1. Introduction
Tracheal resection is the mainstay of treatment for primary tracheal cancers. Long resections with subsequent full tracheal mobilization could be burdened by potentially severe anastomotic complications. Specifically, anastomotic leak, erosion of the brachiocephalic vessels, and tracheoesophageal fistula could be life-threatening conditions [
1]. Nonetheless, there is no universally valid definition of the extent of tracheal resections that would be considered “extended”, even if existing data suggest a “cut-off margin” of 35 to 40 mm referring to the likelihood of complications and necessity of additional mobilization manoeuvres [
1]. Furthermore, the role of induction chemotherapy in tracheal tumours has not yet been assessed, and very few authors recommend such an approach [
2]. Another matter for debate is whether and how to protect the tracheal anastomosis suture from the brachiocephalic vessels [
3].
We reported a case of successful extended tracheal resection (40 mm) following induction chemotherapy with a reversed thymic fat pad utilized to protect the tracheal anastomosis from the brachiocephalic vessels.
2. Case Presentation
A 67-year-old man was referred to our tertiary care centre with the suspicion of a malignant tracheal tumour. The patient had undergone partial left hemiglossectomy and left functional neck dissection followed by chemotherapy due to squamous cell carcinoma 5 years earlier. He presented with acute respiratory failure; bronchoscopy revealed a middle tracheal cancer obstructing the airway. A treatment by Nd-Yag laser and Dumon stent placement was performed.
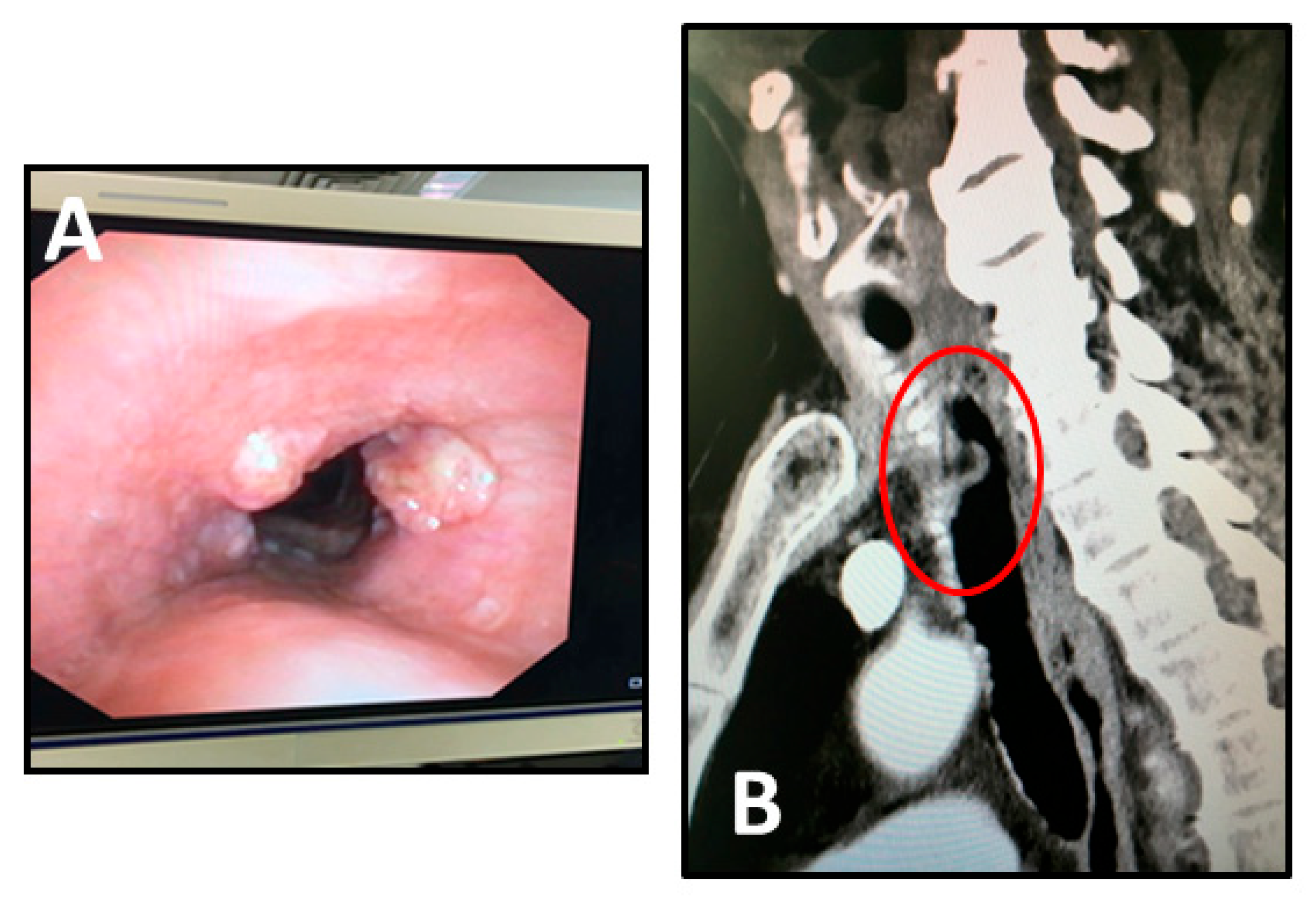
The case was discussed at the local multidisciplinary tumour board (MTB) and, considering the length and thickness extension of the tumour, induction chemotherapy was indicated. After two cycles of Cisplatin and Fluorouracil we observed a partial response with a reduction in the thickness of the tumour at the bronchoscopy (
Figure 1A) and computed tomography (CT) scan imaging (
Figure 1B). The patient was once more discussed at the MTB and scheduled for surgical treatment.
