A Multilayered Dural Repair Technique Using Duragen for Early Cranioplasty Following Decompressive Craniotomy
Abstract
:1. Introduction
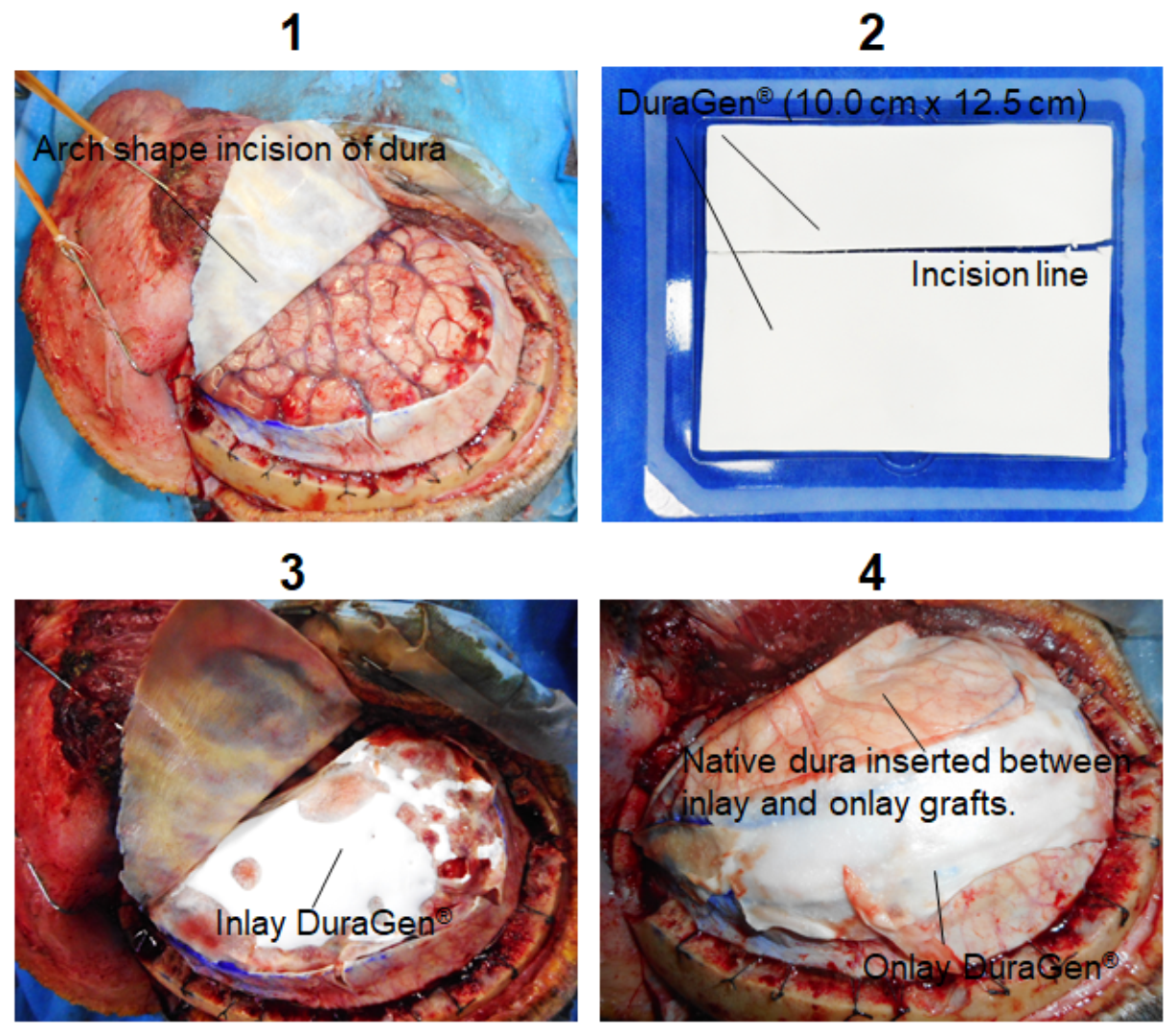
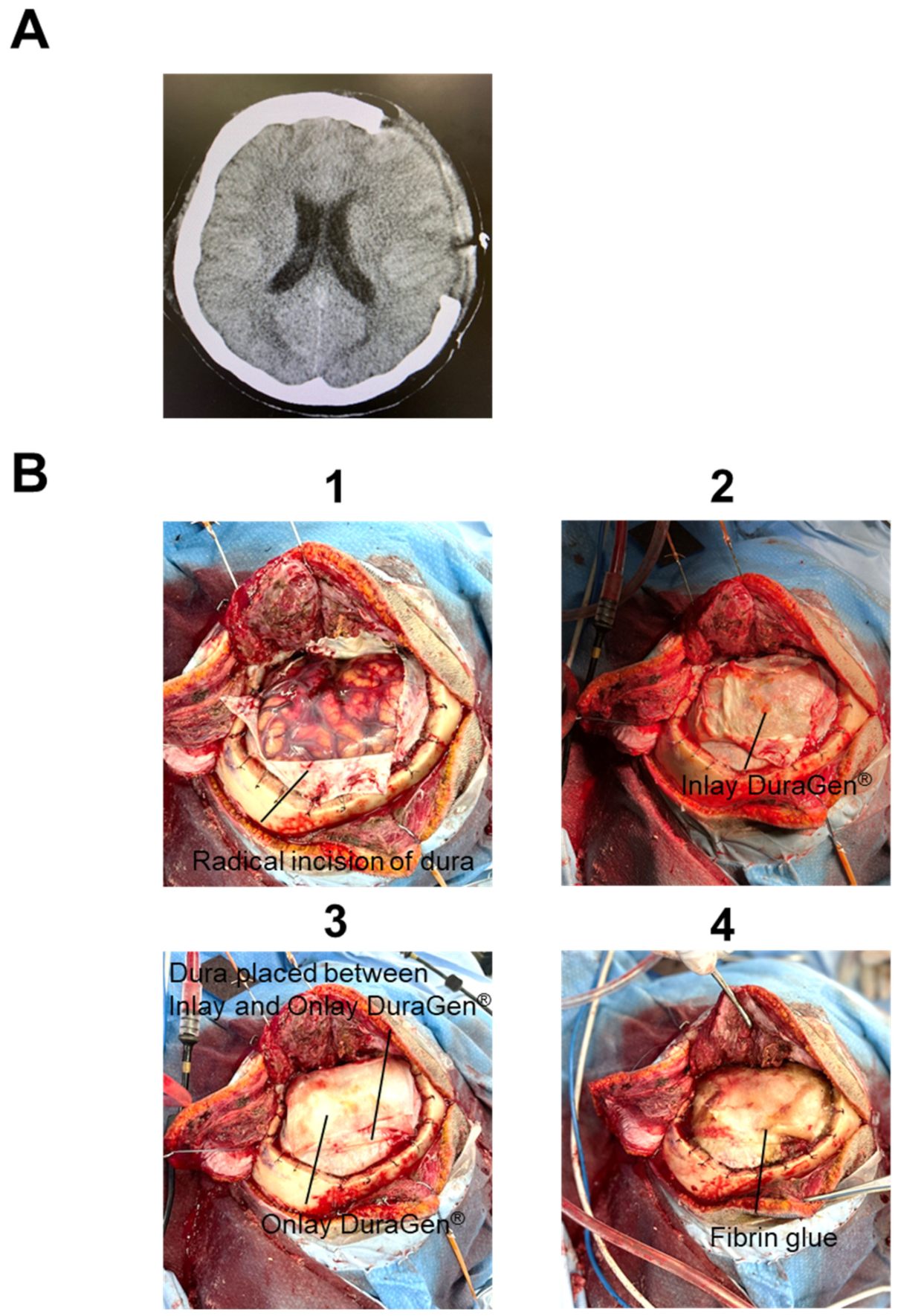
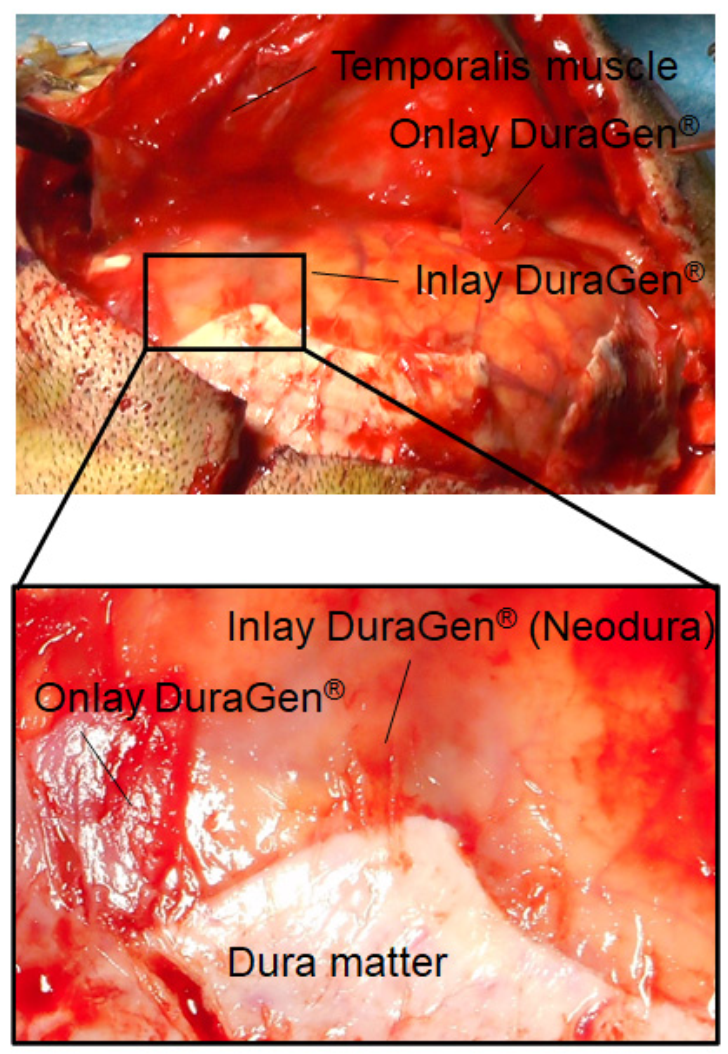
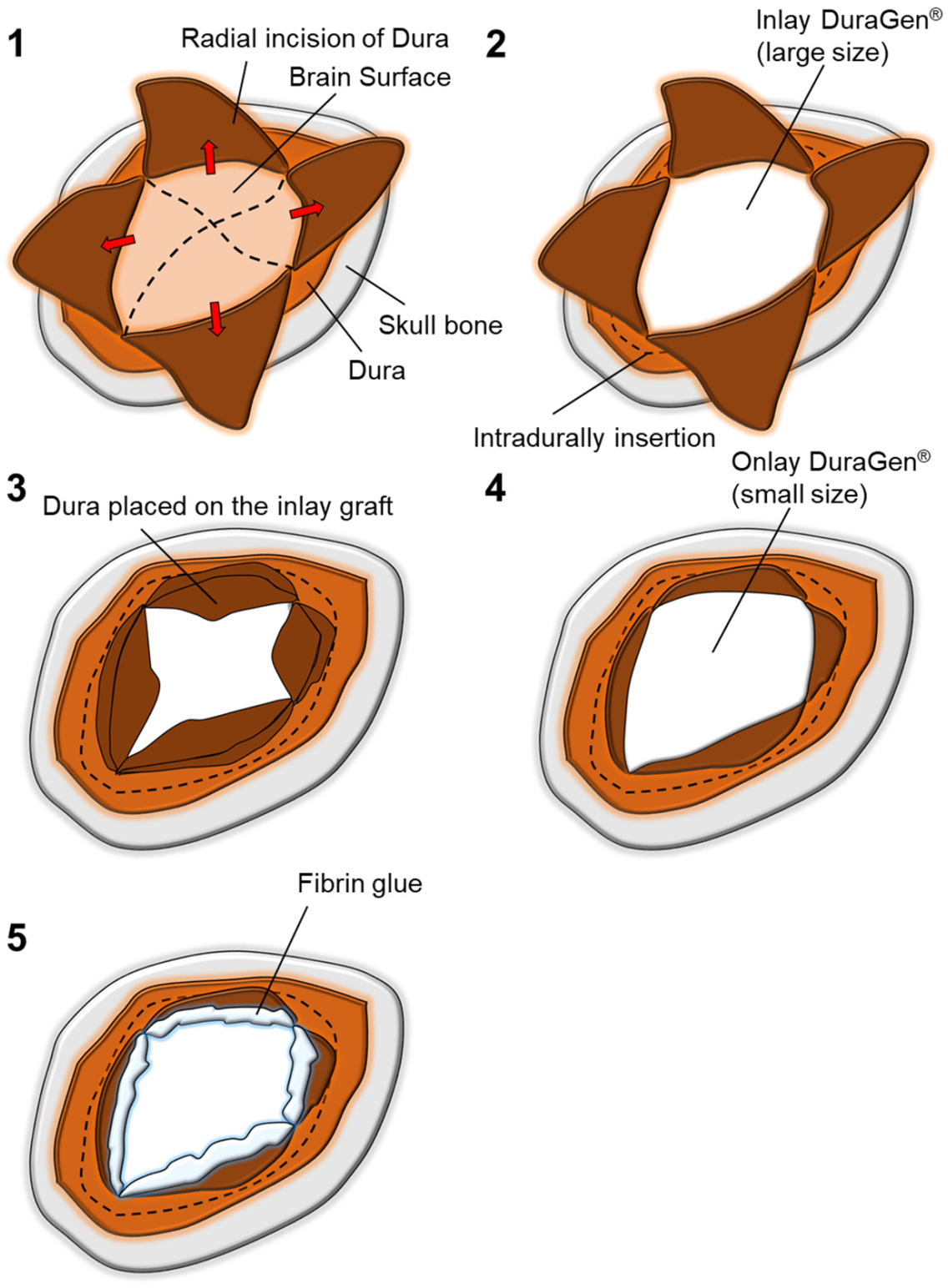
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
References
- Timofeev, I.; Santarius, T.; Kolias, A.G.; Hutchinson, P.J. Decompressive craniectomy-operative technique and perioperative care. In Advances and Technical Standards in Neurosurgery; Springer: Vienna, Austria, 2012; Volume 38, pp. 115–136. [Google Scholar]
- De Bonis, P.; Frassanito, P.; Mangiola, A.; Nucci, C.G.; Anile, C.; Pompucci, A. Cranial repair: How complicated is filling a “hole”? J. Neurotrauma 2012, 29, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Minami, H.; Yamaura, I.; Yoshida, Y. Postoperative subdural hematoma with blood flow from an epidural hematoma through a tear at the suture point of an artificial dura substitute. Acta Neurochir. 