Lasting Impact of COVID-19 on Bariatric Surgery Delivery in North America: A Retrospective International Cohort Study of 349,209 Patients in 902 Centers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design, Patient Population, and Variable Definitions
2.3. Statistical Analysis
3. Results
3.1. Patient Demographics
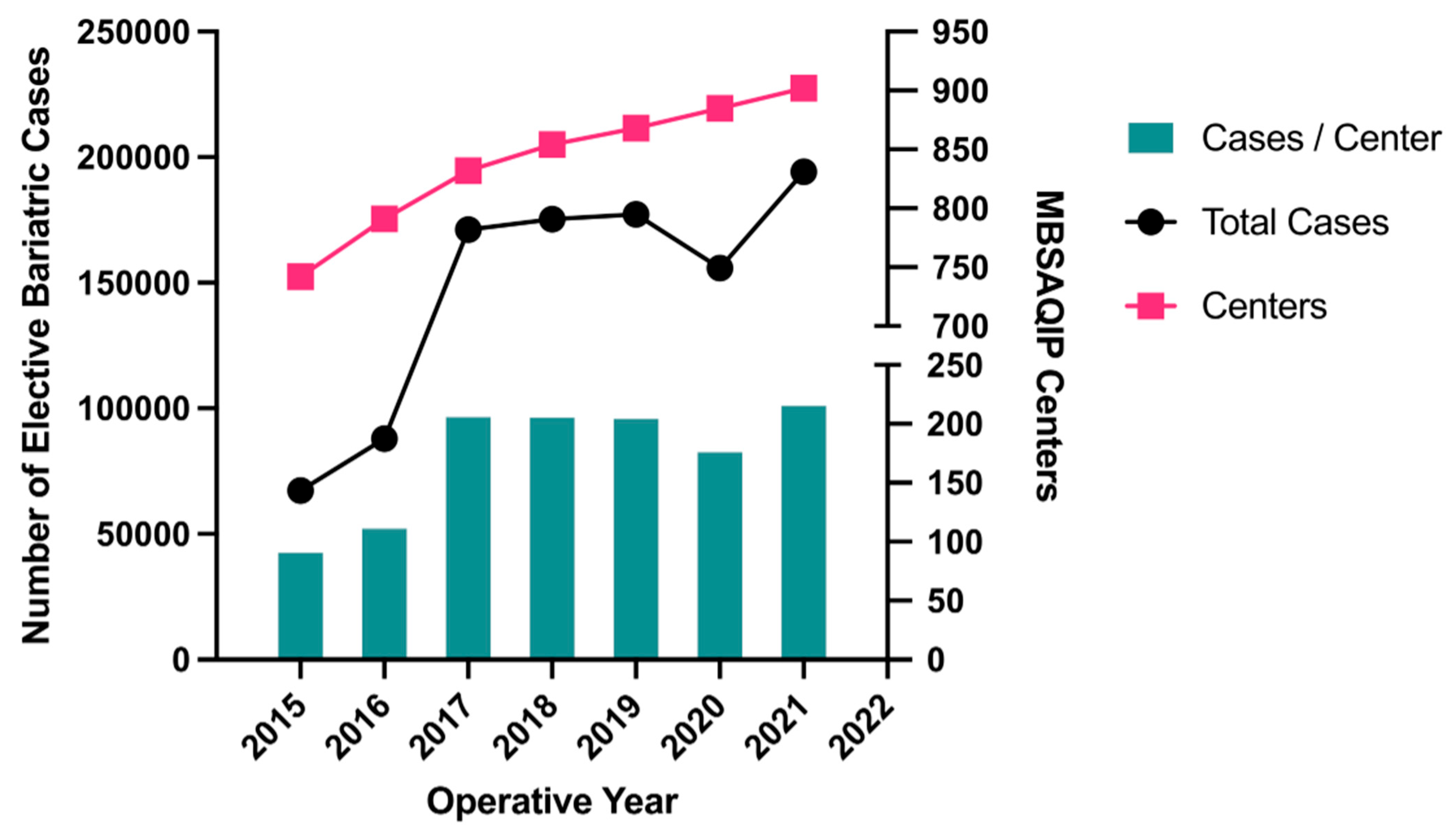
3.2. Operative Volume
3.3. Bivariate Analysis of Post-Operative Outcomes Comparing Patients without Peri-Operative COVID-19 to Those with Pre- or Post-Operative COVID-19
3.4. Multivariable Logistic Regression Evaluating Predictors of Serious Complications and Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Kibbe, M.R. Surgery and COVID-19. JAMA 2020, 324, 1151–1152. [Google Scholar] [CrossRef]
- Verhoeff, K.; Mocanu, V.; Wilson, H.; Switzer, N.; Birch, D.; Karmali, S. Impact of the COVID-19 pandemic on bariatric surgery in North America—A retrospective analysis of 834,647 patients. Surg. Obes. Relat. Dis. 2022, 18, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Abu-Omar, N.; Marcil, G.; Mocanu, V.; Dang, J.T.; Switzer, N.; Kanji, A.; Birch, D.; Karmali, S. The effect of the COVID-19 pandemic on bariatric surgery delivery in Edmonton, Alberta: A single-centre experience. Can. J. Surg. 2021, 64, E307–E309. [Google Scholar] [CrossRef]
- Murtha, J.A.; Alagoz, E.; Breuer, C.R.; Eierman, L.; Jawara, D.; Farrar-Edwards, D.; Voils, C.I.; Funk, L.M. Impact of COVID-19 on the Postoperative Bariatric Surgery Patient Experience. Ann. Surg. 2023, 277, E745–E751. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, V.; Verhoeff, K.; Dang, J.; Birch, D.W.; Karmali, S.; Switzer, N.J. Post-Operative but Not Pre-Operative COVID-19 Predicts Serious Complications and Mortality Following Elective Bariatric Surgery. Obes. Surg. 2023, 33, 1202–1210. [Google Scholar] [CrossRef]
