Designing an In Vivo Preclinical Research Study
Abstract
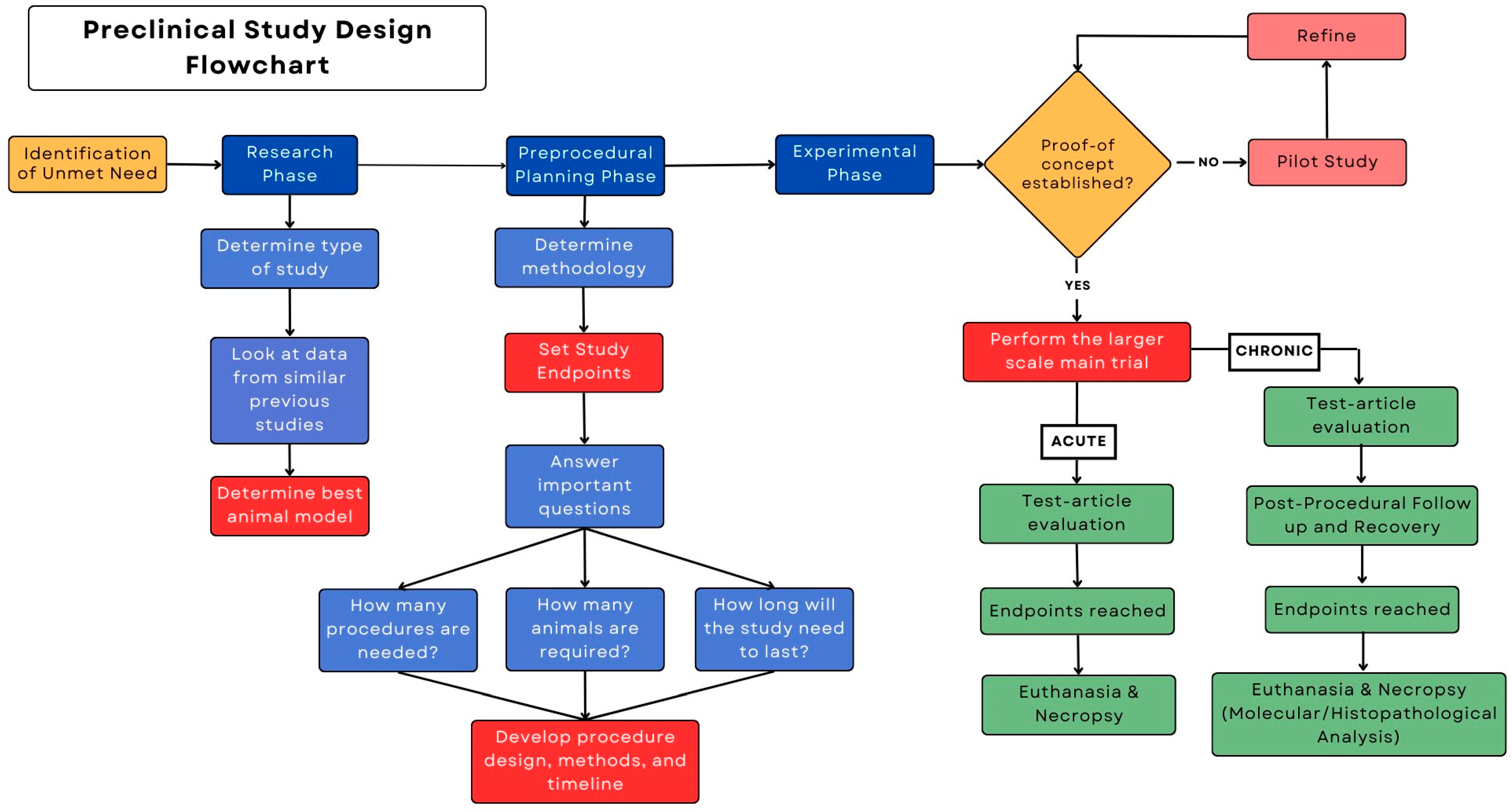
:1. Introduction
2. Procedural Technique Development
3. Research Phase
3.1. Exploratory Studies
3.2. Confirmatory Studies
Good Laboratory Practice (GLP) Studies
3.3. Discussion of Animal Models
3.4. Bioethics in Preclinical Research
4. Preprocedural Planning
4.1. Methodology
4.2. Critical Questions
4.3. Statistics
- Effect size (ES): This represents the magnitude of the difference or effect you expect to find in your study. In other words, it quantifies the practical or clinical significance of the observed difference. Effect size can vary depending on the statistical test being used (e.g., Cohen’s d for t-tests, eta-squared for an analysis of variance (ANOVA)).