2019, 161, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Narotam, P.K.; van Dellen, J.R.; Bhoola, K.D. A clinicopathological study of collagen sponge as a dural graft in neurosurgery. J. Neurosurg. 1995, 82, 406–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danish, S.F.; Samdani, A.; Hanna, A.; Storm, P.; Sutton, L. Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations. J. Neurosurg. 2006, 104, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Narotam, P.K.; Reddy, K.; Fewer, D.; Qiao, F.; Nathoo, N. Collagen matrix duraplasty for cranial and spinal surgery: A clinical and imaging study. J. Neurosurg. 2007, 106, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjornson, A.; Tajsic, T.; Kolias, A.G.; Wells, A.; Naushahi, M.J.; Anwar, F.; Helmy, A.; Timofeev, I.; Hutchinson, P.J. A case series of early and late cranioplasty-comparison of surgical outcomes. Acta Neurochir. 2019, 161, 467–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Hwang, S.Y.; Kwon, T.H.; Chong, K.; Yoon, W.K.; Kim, J.H. Defining “early” cranioplasty to achieve lower complication rates of bone flap failure: Resorption and infection. Acta Neurochir. 2019, 161, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Park, T.; Lee, S.P.; Baek, J.W.; Ryou, K.S.; Kim, S.H. Optimal timing and complications of cranioplasty: A single-center retrospective review of 109 cases. J. Neurointensive Care 2020, 3, 48–57. [Google Scholar] [CrossRef]
- Stendel, R.; Danne, M.; Fiss, I.; Klein, I.; Schilling, A.; Hammersen, S.; Pietilae, T.; Jänisch, W.; Hopfenmüller, W. Efficacy and safety of a collagen matrix for cranial and spinal dural reconstruction using different fixation techniques. J. Neurosurg. 2008, 109, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Narotam, P.K.; José, S.; Nathoo, N.; Taylon, C.; Vora, Y. Collagen matrix (DuraGen) in dural repair: Analysis of a new modified technique. Spine 2004, 29, 2861–2867. [Google Scholar] [CrossRef] [PubMed]
- Sade, B.; Oya, S.; Lee, J.H. Non-watertight dural reconstruction in meningioma surgery: Results in 439 consecutive patients and a review of the literature. Clinical article. J. Neurosurg. 