- Singhal, R.; Tahrani, A.A.; Ludwig, C.; Mahawar, K.; Abou-Mrad-Fricquegnon, A.; Alasfur, A.; Alexandrou, A.; Barbosa, A.; Bashir, A.; Bosco, A.; et al. Global 30-day outcomes after bariatric surgery during the COVID-19 pandemic (GENEVA): An international cohort study. Lancet Diabetes Endocrinol. 2021, 9, 7–9. [Google Scholar] [CrossRef]
- Søreide, K.; Hallet, J.; Matthews, J.B.; Schnitzbauer, A.A.; Line, P.D.; Lai, P.B.S.; Otero, J.; Callegaro, D.; Warner, S.G.; Baxter, N.N.; et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br. J. Surg. 2020, 107, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Nandhra, S.; Benson, R.A. The persistent challenges faced by vascular surgery services during the UK coronavirus pandemic: A snapshot qualitative survey. Ann. R. Coll. Surg. Engl. 2021, 104, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, S.M.; Crump, R.T.; Sutherland, J.M. Surgical wait list management in Canada during a pandemic: Many challenges ahead. Can. J. Surg. 2020, 63, E226–E228. [Google Scholar] [CrossRef]
- The Lancet Rheumatology. Too long to wait: The impact of COVID-19 on elective surgery. Lancet Rheumatol. 2021, 3, e83. [Google Scholar] [CrossRef]
- American College of Surgeons. MBSAQIP Participant Use Data File (PUF); American College of Surgeons: Chicago, IL, USA, 2019; Available online: https://www.facs.org/quality-programs/mbsaqip/participant-use (accessed on 14 September 2019).