- Significance level (α or alpha): This is the probability of making a Type I error, which is the likelihood of incorrectly rejecting the null hypothesis when it is true. The most common alpha level is 0.05, indicating a 5% chance of making a Type I error.
- Sample size (N): This is the number of participants or observations in your study.
- Parametric tests are used when the data follow a normal distribution. These tests are often more powerful (i.e., have a better chance of detecting true effects) when the assumptions are met than when they are not met. Key parametric tests include those that are described below.
- ○
- t-test: Used to compare means between two groups. There are different versions of the t-test, including the independent samples t-test and the paired samples t-test.
- ○
- ANOVA: Used to compare means among three or more groups. ANOVA can be one-way (comparing one factor) or two-way (comparing two factors).
- ○
- Linear regression: Used to model the relationship between a dependent variable and one or more independent variables.
- Nonparametric tests are used when data do not meet the assumptions of normality or when data are categorical or ordinal. These tests make fewer assumptions about the data distribution and are often referred to as distribution-free tests. Key nonparametric tests include those that are described below.
- ○
- Mann–Whitney U test (Wilcoxon Rank-Sum test): Used to compare two independent groups when the data are not normally distributed. It assesses whether one group’s values tend to be higher or lower than the other group’s values.
- ○
- Kruskal–Wallis test: A nonparametric alternative to one-way ANOVA, used to compare three or more independent groups.
- ○
- Chi-square test: Used to test the association between categorical variables. The chi-square test includes the Pearson chi-square test for independence and the chi-square goodness-of-fit test.
- ○
- Spearman’s rank correlation: Used to assess the strength and direction of a monotonic relationship between two variables. It does not assume linearity as in Pearson’s correlation.
4.4. Animal Care
5. Experimental Phase
Necropsy and Euthanasia
- Intravenous injection: The euthanasia solution (e.g., pentobarbital) is administered directly into an appropriately sized vein.
- Intraperitoneal injection: When IV access is not possible, the euthanasia solution is injected into the abdominal cavity.
- Carbon dioxide (CO2) gas: Animals are placed in a chamber where the gas is gradually introduced to displace oxygen. CO2 inhalation is a widely accepted method for the euthanasia of rodents.
- Inhaled anesthetics: Isoflurane or sevoflurane are accepted agents. The animals are placed in a chamber or mask, and the gas is administered until they are rendered unconscious.
- Cervical dislocation: The neck is broken to cause immediate loss of consciousness and death. This is acceptable for mice and rats under 200 g.
- Decapitation: A specialized rodent guillotine is used. These must be kept clean and in good condition, with sharp blades. Other sharp instruments may also be used. With the appropriate equipment and trained personnel, this is acceptable for mice and rats.
- Exsanguination: Usually, this is accomplished via an incision of the ventral aspect of the throat or neck transecting all tissues including the jugular vein and carotid artery. This is not recommended as a sole method of euthanasia but may be performed as an adjunctive measure to ensure death, when necessary, in an unconscious animal.
6. Pitfalls to Avoid
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ioannidis, J.P.; Greenland, S.; Hlatky, M.A.; Khoury, M.J.; Macleod, M.R.; Moher, D.; Schulz, K.F.; Tibshirani, R. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014, 383, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Percie du Sert, N.; Vollert, J.; Rice, A.S.C. General principles of preclinical study design. Handb. Exp. Pharmacol. 2020, 257, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Kimmelman, J.; Mogil, J.S.; Dirnagl, U. Distinguishing between exploratory and confirmatory preclinical research will improve translation. PLoS Biol. 2014, 12, e1001863. [Google Scholar] [CrossRef] [PubMed]
- Wagenmakers, E.J.; Wetzels, R.; Borsboom, D.; van der Maas, H.L.; Kievit, R.A. An agenda for purely confirmatory research. Perspect. Psychol. Sci. 2012, 7, 632–638. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, J.S.; Jeon, M.S.; Lee, K.; Chung, M.K.; Song, C.W. Basic principles of the validation for good laboratory practice institutes. Toxicol. Res. 2009, 25, 1–8. [Google Scholar] [CrossRef]
- World Health Organization. Good Laboratory Practice; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Andrade, E.L.; Bento, A.F.; Cavalli, J.; Oliveira, S.K.; Schwanke, R.C.; Siqueira, J.M.; Freitas, C.S.; Marcon, R.; Calixto, J.B. Non-clinical studies in the process of new drug development—Part II: Good laboratory practice, metabolism, pharmacokinetics, safety and dose translation to clinical studies. Braz. J. Med. Biol. Res. 2016, 49, e5646. [Google Scholar] [CrossRef]
- van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O’Collins, V.; Macleod, M.R. Can animal models of disease reliably inform human studies? PLoS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef]
- Pubrica Academy. Why Is It Important to Do a Literature Review in Research? Available online: https://pubrica.com/academy/research/why-is-it-important-to-do-a-literature-review-in-research/ (accessed on 12 July 2023).
