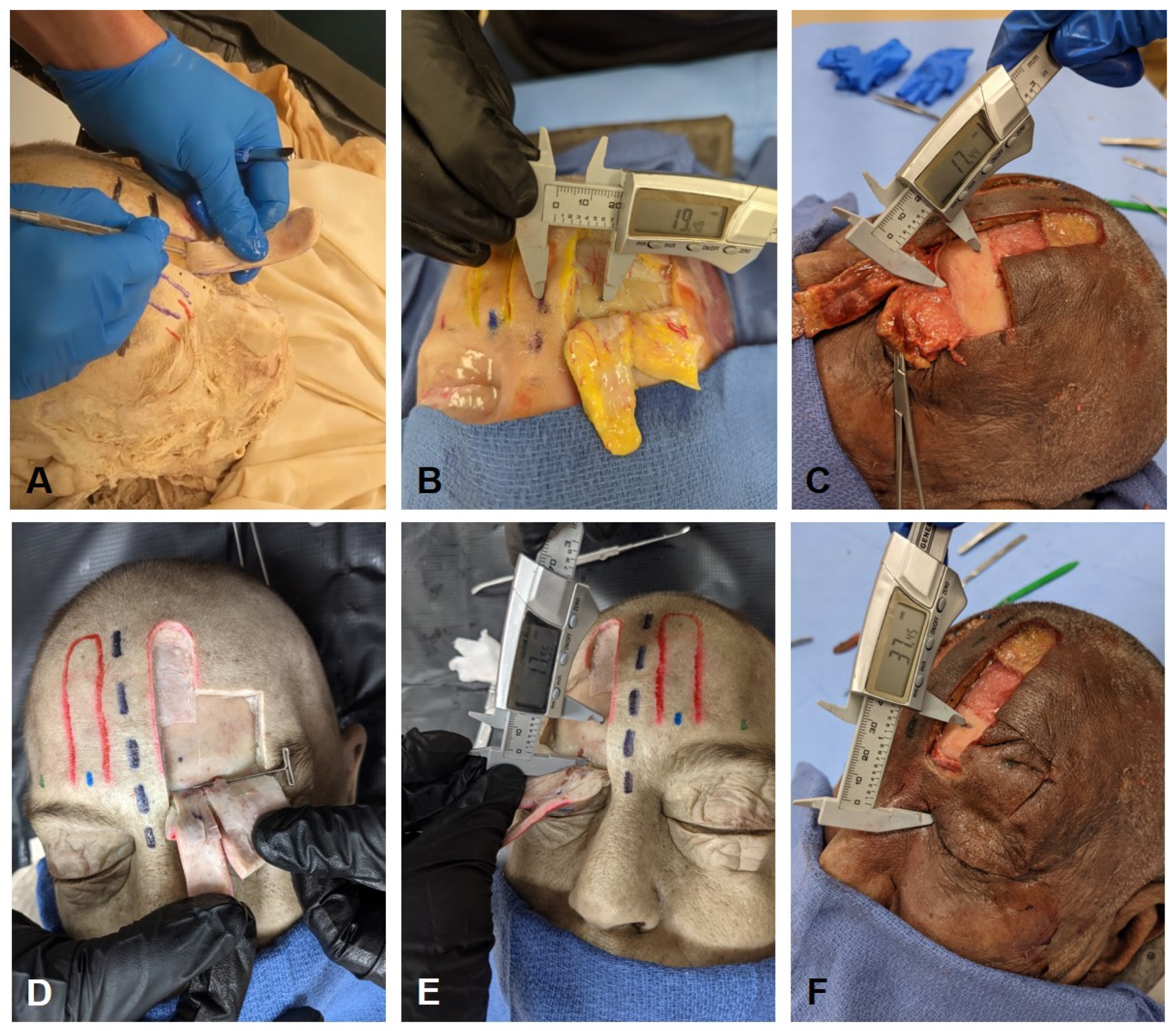
2.2. Dissection Approach
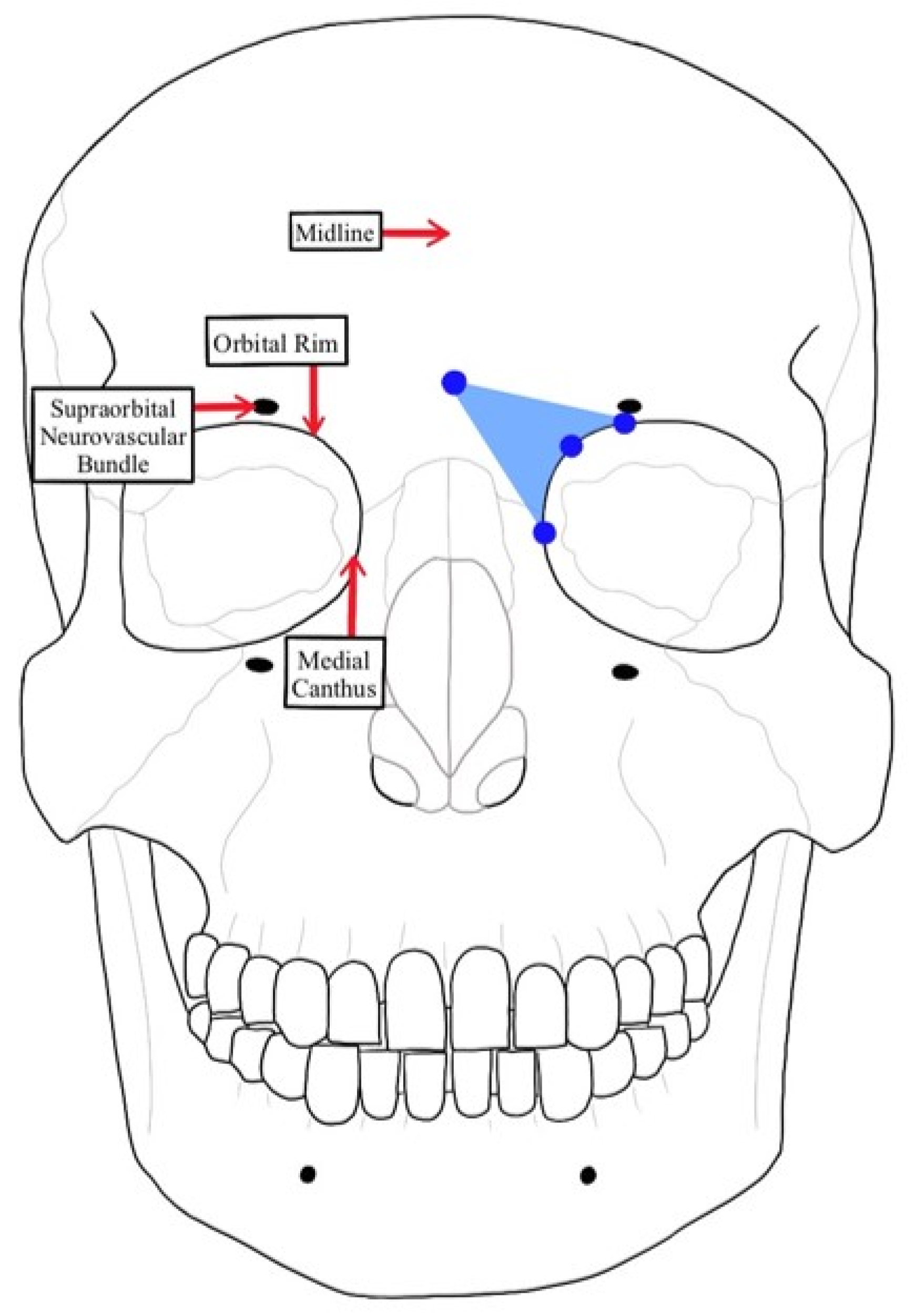
2.3. Measurement of STA Pedicle to Anatomical Landmarks
2.4. Statistical Analysis
3. Results
3.1. Facial Midline to the STA Pedicle
3.2. Supraorbital Neurovascular Bundle to the STA Pedicle
3.3. Bony Orbital Rim to the STA Pedicle
3.4. Medial Canthus to the STA Pedicle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalley, A.F.I.A.; Anne, M.R. Moore’s Clinically Oriented Anatomy, 9th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2022. [Google Scholar]
- Shumrick, K.A.; Smith, T.L. The anatomic basis for the design of forehead flaps in nasal reconstruction. Arch. Otolaryngol. Head Neck Surg. 1992, 118, 373–379. [Google Scholar] [CrossRef]
- Yu, D.; Weng, R.; Wang, H.; Mu, X.; Li, Q. Anatomical study of forehead flap with its pedicle based on cutaneous branch of supratrochlear artery and its application in nasal reconstruction. Ann. Plast. Surg. 2010, 65, 183–187. [Google Scholar] [CrossRef]
- Kelly, C.P.; Yavuzer, R.; Keskin, M.; Bradford, M.; Govila, L.; Jackson, I.T. Functional anastomotic relationship between the supratrochlear and facial arteries: An anatomical study. Plast. Reconstr. Surg. 2008, 121, 458–465. [Google Scholar] [CrossRef]