- Kizy, S.; Jahansouz, C.; Downey, M.C.; Hevelone, N.; Ikramuddin, S.; Leslie, D. National Trends in Bariatric Surgery 2012–2015: Demographics, Procedure Selection, Readmissions, and Cost. Obes. Surg. 2017, 27, 2933–2939. [Google Scholar] [CrossRef]
- Daniels, N.F.; Burrin, C.; Chan, T.; Fusco, F. A Systematic Review of the Impact of the First Year of COVID-19 on Obesity Risk Factors: A Pandemic Fueling a Pandemic? Curr. Dev. Nutr. 2022, 6, nzac011. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Birkenfeld, A.L.; Schulze, M.B. Global pandemics interconnected—Obesity, impaired metabolic health and COVID-19. Nat. Rev. Endocrinol. 2021, 17, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R.; Nahid, M.; Ringel, J.B.; et al. Clinical Characteristics of COVID-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef]
- Shaikh, C.F.; Palmer Kelly, E.; Paro, A.; Cloyd, J.; Ejaz, A.; Beal, E.W.; Pawlik, T.M. Burnout Assessment Among Surgeons and Surgical Trainees During the COVID-19 Pandemic: A Systematic Review. J. Surg. Educ. 2022, 79, 1206–1220. [Google Scholar] [CrossRef] [PubMed]
- Houdmont, J.; Daliya, P.; Theophilidou, E.; Adiamah, A.; Hassard, J.; Lobo, D.N.; Ahmed, J.; Babu, V.; Baker, D.; Bartlett, D.; et al. Burnout Among Surgeons in the UK During the COVID-19 Pandemic: A Cohort Study. World J. Surg. 2022, 46, 1–9. [Google Scholar] [CrossRef]
- Magliah, S.F.; Alzahrani, A.M.; Sabban, M.F.; Abulaban, B.A.; Turkistani, H.A.; Magliah, H.F.; Jaber, T.M. Psychological impact of the COVID-19 pandemic on waitlisted pre-bariatric surgery patients in Saudi Arabia: A cross-sectional study. Ann. Med. Surg. (Lond.) 2022, 82, 104767. [Google Scholar] [CrossRef]
- Gagliardi, A.R.; Yip, C.Y.Y.; Irish, J.; Wright, F.C.; Rubin, B.; Ross, H.; Green, R.; Abbey, S.; McAndrews, M.P.; Stewart, D.E. The psychological burden of waiting for procedures and patient-centred strategies that could support the mental health of wait-listed patients and caregivers during the COVID-19 pandemic: A scoping review. Health Expect. 2021, 24, 978–990. [Google Scholar] [CrossRef]
- Vanetta, C.; Dreifuss, N.H.; Angeramo, C.A.; Baz, C.; Cubisino, A.; Schlottmann, F.; Masrur, M.A. Outcomes of same-day discharge sleeve gastrectomy and Roux-en-Y gastric bypass: A systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2022. [Google Scholar] [CrossRef]
- Houlder, K.; Mocanu, V.; Verhoeff, K.; Marcil, G.; Birch, D.W.; Karmali, S.; Switzer, N.J. Trends, Outcomes, and Impact of Early Discharge Following Bariatric Surgery: A Retrospective MBSAQIP Analysis of 748,955 Patients. Obes. Surg. 2022, 32, 2572–2581. [Google Scholar] [CrossRef]
- Ignat, M.; Ansiaux, J.; Osailan, S.; D’Urso, A.; Morainvillers-Sigwalt, L.; Vix, M.; Mutter, D. A Cost Analysis of Healthcare Episodes Including Day-Case Bariatric Surgery (Roux-en-Y Gastric Bypass and Sleeve Gastrectomy) Versus Inpatient Surgery. Obes. Surg. 2022, 32, 2504–2511. [Google Scholar] [CrossRef]
- Inaba, C.S.; Koh, C.Y.; Sujatha-Bhaskar, S.; Zhang, L.; Nguyen, N.T. Same-Day Discharge after Laparoscopic Roux-en-Y Gastric Bypass: An Analysis of the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program Database. J. Am. Coll. Surg. 2018, 226, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Leepalao, M.C.; Arredondo, D.; Speights, F.; Duncan, T.D. Same-day discharge on laparoscopic Roux-en-Y gastric bypass patients: An outcomes review. Surg. Endosc. 2020, 34, 3614–3617. [Google Scholar] [CrossRef] [PubMed]