Following the Dumon stent removal, an extended (40 mm) middle tracheal resection according to Grillo’s technique [
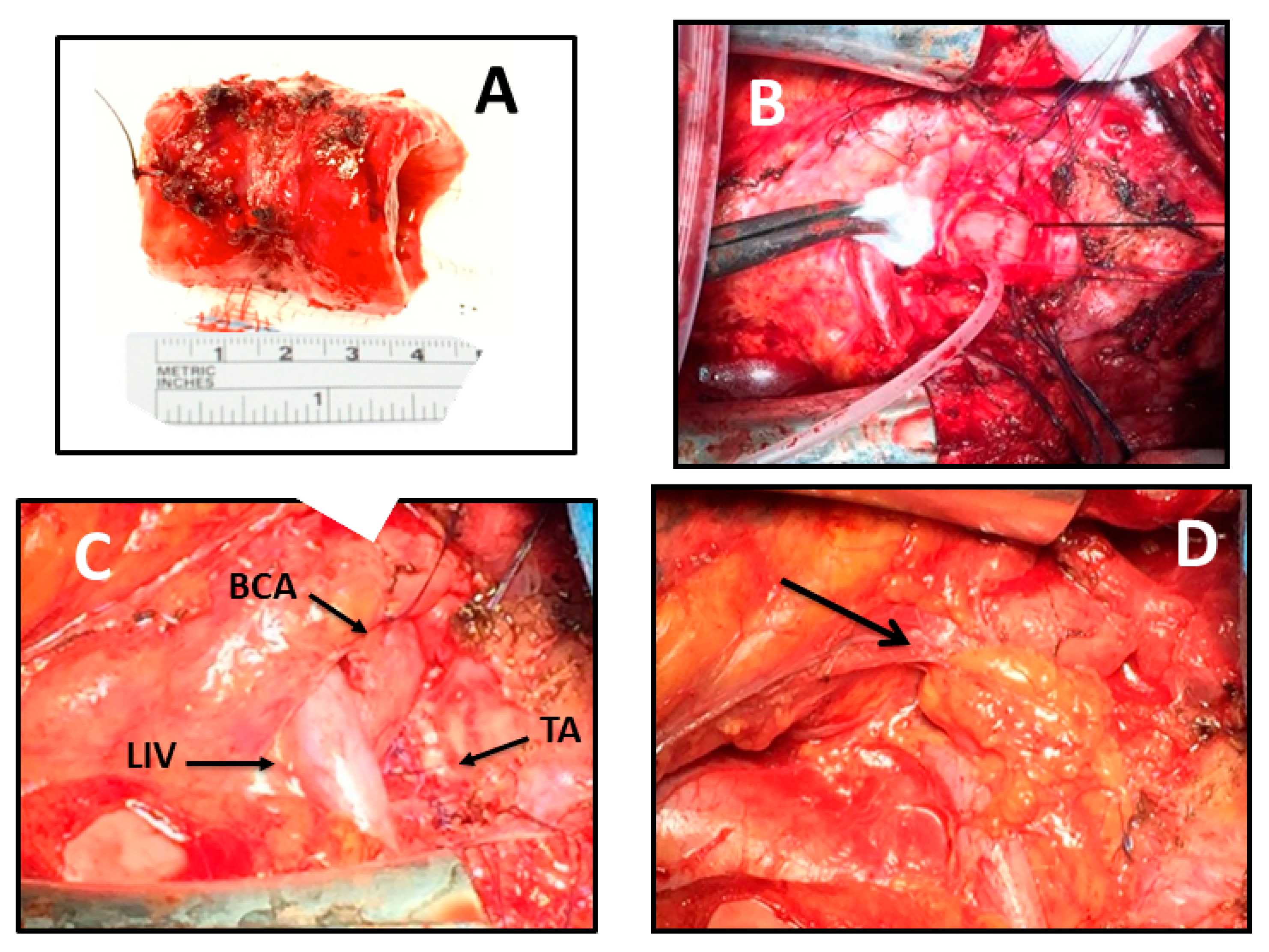
4] via cervicosternotomy was performed. The procedure was made with the patient in the supine position, with a roll placed behind the shoulders to extend the neck. A cervical collar incision with median sternotomy was made to expose the entire trachea. The pretracheal plane was dissected distally toward the carina and proximally to the cervical level to fully mobilize the overall length of the trachea to reduce anastomotic tension. Laterally, the dissection was performed taking special care to avoid injury to the blood supply of the trachea. Proximal and distal margins for segmental tracheal resection were intraoperatively determined via flexible bronchoscopy. The resected specimen measured 40 mm (5 tracheal cartilage rings) longitudinally (
Figure 2A). A low-tension end-to-end anastomosis using running 4-0 polydioxanone suture for the posterior membranous wall and interrupted 3-0 polyglactin sutures for the anterior cartilaginous wall was performed. No further tracheal release manoeuvres were needed. Tracheal anastomosis was completed in 38 min of apnoeic hyperoxygenation with mild hypercapnia (PaCO
2 48–50 mmHg), which was promptly resolved in less than 2 min (
Figure 2B). The patient had been preoxygenated to a PaO
2 of 400 mmHg at the beginning of surgical procedure; Near Infrared Spectroscopy (NIRS) was used to monitor cerebral oxygenation, and serial blood gases analyses were performed to control the pH during the apnoeic hyperoxygenation. The suture line was protected with a reverse thymic fat pad interposed between the trachea and the brachiocephalic vessels. Dissection of the thymus began at the inferior margin of the gland, and it was carried laterally to within 2 cm of the phrenic nerve and superiorly to above the innominate vein, until the entire gland was freed. The flap was pedicled on the thymic branches arising from the internal mammary artery. (
Figure 2C,D). A water suture test was done. The cervicosternotomy incision was closed in the standard fashion. A “guardian stitch” was placed in the midline from the submental crease to the anterior chest at about the angle of Louis, to maintain neck flexion for the first postoperative week. The patient was extubated in the operating room under bronchoscopic control.
Pathological examination revealed a squamous cell carcinoma; tumour invasion reached up to muscular tonaca. On the 11th postoperative day, the patient was discharged; no complications have occurred. At discharge, bronchoscopy confirmed the integrity of the tracheal anastomosis (