- Upstate University of South Carolina Library. Literature Review: Purpose of a Literature Review. Available online: https://uscupstate.libguides.com/c.php?g=627058&p=4389968#:~:text=The%20purpose%20of%20a%20literature,questions%20left%20from%20other%20research (accessed on 12 July 2023).
- Moran, C.J.; Ramesh, A.; Brama, P.A.; O’Byrne, J.M.; O’Brien, F.J.; Levingstone, T.J. The benefits and limitations of animal models for translational research in cartilage repair. J. Exp. Orthop. 2016, 3, 1. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef]
- Walters, E.M.; Wells, K.D.; Bryda, E.C.; Schommer, S.; Prather, R.S. Swine models, genomic tools and services to enhance our understanding of human health and diseases. Lab Anim. 2017, 46, 167–172. [Google Scholar] [CrossRef]
- Monreal, G.; Sherwood, L.C.; Sobieski, M.A.; Giridharan, G.A.; Slaughter, M.S.; Koenig, S.C. Large animal models for left ventricular assist device research and development. ASAIO J. 2014, 60, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Gonzalez, L.; Blikslager, A. Large animal models: The key to translational discovery in digestive disease research. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.K.; Grace Cherian, S.; Babu Phaniti, P.; Babu Chidambaram, S.; Rachel Vasanthi, A.H.; Arumugam, M. Preclinical animal studies in ischemic stroke: Challenges and some solutions. Anim. Models Exp. Med. 2021, 4, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Zomer, H.D.; Trentin, A.G. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Models Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef]
- Vandamme, T.F. Use of rodents as models of human diseases. J. Pharm. Bioallied Sci. 2014, 6, 2–9. [Google Scholar] [CrossRef]
- Mapara, M.; Thomas, B.S.; Bhat, K.M. Rabbit as an animal model for experimental research. Dent. Res. J. 2012, 9, 111–118. [Google Scholar] [CrossRef]
- Canning, B.J.; Chou, Y. Using guinea pigs in studies relevant to asthma and COPD. Pulm. Pharmacol. Ther. 2008, 21, 702–720. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Y.; Dunmall, L.S.C.; Lemoine, N.R.; Wang, P.; Wang, Y. Syrian hamster as an ideal animal model for evaluation of cancer immunotherapy. Front. Immunol. 2023, 14, 1126969. [Google Scholar] [CrossRef]
- Burmeister, D.M.; Supp, D.M.; Clark, R.A.; Tredget, E.E.; Powell, H.M.; Enkhbaatar, P.; Bohannon, J.K.; Cancio, L.C.; Hill, D.M.; Nygaard, R.M. Advantages and disadvantages of using small and large animals in burn research: Proceedings of the 2021 research special interest group. J. Burn Care Res. 2022, 43, 1032–1041. [Google Scholar] [CrossRef]
- Dias, I.E.; Viegas, C.A.; Requicha, J.F.; Saavedra, M.J.; Azevedo, J.M.; Carvalho, P.P.; Dias, I.R. Mesenchymal stem cell studies in the goat model for biomedical research-a review of the scientific literature. Biology 2022, 11, 1276. [Google Scholar] [CrossRef]
- Gregory, M.H.; Capito, N.; Kuroki, K.; Stoker, A.M.; Cook, J.L.; Sherman, S.L. A review of translational animal models for knee osteoarthritis. Arthritis 2012, 2012, 764621. [Google Scholar] [CrossRef] [PubMed]