- Nijland, L.M.G.; de Castro, S.M.M.; Vogel, M.; Coumou, J.W.F.; van Rutte, P.W.J.; van Veen, R.N. Feasibility of Same-Day Discharge After Laparoscopic Roux-en-Y Gastric Bypass Using Remote Monitoring. Obes. Surg. 2021, 31, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; De Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg. Obes. Relat. Dis. 2022, 18, 1345–1356. [Google Scholar] [CrossRef]
- Sugerman, H.J.; Wolfe, L.G.; Sica, D.A.; Clore, J.N. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann. Surg. 2003, 237, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Bloomston, M.; Zervos, E.E.; Camps, M.A.; Goode, S.E.; Rosemurgy, A.S. Outcome following bariatric surgery in super versus morbidly obese patients: Does weight matter? Obes. Surg. 1997, 7, 414–419. [Google Scholar] [CrossRef]
- Verhoeff, K.; Mocanu, V.; Dang, J.; Purich, K.; Switzer, N.J.; Birch, D.W.; Karmali, S. Five Years of MBSAQIP Data: Characteristics, Outcomes, and Trends for Patients with Super-obesity. Obes. Surg. 2021, 32, 406–415. [Google Scholar] [CrossRef]
- Sharples, A.J.; Mahawar, K. Systematic Review and Meta-Analysis of Randomised Controlled Trials Comparing Long-Term Outcomes of Roux-En-Y Gastric Bypass and Sleeve Gastrectomy. Obes. Surg. 2020, 30, 664–672. [Google Scholar] [CrossRef]
- Shoar, S.; Saber, A.A. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: A systematic review and meta-analysis of comparative studies. Surg. Obes. Relat. Dis. 2017, 13, 170–180. [Google Scholar] [CrossRef]
- Peterli, R.; Wölnerhanssen, B.K.; Peters, T.; Vetter, D.; Kröll, D.; Borbély, Y.; Schultes, B.; Beglinger, C.; Drewe, J.; Schiesser, M.; et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients with Morbid Obesity: The SM-BOSS Randomized Clinical Trial. JAMA 2018, 319, 255–265. [Google Scholar] [CrossRef]
- Roslin, M.; Tugertimur, B.; Zarabi, S.; Cottam, D. Is There a Better Design for a Bariatric Procedure? The Case for a Single Anastomosis Duodenal Switch. Obes. Surg. 2018, 28, 4077–4086. [Google Scholar] [CrossRef]
- Gonzalez-Heredia, R.; Sanchez-Johnsen, L.; Valbuena, V.S.; Masrur, M.; Murphey, M.; Elli, E. Surgical management of super-super obese patients: Roux-en-Y gastric bypass versus sleeve gastrectomy. Surg. Endosc. 2016, 30, 2097–2102. [Google Scholar] [CrossRef]
- Boyers, D.; Retat, L.; Jacobsen, E.; Avenell, A.; Aveyard, P.; Corbould, E.; Jaccard, A.; Cooper, D.; Robertson, C.; Aceves-Martins, M.; et al. Cost-effectiveness of bariatric surgery and non-surgical weight management programmes for adults with severe obesity: A decision analysis model. Int. J. Obes. 2021, 45, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Lester, E.L.W.; Padwal, R.S.; Birch, D.W.; Sharma, A.M.; So, H.; Ye, F.; Klarenbach, S.W. The real-world cost-effectiveness of bariatric surgery for the treatment of severe obesity: A cost–utility analysis. CMAJ Open 2021, 9, E673. [Google Scholar] [CrossRef]
- Miranda, W.R.; Batsis, J.A.; Sarr, M.G.; Collazo-Clavell, M.L.; Clark, M.M.; Somers, V.K.; Lopez-Jimenez, F. Impact of bariatric surgery on quality of life, functional capacity, and symptoms in patients with heart failure. Obes. Surg. 2013, 23, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Gokce, N.; Karki, S.; Dobyns, A.; Zizza, E.; Sroczynski, E.; Palmisano, J.N.; Mazzotta, C.; Hamburg, N.M.; Pernar, L.I.; Carmine, B.; et al. Association of Bariatric Surgery With Vascular Outcomes. JAMA Netw. Open 2021, 4, e2115267. [Google Scholar] [CrossRef] [PubMed]