- Salzano, G.; Maffìa, F.; Vaira, L.A.; Committeri, U.; Copelli, C.; Maglitto, F.; Manfuso, A.; Abbate, V.; Bonavolontà, P.; Scarpa, A.; et al. Locoregional Flaps for the Reconstruction of Midface Skin Defects: A Collection of Key Surgical Techniques. Clin. Med. 2023, 12, 3700. [Google Scholar] [CrossRef]
- Hammer, D.; Williams, F.; Kim, R. Paramedian Forehead Flap. Oral Maxillofac. Surg. Clin. 2020, 28, 23–28. [Google Scholar] [CrossRef]
- Menick, F.J. A 10-year experience in nasal reconstruction with the three-stage forehead flap. Plast. Reconstr. Surg. 2002, 109, 1839–1855, discussion 1856-1861. [Google Scholar] [CrossRef]
- Shokri, T.; Kadakia, S.; Saman, M.; Habal, M.B.; Kohlert, S.; Sokoya, M.; Ducic, Y.; Wood-Smith, D. The Paramedian Forehead Flap for Nasal Reconstruction: From Antiquity to Present. J. Craniofacial Surg. 2019, 30, 330–333. [Google Scholar] [CrossRef]
- Apaydin, F.; Kaya, I.; Uslu, M.; Berber, V. Paramedia Forehead Flap in Large Nasal Skin Defects: Twenty-years’ Experience. Turk. Arch. Otorhinolaryngol. 2022, 60, 155–160. [Google Scholar] [CrossRef]
- Care UoIH. Iowa Head and Neck Protocols: Paramedian Forehead Flap. University of Iowa. 2022. Available online: https://medicine.uiowa.edu/iowaprotocols/paramedian-forehead-flap (accessed on 1 December 2023).