Figure 3A). Concurrent chemoradiation (CDDP four cycles + 50 Gy) was administered two months later, after which bronchoscopy confirmed satisfactory epithelization of the anastomotic tract. At the followup at six months, the chest CT confirmed the absence of residual disease (
Figure 3B) and the persistence of the reverse thymic fat pad interposed between the trachea and the brachiocephalic vessels (
Figure 3C).
3. Discussion
Surgical resection followed by primary reconstruction represents the best curative treatment for tracheal cancer to date, in the absence of distant metastasis and if local resectability is provided [
2]. Patients surgically treated have a better prognosis than unresected ones, with 5- and 10-year survival rates of 50% and 35%, respectively, compared to 5–15% and 6–7%, correspondingly, of patients not undergoing surgery [
5].
Controversies still exist among surgeons about the length of tracheal resectability. Extended tracheal resections are generally considered to be burdened by a greater rate of postoperative complications than standard resections, whose cut-off margin can be considered as a length of less than 40 mm. However, most publications presented in the literature are focused on postoperative results of tracheal resection in general, with scarce attention to extended tracheal surgery specifically [
1]. Mulliken and Grillo demonstrated that approximately half of the trachea could be resected and reconstructed with primary anastomosis by utilizing several release manoeuvres to reduce anastomotic tension [
6]. Conversely, Wright and colleagues showed that resections > 40 mm were associated with a drastic increase in anastomotic complications, due to tracheal devascularization and excessive anastomotic tension [
7]. In our case, requiring a relatively long resection of the trachea, which reached the safety limit, a cervical collar incision with median sternotomy provided a good exposure for mobilizing the entire trachea to achieve tension-limited anastomosis. No further release techniques were needed. Neck flexion and dissection of the pretracheal plane typically provide enough relief of anastomotic tension, according to Mulliken and Grillo. In cadaveric experiments, they showed that with 15° to 35° of neck flexion, a 4.5 cm length of trachea could be resected with a tension-free primary anastomosis [
6]. Furthermore, careful release manoeuvres should be performed to minimize tracheal devascularization and consequent life-threatening complications. This is even more true in such a case as our patient, with a previous history of left hemiglossectomy and neck dissection. Despite the consequent fibrosis of the surgical field, which made this procedure technically more challenging, it was successfully performed.