| Patients Operated on in 2020 n = 154,960 n (%) | Patients Operated on in 2021 n = 194,249 n (%) | p-Value | |
|---|---|---|---|
| Age, years | |||
| mean ± sd | 43.9 ± 11.9 | 42.4 ± 11.7 | <0.001 |
| <18 | 290 (0.2) | 392 (0.2) | <0.001 |
| 18–29 | 17,276 (11.2) | 22,644 (11.7) | |
| 30–39 | 41,774 (30.0) | 54,460 (28.0) | |
| 40–49 | 45,190 (29.2) | 57,050 (29.4) | |
| 50–59 | 33,666 (21.7) | 41,087 (21.2) | |
| ≥60 | 16,764 (10.8) | 18,616 (9.6) | |
| Gender | |||
| Female | 126,373 (81.6) | 161,232 (83.0) | <0.001 |
| BMI, Kg/m2 | |||
| mean ± sd | 44.7 ± 7.7 | 44.8 ± 7.7 | 0.154 |
| <35 | 7652 (4.9) | 9792 (5.0) | 0.392 |
| 35–39 | 36,024 (23.3) | 45,424 (23.4) | |
| 40–45 | 47,505 (30.7) | 59,467 (30.6) | |
| 45–50 | 31,070 (20.1) | 39,007 (20.1) | |
| 50–60 | 27,752 (16.6) | 32,015 (16.5) | |
| >60 | 6953 (4.5) | 8539 (4.4) | |
| Functional Status | <0.001 | ||
| Independent | 153,928 (99.5) | 193,222 (99.6) | |
| Partially dependent | 816 (0.5) | 849 (0.4) | |
| Fully dependent | 39 (0.03) | 30 (0.02) | |
| ASA Category | <0.001 | ||
| 1–2 | 30,377 (19.7) | 37,259 (19.2) | |
| 3 | 118,275 (76.6) | 150,100 (77.3) | |
| 4–5 | 5859 (3.8) | 6721 (3.5) | |
| Smoker | 10,598 (6.8) | 12,569 (6.5) | <0.001 |
| Diabetes | |||
| No or diet-controlled | 119,598 (77.2) | 152,450 (78.5) | <0.001 |
| Non-insulin-dependent | 25,371 (16.4) | 30,490 (15.7) | |
| Insulin-dependent | 9991 (6.5) | 11,309 (5.8) | |
| Hypertension | 68,785 (44.4) | 83,068 (42.8) | <0.001 |
| GERD | 49,307 (31.8) | 62,747 (32.3) | 0.062 |
| COPD | 1870 (1.2) | 2156 (1.1) | 0.008 |
| Hyperlipidemia | 34,675 (22.4) | 41,878 (21.6) | <0.001 |
| Renal insufficiency | 879 (0.6) | 989 (0.5) | 0.019 |
| Dialysis dependent | 483 (0.3) | 559 (0.3) | 0.198 |
| History of DVT | 3984 (2.6) | 4863 (2.5) | 0.207 |
| Venous stasis | 1090 (0.7) | 1142(0.6) | <0.001 |
| Pre-operative therapeutic anticoagulation | 4458 (2.9) | 5141 (2.7) | <0.001 |
| Sleep apnea | 57,197 (36.9) | 69,163 (35.6) | <0.001 |
| History of MI | 1624 (1.1) | 1849 (1.0) | 0.004 |
| Previous major cardiac surgery | 1413 (0.9) | 1552 (0.8) | <0.001 |
| Previous PCI | 2302 (1.5) | 2341 (1.2) | <0.001 |
| SG | 108,554 (70.1) | 135,041 (69.5) | 0.001 |
| RYGB | 46,406 (30.0) | 59,208 (30.5) | 0.001 |
| Operative time, minutes | |||
| mean ± sd | 89.7 ± 54.1 | 88.6 ± 53.3 | <0.001 |
| Patients Operated on in 2020 n = 154,960 n (%) | Patients Operated on in 2021 n = 194,249 n (%) | p-Value | |
|---|---|---|---|
| Length of stay (days) | 1.4 ± 1.2 | 1.3 ± 1.2 | <0.001 |
| Reoperation | 1666 (1.1) | 2099 (1.1) | 0.877 |
| Reintervention | 1205 (0.8) | 1640 (0.8) | 0.029 |
| Readmission | 4736 (3.1) | 6212 (3.2) | 0.017 |
| Deep SSI | 545 (0.35) | 770 (0.4) | 0.032 |
| Wound disruption | 79 (0.05) | 109 (0.06) | 0.124 |
| Sepsis | 157 (0.1) | 229 (0.1) | 0.143 |
| Pneumonia | 338 (0.2) | 406 (0.2) | 0.562 |
| Unplanned intubation | 155 (0.1) | 173 (0.1) | 0.185 |
| VTE | 611 (0.4) | 706 (0.4) | 0.140 |
| Acute kidney injury | 165 (0.1) | 180 (0.1) | 0.197 |
| MI | 176 (0.1) | 215 (0.1) | 0.799 |
| Cerebral vascular accidents | 18 (0.01) | 31 (0.02) | 0.282 |
| Leak | 425 (0.3) | 543 (0.3) | 0.768 |
| Bleed | 1548 (1.0) | 1835 (0.9) | 0.104 |
| Serious complication | 4883 (3.2) | 6074 (3.1) | 0.683 |