- Mellette, J.R.; Ho, D.Q. Interpolation flaps. Dermatol. Clin. 2005, 23, 87–112. [Google Scholar] [CrossRef]
- Correa, B.J.; Weathers, W.M.; Wolfswinkel, E.M.; Thornton, J.F. The forehead flap: The gold standard of nasal soft tissue reconstruction. Semin. Plast. Surg. 2013, 27, 96–103. [Google Scholar] [CrossRef]
- Reece, E.M.; Schaverien, M.; Rohrich, R. The paramedian forehead flap: A dynamic anatomical vascular study verifying safety and clinical implications. Plast. Reconstr. Surg. 2008, 121, 1956–1963. [Google Scholar] [CrossRef]
- Gilroy, A.; MacPherson, B.; Wikenheiser, J.; Schunke, M.; Schulte, E.; Schumacher, U.; Voll, M.; Wesker, K. Atlas of Anatomy, 4th ed.; Thieme: New York, NY, USA, 2020. [Google Scholar]
- Erdogmus, S.; Govsa, F. Anatomy of the supraorbital region and the evaluation of it for the reconstruction of facial defects. J. Craniofacial Surg. 2007, 18, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Detton, A.J. Grant’s Dissector, 17th ed.; LWW: Philadelphia, PA, USA, 2020. [Google Scholar]
- Balta, J.Y.; Twomey, M.; Moloney, F.; Duggan, O.; Murphy, K.P.; O’Connor, O.J.; Cronin, M.; Cryan, J.F.; Maher, M.M.; O’Mahony, S.M. A comparison of embalming fluids on the structures and properties of tissue in human cadavers. Anat. Histol. Embryol. 2019, 48, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Knott, P.D.; Seth, R. Sex-Related Characteristics of the Face. Otolaryngol. Clin. North Am. 2022, 55, 775–783. [Google Scholar] [CrossRef]
- Peters, F.; Mücke, M.; Möhlhenrich, S.C.; Bock, A.; Stromps, J.P.; Kniha, K.; Hölzle, F.; Modabber, A. Esthetic outcome after nasal reconstruction with paramedian forehead flap and bilobed flap. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.K.; Acharya, S.; Midya, M.; Chakraborty, S.S. Expanded Paramedian Forehead Flap for Nasal Reconstruction Following Congenital Nevus Excision. Plast. Aesthetic Nurs. 2022, 42, 163–166. [Google Scholar] [CrossRef]
- Rajan, S.; Akhtar, N.; Kumar, V.; Gupta, S.; Misra, S.; Chaturvedi, A.; Chaudhary, S.; Suryavanshi, P. Paramedian forehead flap reconstruction for skin tumors involving central subunit of face: An analysis of 37 cases. J. Oral Biol. Craniofacial Res. 2020, 10, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; John, J.; Hart, J.; Chaiyasate, K. Medial Canthus Reconstruction with the Paramedian Forehead Flap. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4419. [Google Scholar] [CrossRef]
- Immaneni, S.; Harvey, D.T.; Delgado, F. Reuse of the paramedian forehead flap pedicle: A case report. SAGE Open Med. Case Rep. 2023, 11, 2050313X231160913. [Google Scholar] [CrossRef]
- Tripathy, S.; Garg, A.; John, J.R.; Sharma, R.K. Use of Modified Islanded Paramedian Forehead Flap for Complex Periocular Facial Reconstruction. J. Craniofac. Surg. 2019, 30, e117–e119. [Google Scholar] [CrossRef]
- Itani, Y.; Yotsuyanagi, T.; Yamauchi, M.; Sugai, A.; Kato, S.; Yamashita, K.; Isogai, N. The Laterally Extended Paramedian Forehead Flap for Nasal Reconstruction: The Delay Technique Revisited. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2871. [Google Scholar] [CrossRef] [PubMed]