- Banstola, A.; Reynolds, J.N.J. The sheep as a large animal model for the investigation and treatment of human disorders. Biology 2022, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Berset, C.M.; Lanker, U.; Zeiter, S. Survey on sheep usage in biomedical research. Animals 2020, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Hamernik, D.L. Farm animals are important biomedical models. Anim. Front. 2019, 9, 3–5. [Google Scholar] [CrossRef]
- Carlsson, H.E.; Schapiro, S.J.; Farah, I.; Hau, J. Use of primates in research: A global overview. Am. J. Primatol. 2004, 63, 225–237. [Google Scholar] [CrossRef]
- Friedman, H.; Ator, N.; Haigwood, N.; Newsome, W.; Allan, J.S.; Golos, T.G.; Kordower, J.H.; Shade, R.E.; Goldberg, M.E.; Bailey, M.R.; et al. The critical role of nonhuman primates in medical research. Pathog. Immun. 2017, 2, 352–365. [Google Scholar] [CrossRef]
- Shively, C.A.; Clarkson, T.B. The unique value of primate models in translational research. Nonhuman primate models of women’s health: Introduction and overview. Am. J. Primatol. 2009, 71, 715–721. [Google Scholar] [CrossRef]
- Gorzalczany, S.B.; Rodriguez Basso, A.G. Strategies to apply 3Rs in preclinical testing. Pharmacol. Res. Perspect. 2021, 9, e00863. [Google Scholar] [CrossRef]
- Fenwick, N.; Griffin, G.; Gauthier, C. The welfare of animals used in science: How the “Three Rs” ethic guides improvements. Can. Vet. J. 2009, 50, 523–530. [Google Scholar]
- Lewis, D.I. Animal experimentation: Implementation and application of the 3Rs. Emerg. Top. Life Sci. 2019, 3, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.B.; Jennings, M.; Buckwell, A.; Ewbank, R.; Godfrey, C.; Holgate, B.; Inglis, I.; James, R.; Page, C.; Sharman, I.; et al. Refining procedures for the administration of substances. Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. British Veterinary Association Animal Welfare Foundation/Fund for the Replacement of Animals in Medical Experiments/Royal Society for the Prevention of Cruelty to Animals/Universities Federation for Animal Welfare. Lab. Anim. 2001, 35, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.R. Fundamentals of clinical trial design. J. Exp. Stroke Transl. Med. 2010, 3, 19–27. [Google Scholar] [CrossRef]
- Festing, M.F.; Altman, D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002, 43, 244–258. [Google Scholar] [CrossRef]
- Ko, M.J.; Lim, C.Y. General considerations for sample size estimation in animal study. Korean J. Anesthesiol. 2021, 74, 23–29. [Google Scholar] [CrossRef]
- Kiani, A.K.; Naureen, Z.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; et al. Methodology for clinical research. J. Prev. Med. Hyg. 2022, 63, E267–E278. [Google Scholar] [CrossRef]
- Suresh, K.; Chandrashekara, S. Sample size estimation and power analysis for clinical research studies. J. Hum. Reprod. Sci. 2012, 5, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, T. Parametric and Nonparametric: Demystifying the Terms. Available online: https://www.mayo.edu/research/documents/parametric-and-nonparametric-demystifying-the-terms/doc-20408960 (accessed on 12 July 2023).