| Mortality | 104 (0.07) | 143 (0.07) | 0.473 |
| Risk Factor | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| 2021 operative year (compared to 2020) | 1.00 | 0.96–1.04 | 0.995 |
| Age | 1.06 | 1.03–1.08 | <0.001 |
| Female gender | 1.00 | 0.92–1.08 | 0.961 |
| GERD | 1.24 | 1.16–1.33 | <0.001 |
| BMI | 1.00 | 0.99–1.00 | <0.001 |
| Hypertension | 1.13 | 1.08–1.18 | <0.001 |
| Hyperlipidemia | 1.04 | 0.99–1.09 | 0.165 |
| Diabetes | |||
| Non-insulin-dependent | 0.95 | 0.90–1.01 | 0.078 |
| Insulin-dependent | 1.10 | 1.02–1.18 | 0.015 |
| Previous VTE | 1.92 | 1.76–2.09 | <0.001 |
| History of MI | 1.62 | 1.41–1.86 | <0.001 |
| Renal insufficiency | 2.19 | 1.85–2.58 | <0.001 |
| COPD | 1.47 | 1.29–1.68 | <0.001 |
| Operative duration | 1.00 | 1.00–1.00 | <0.001 |
| Sleep apnea | 1.00 | 0.96–1.04 | 0.886 |
| Race category | |||
| Black (compared to White) | 1.30 | 1.24–1.37 | <0.001 |
| Other (compared to White) | 0.95 | 0.89–1.01 | 0.078 |
| RYGB (compared to SG) | 2.04 | 1.89–2.19 | <0.001 |
| Functional status | |||
| Partially dependent | 1.72 | 1.42–2.08 | <0.001 |
| Dependent | 2.07 | 0.87–4.93 | 0.099 |
| Risk Factor | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| 2021 operative year (compared to 2020) | 1.20 | 0.93–1.55 | 0.150 |
| Age | 1.84 | 1.62–2.09 | <0.001 |
| Female gender | 0.39 | 0.30–0.51 | <0.001 |
| GERD | 1.29 | 0.99–1.68 | 0.053 |
| BMI | 1.05 | 1.03–1.07 | <0.001 |
| Hyperlipidemia | 0.98 | 0.73–1.33 | 0.901 |
| Diabetes | |||
| Non-insulin-dependent | 1.05 | 0.75–1.47 | 0.758 |
| Insulin-dependent | 1.52 | 1.05–2.21 | 0.028 |
| Previous VTE | 2.35 | 1.56–3.54 | <0.001 |
| History of MI | 3.52 | 2.21–5.61 | <0.001 |
| Renal insufficiency | 2.20 | 1.13–4.29 | 0.021 |
| COPD | 1.36 | 0.76–2.43 | 0.304 |
| Operative duration | 1.00 | 1.00–1.00 | <0.001 |
| Sleep apnea | 0.73 | 0.56–0.96 | 0.023 |
| Race category | |||
| Black (compared to White) | 1.40 | 1.03–1.91 | 0.034 |
| Other (compared to White) | 0.92 | 0.59–1.44 | 0.723 |
| RYGB (compared to SG) | 1.54 | 1.16–2.05 | 0.003 |
| Functional status | |||
| Partially dependent | 2.66 | 1.41–5.04 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verhoeff, K.; Mocanu, V.; Dang, J.; Birch, D.W.; Karmali, S.; Switzer, N.J. Lasting Impact of COVID-19 on Bariatric Surgery Delivery in North America: A Retrospective International Cohort Study of 349,209 Patients in 902 Centers. Surgeries 2023, 4, 342-353. https://doi.org/10.3390/surgeries4030035
Verhoeff K, Mocanu V, Dang J, Birch DW, Karmali S, Switzer NJ. Lasting Impact of COVID-19 on Bariatric Surgery Delivery in North America: A Retrospective International Cohort Study of 349,209 Patients in 902 Centers. Surgeries. 2023; 4(3):342-353. https://doi.org/10.3390/surgeries4030035
Chicago/Turabian StyleVerhoeff, Kevin, Valentin Mocanu, Jerry Dang, Daniel W. Birch, Shahzeer Karmali, and Noah J. Switzer. 2023. "Lasting Impact of COVID-19 on Bariatric Surgery Delivery in North America: A Retrospective International Cohort Study of 349,209 Patients in 902 Centers" Surgeries 4, no. 3: 342-353. https://doi.org/10.3390/surgeries4030035
APA StyleVerhoeff, K., Mocanu, V., Dang, J., Birch, D. W., Karmali, S., & Switzer, N. J. (2023). Lasting Impact of COVID-19 on Bariatric Surgery Delivery in North America: A Retrospective International Cohort Study of 349,209 Patients in 902 Centers. Surgeries, 4(3), 342-353. https://doi.org/10.3390/surgeries4030035