- Lo Torto, F.; Redi, U.; Cigna, E.; Losco, L.; Marcasciano, M.; Casella, D.; Ciudad, P.; Ribuffo, D. Nasal Reconstruction With Two Stages Versus Three Stages Forehead Fap: What is Better for Patients With High Vascular Risk? J. Craniofacial Surg. 2020, 31, e57–e60. [Google Scholar] [CrossRef] [PubMed]
- Little, S.C.; Hughley, B.B.; Park, S.S. Complications with forehead flaps in nasal reconstruction. Laryngoscope 2009, 119, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.S.; Goel, A.D.; Sahu, R.K.; Midya, M.; Acharya, S.; Shakrawal, N. Effectiveness of Nasolabial Flap Versus Paramedian Forehead Flap for Nasal Reconstruction: A Systematic Review and Meta-analysis. Aesthetic Plast. Surg. 2023, 47, 313–329. [Google Scholar] [CrossRef]
- Chen, C.L.; Most, S.P.; Branham, G.H.; Spataro, E.A. Postoperative Complications of Paramedian Forehead Flap Reconstruction. JAMA Facial Plast. Surg. 2019, 21, 298–304. [Google Scholar] [CrossRef]
- Eskiizmir, G.; Tanyeri Toker, G.; Ozgur, E.; Tarhan, S.; Cengiz Ozyurt, B. Hemodynamic Changes in Paramedian Forehead Flap. J. Craniofacial Surg. 2018, 29, 159–162. [Google Scholar] [CrossRef]
- Society, A.C. Cancer Facts & Figures 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html (accessed on 1 December 2023).
| Formalin-Embalmed Cadavers versus Fresh Disarticulated Head and Neck Cadavers | |||
| Embalmed Cadaver Mean ± SD | Fresh Cadaver Mean ± SD | p-value * | |
| Facial Midline to the STA Pedicle | 1.68 ± 0.14 | 1.81 ± 0.10 | <0.05 |
| Supraorbital Neurovascular Bundle to the STA Pedicle | 1.48 ± 0.33 | 1.73 ± 0.73 | 0.265 |
| Bony Orbital Rim to the STA Pedicle | 1.54 ± 0.37 | 1.52 ± 0.56 | 0.684 |
| Medial Canthus to the STA Pedicle | 3.04 ± 0.36 | 3.23 ± 0.51 | 0.283 |
| STA Measurements in Male versus Female Cadavers | |||
| Male Cadaver Mean ± SD | Female Cadaver Mean ± SD | p-value ** | |
| Facial Midline to the STA Pedicle | 1.75 ± 0.11 | 1.63 ± 0.16 | <0.05 |
| Supraorbital Neurovascular Bundle to the STA Pedicle | 1.58 ± 0.37 | 1.40 ± 0.36 | <0.05 |
| Bony Orbital Rim to the STA Pedicle | 1.65 ± 0.39 | 1.42 ± 0.35 | <0.05 |
| Medial Canthus to the STA Pedicle | 3.17 ± 0.39 | 2.92 ± 0.31 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limback, K.A.; Kendell, A.H.; Motzko, M.; Surek, C.C.; Dennis, J.F. Danger Zone for Paramedian Forehead Flap Elevation: Maximizing Flap Length and Viability. Surgeries 2024, 5, 13-23. https://doi.org/10.3390/surgeries5010004
Limback KA, Kendell AH, Motzko M, Surek CC, Dennis JF. Danger Zone for Paramedian Forehead Flap Elevation: Maximizing Flap Length and Viability. Surgeries. 2024; 5(1):13-23. https://doi.org/10.3390/surgeries5010004
Chicago/Turabian StyleLimback, Kylie A., Alyssa H. Kendell, Micaela Motzko, Christopher C. Surek, and Jennifer F. Dennis. 2024. "Danger Zone for Paramedian Forehead Flap Elevation: Maximizing Flap Length and Viability" Surgeries 5, no. 1: 13-23. https://doi.org/10.3390/surgeries5010004
APA StyleLimback, K. A., Kendell, A. H., Motzko, M., Surek, C. C., & Dennis, J. F. (2024). Danger Zone for Paramedian Forehead Flap Elevation: Maximizing Flap Length and Viability. Surgeries, 5(1), 13-23. https://doi.org/10.3390/surgeries5010004