- National Research Council Institute for Laboratory Animal Research. The National Academies Collection: Reports funded by National Institutes of Health. In Guidance for the Description of Animal Research in Scientific Publications; National Academy of Sciences: Washington, DC, USA, 2011. [Google Scholar]
- McCulloch, P. Developing appropriate methodology for the study of surgical techniques. J. R. Soc. Med. 2009, 102, 51–55. [Google Scholar] [CrossRef]
- Li, K.; Wagner, L.; Moctezuma-Ramirez, A.; Vela, D.; Perin, E. A robust percutaneous myocardial infarction model in pigs and its effect on left ventricular function. J. Cardiovasc. Transl. Res. 2021, 14, 1075–1084. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Wagner Florencio, L.; Tang, L.; Heallen, T.R.; Leach, J.P.; Wang, Y.; Grisanti, F.; Willerson, J.T.; Perin, E.C.; et al. Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction. Sci. Transl. Med. 2021, 13, eabd6892. [Google Scholar] [CrossRef]
- Henderson, V.C.; Kimmelman, J.; Fergusson, D.; Grimshaw, J.M.; Hackam, D.G. Threats to validity in the design and conduct of preclinical efficacy studies: A systematic review of guidelines for in vivo animal experiments. PLoS Med. 2013, 10, e1001489. [Google Scholar] [CrossRef] [PubMed]
- Gelijns, A.C. Technological Innovation: Comparing Development of Drugs, Devices, and Procedures in Medicine; National Academies Press (US): Washington, DC, USA, 1989. [Google Scholar]
| Animal Model | Best Uses | Challenges | References |
|---|---|---|---|
| Pig | Human cardiovascular anatomy, device implants, Alzheimer’s disease, atherosclerosis, Type 2 diabetes mellitus, breast cancer, toxicology | Higher purchase costs than rodents or rabbits, need for more storage space, keeping the animal settled after surgery, require specialized husbandry | [11,12,13] |
| Cattle | Mechanical circulatory support devices, female reproductive model, pregnancy-related issues, tuberculosis | Largely increased purchase and maintenance costs, including feed, veterinary care, and surgery costs. Longer reproductive cycle, creating slow and expensive experiments. Calves are the most comparable to humans in size, but their quick growth limits plausible study duration | [12,14,15] |
| Rodents | Genetics and genomics studies of knockout or transgenic mice/rats created to mimic human genetic conditions; drug testing; cancer research studies involving humanized mice (patient-derived xenograft models); immunology studies of asthma, psoriasis, or multiple sclerosis; neuroscience; metabolic/obesity research; cardiovascular research; developmental biology; infectious disease research; aging research; toxicology and safety testing | Large differences between mice and humans in physiology and brain composition | [12,16,17,18,19] |
| Rabbits | Wound healing model, drug therapy, asthma, cholesterol, cardiovascular disease, Alzheimer’s disease, stroke, cartilage repair | Different microstructure than humans, lack of literature on required care, lack of well-equipped host facilities and expert handlers | [11,12,16,20] |
| Guinea Pigs | Cholesterol metabolism, asthma COPD, feto-placental development, Alzheimer’s disease, tuberculosis, vaccines | Fewer syngenetic tumor cell lines and lack of specific immune reagents | [12,21] |
| Hamster | Reproductive system, micro-circulation, cancer, infection (leptospirosis), vaccines | Fewer syngenetic tumor cell lines and lack of specific immune reagents | [12,22] |
| Goat | Orthopedics, mechanical circulatory support devices, stem cell and locomotor system studies | Not prone to spontaneous arthritis like rodents, prone to spontaneous arthritis similar to rodents, shortage in antibodies | [12,23,24,25] |
| Sheep | Surgical bone-to-bone healing, asthma, heart pathology, vaccines, cartilage repair, smoke inhalation, pulmonary edema, medical device testing, osteoporosis, study of main physiologic systems, abnormal fetal development, and congenital birth defects | Relatively small chest cavity compared with humans, often have health issues not related to the study, limited availability of physiologic databases for mapping to humans | [11,12,23,25,26,27,28] |
| Nonhuman Primates | Cancer, AIDS, Alzheimer’s disease, Parkinson’s disease, obesity and diabetes, transplants, pregnancy complications | Ethical concerns, strict regulatory guidelines, high cost of husbandry and experiments, need for specialized personnel | [29,30,31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moctezuma-Ramirez, A.; Dworaczyk, D.; Whitehorn, J.; Li, K.; Cardoso, C.d.O.; Elgalad, A. Designing an In Vivo Preclinical Research Study. Surgeries 2023, 4, 544-555. https://doi.org/10.3390/surgeries4040053
Moctezuma-Ramirez A, Dworaczyk D, Whitehorn J, Li K, Cardoso CdO, Elgalad A. Designing an In Vivo Preclinical Research Study. Surgeries. 2023; 4(4):544-555. https://doi.org/10.3390/surgeries4040053
Chicago/Turabian StyleMoctezuma-Ramirez, Angel, David Dworaczyk, Julia Whitehorn, Ke Li, Cristiano de Oliveira Cardoso, and Abdelmotagaly Elgalad. 2023. "Designing an In Vivo Preclinical Research Study" Surgeries 4, no. 4: 544-555. https://doi.org/10.3390/surgeries4040053
APA StyleMoctezuma-Ramirez, A., Dworaczyk, D., Whitehorn, J., Li, K., Cardoso, C. d. O., & Elgalad, A. (2023). Designing an In Vivo Preclinical Research Study. Surgeries, 4(4), 544-555. https://doi.org/10.3390/surgeries4040053