According to Sanchez-Lorente et al., we performed airway resection and reconstruction under apnoeic hyperoxygenation to provide adequate oxygenation during the apnoeic time period of anastomosis by an increase in intrapulmonary oxygen storage [
8,
9]. This technique makes it easier to perform anastomosis, due to an optimal surgical exposure, without the obstacle of the endotracheal tube or cross-field ventilation, which always remains available on the surgical table in our practice. In addition, compared to extracorporeal membrane oxygenation (ECMO), there is no need of heparinization, with a significantly lower risk of bleeding. ECMO is used in the management of severe cardiopulmonary failure, but the indication in the oncologic population has not been clearly established [
10]. The outcome for ECMO among patients with a thoracic neoplasm is variable, especially in tracheobronchial surgery. Moreover, this procedure is still burdened by potentially severe complications, such as bleeding and thrombosis, including disseminated intravascular coagulation, haemolysis, fibrinolysis, cannulation and surgical site bleeding, intracranial bleeding, and gastrointestinal and pulmonary haemorrhage [
11].
To date, primary resection is the mainstay of tracheal cancer treatment, while the value of induction chemotherapy remains unclear [
2]. There is no consensus about the benefits of chemotherapy in a neoadjuvant setting compared to the higher risk of postoperative complications and mortality. The presented case is almost unique in the literature, given the MTB indication to neoadjuvant therapy. We decided upon this strategy rather than upfront surgery because of the tumour’s longitudinal extension of more than 5 cm and cartilaginous ring infiltration. The patient’s history of prior neck surgery was another key point in our decision making: tumour shrinkage was the goal of multimodal treatment to minimize the surgical complications of a redo procedure. Our strategy successfully reduced the tumour load and lessened the extent of tracheal resection.
Additionally, the coverage of the tracheal anastomotic line with a vascularized tissue flap to prevent erosion of contiguous brachiocephalic vessels is still a matter of debate [
3]. Omentoplasty, myoplasty, or flaps with a blood supply arising from the branches of the internal thoracic artery, such as the pericardial fat graft or the thymus flap can be used [
12]. The reverse thymic fat pad proved to be a good tool to protect the suture line. It has been demonstrated that omentum enhances blood supply, processing an angiogenic factor, and it improves healing by new fibroblast growth [
13]. Furthermore, in our experience, postoperative concurrent chemoradiation did not seem to add risk for the integrity of the anastomosis. Regarding the surgical technique, performing a thymic flap via sternotomy is more feasible and easier than omentoplasty or pectoral myoplasty, which have longer pedicles and thus present a higher risk of terminal devascularization. Indeed, thymic vessels arise from the internal thoracic artery branches and thus, reversing the thymic flap is enough, after it is pedunculated. With this technique, a well-vascularized flap of about 15 cm long with a thickness of approximately 4 cm is developed, preserving superior and lateral arterial supply from the internal mammary artery. Similar to the omentum, thymic fat produces an angiogenic factor that stimulates the development of new blood supply in avascular areas and provides new fibroblast growth to enhance healing. These fat-derived mesenchymal stem cells are not present in a muscle flap which, conversely, undergoes scar fibrosis. According to the “omentum model”, we suppose that a thymic fat flap might confer similar biological advantages and, consequently, add a benefit in terms of the better trophism of the tracheal anastomotic suture line. Moreover, when properly harvested, the lobes of the thymus generate two pedicles, which are versatile enough to be applied to any part of the cervical or thoracic trachea. They may represent a suitable option to wrap a tracheal anastomosis both anteriorly and posteriorly, to prevent tracheoesophageal fistula. This feared complication is a consequence of breakdown of the membranous wall of the anastomosis. The interposition of a well-vascularized and angiogenetic flap may lead to a better membranous repair and, consequently, this phenomenon may be effective in preventing posterior anastomotic leakage, the cause of a tracheoesophageal fistula.
To conclude, the presented case suggests that extended tracheal resection following induction chemotherapy may be safely performed. Avoiding devascularization and ensuring low-tension anastomosis are paramount to achieving excellent postoperative outcomes. The reverse thymic fat flap proved to be an effective tissue to protect and enhance anastomosis healing.