2011, 114, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Neulen, A.; Gutenberg, A.; Takács, I.; Wéber, G.; Wegmann, J.; Schulz-Schaeffer, W.; Giese, A. Evaluation of efficacy and biocompatibility of a novel semisynthetic collagen matrix as a dural onlay graft in a large animal model. Acta Neurochir. 2011, 153, 2241–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.F.; Yip, C.M.; Lin, Y.H. Endoscopic transpterygoid approach to repair lateral sphenoid recess cerebrospinal fluid leakage by multilayered reconstruction. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Shitara, S.; Shima, A.; Goto, Y.; Fukushima, T. Double collagen matrix grafting for dural closure in microvascular decompression: An alternative use of autologous fascial grafting. Acta Neurochir. 2021, 163, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Oladunjoye, A.O.; Schrot, R.J.; Zwienenberg-Lee, M.; Muizelaar, J.P.; Shahlaie, K. Decompressive craniectomy using gelatin film and future bone flap replacement. J. Neurosurg. 2013, 118, 776–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przybylowski, C.J.; So, V.; DeTranaltes, K.; Walker, C.; Baranoski, J.F.; Chapple, K.; Sanai, N. Sterile Gelatin Film Reduces Cortical Injury Associated with Brain Tumor Re-Resection. Oper. Neurosurg. 2021, 20, 383–388. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, R.; Kuranari, Y.; Mishima, M.; Katayama, M. A Multilayered Dural Repair Technique Using Duragen for Early Cranioplasty Following Decompressive Craniotomy. Surgeries 2021, 2, 371-377. https://doi.org/10.3390/surgeries2040036
Tamura R, Kuranari Y, Mishima M, Katayama M. A Multilayered Dural Repair Technique Using Duragen for Early Cranioplasty Following Decompressive Craniotomy. Surgeries. 2021; 2(4):371-377. https://doi.org/10.3390/surgeries2040036
Chicago/Turabian StyleTamura, Ryota, Yuki Kuranari, Maki Mishima, and Makoto Katayama. 2021. "A Multilayered Dural Repair Technique Using Duragen for Early Cranioplasty Following Decompressive Craniotomy" Surgeries 2, no. 4: 371-377. https://doi.org/10.3390/surgeries2040036
APA StyleTamura, R., Kuranari, Y., Mishima, M., & Katayama, M. (2021). A Multilayered Dural Repair Technique Using Duragen for Early Cranioplasty Following Decompressive Craniotomy. Surgeries, 2(4), 371-377. https://doi.org/10.3390/surgeries2040036





