Functional Outcome in Patients with Dural Arteriovenous Fistulae after Surgical Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
Hospital Based Cohort Study
2.2. Statistical Analysis
2.3. Ethical Approval
3. Results
3.1. Hospital-Based Cohort Study
3.2. Treatment
3.3. Functional Outcome on Discharge and Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Declaration
References
- Spetzler, F.R.; Detwiler, P.W.; Riina, H.A.; Porter, R.W. Modified Classification of Spinal Cord Vascular Lesions. J. Neurosurg. 2002, 96, 145–156. [Google Scholar] [CrossRef]
- Ropper, E.A.; Gross, B.A.; Du, R. Surgical Treatment of Type I Spinal Dural Arteriovenous Fistulas. Neurosurg. Focus 2012, 32, E3. [Google Scholar] [CrossRef]
- Jellema, K.; Tijssen, C.C.; van Gijn, J. Spinal Dural Arteriovenous Fistulas: A Congestive Myelopathy That Initially Mimics a Peripheral Nerve Disorder. Brain 2006, 129, 3150–3164. [Google Scholar] [CrossRef] [PubMed]
- Reinges, M.H.; Thron, A.; Mull, M.; Huffmann, B.C.; Gilsbach, J.M. Dural Arteriovenous Fistulae at the Foramen Magnum. J. Neurol. 2001, 248, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Krings, T.; Geibprasert, S. Spinal Dural Arteriovenous Fistulas. AJNR Am. J. Neuroradiol. 2009, 30, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Higashida, R.T.; Halbach, V.V.; Dowd, C.F.; Tsuura, M.; Komai, N.; Wilson, C.B.; Hieshima, G.B. Development of Acquired Arteriovenous Fistulas in Rats Due to Venous Hypertension. J. Neurosurg. 1994, 80, 884–889. [Google Scholar] [CrossRef]
- Herman, J.M.; Spetzler, R.F.; Bederson, J.B.; Kurbat, J.M.; Zabramski, J.M. Genesis of a Dural Arteriovenous Malformation in a Rat Model. J. Neurosurg. 1995, 83, 539–545. [Google Scholar] [CrossRef]
- Aghakhani, N.; Parker, F.; David, P.; Lasjaunias, P.; Tadie, M. Curable Cause of Paraplegia: Spinal Dural Arteriovenous Fistulae. Stroke 2008, 39, 2756–2759. [Google Scholar] [CrossRef]
- Sorenson, T.; Giordan, E.; Cannizzaro, D.; Lanzino, G. Surgical Ligation of Spinal Dural Arteriovenous Fistula. Acta Neurochir. 2018, 160, 191–194. [Google Scholar] [CrossRef]
- Endo, T.; Endo, H.; Sato, K.; Matsumoto, Y.; Tominaga, T. Surgical and Endovascular Treatment for Spinal Arteriovenous Malformations. Neurol. Med. Chir. 2016, 56, 457–464. [Google Scholar] [CrossRef]
- Kataoka, H.; Miyamoto, S.; Nagata, I.; Ueba, T.; Hashimoto, N. Venous Congestion Is a Major Cause of Neurological Deterioration in Spinal Arteriovenous Malformations. Neurosurgery 2001, 48, 1224–1229. [Google Scholar]
- Hurst, R.W.; Kenyon, L.C.; Lavi, E.; Raps, E.C.; Marcotte, P. Spinal Dural Arteriovenous Fistula: The Pathology of Venous Hypertensive Myelopathy. Neurology 1995, 45, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Jellema, K.; Canta, L.R.; Tijssen, C.C.; van Rooij, W.J.; Koudstaal, P.J.; van Gijn, J. Spinal Dural Arteriovenous Fistulas: Clinical Features in 80 Patients. J. Neurol. Neurosurg. Psychiatry. 2003, 74, 1438–1440. [Google Scholar] [CrossRef] [PubMed]
- Kai, Y.; Hamada, J.; Morioka, M.; Yano, S.; Mizuno, T.; Kuratsu, J. Arteriovenous Fistulas at the Cervicomedullary Junction Presenting with Subarachnoid Hemorrhage: Six Case Reports with Special Reference to the Angiographic Pattern of Venous Drainage. AJNR Am. J. Neuroradiol. 2005, 26, 1949–1954. [Google Scholar] [PubMed]
- Manzano, G.; Green, B.A.; Vanni, S.; Levi, A.D. Contemporary Management of Adult Intramedullary Spinal Tumors-Pathology and Neurological Outcomes Related to Surgical Resection. Spinal Cord 2008, 46, 540–546. [Google Scholar] [CrossRef]
- Benzel, E.C.; Lancon, J.; Kesterson, L.; Hadden, T. Cervical Laminectomy and Dentate Ligament Section for Cervical Spondylotic Myelopathy. J. Spinal Disord. 1991, 4, 286–295. [Google Scholar] [CrossRef]
- McCormick, P.C.; Torres, R.; Post, K.D.; Stein, B.M. Intramedullary Ependymoma of the Spinal Cord. J. Neurosurg. 1990, 72, 523–532. [Google Scholar] [CrossRef]
- Goyal, A.; Cesare, J.; Lu, V.M.; Alvi, M.A.; Kerezoudis, P.; Brinjikji, W.; Nasr, D.; Lanzino, G.; Bydon, M. Outcomes Following Surgical Versus Endovascular Treatment of Spinal Dural Arteriovenous Fistula: A Systematic Review and Meta-Analysis. J. Neurol. Neurosurg. Psychiatry. 2019, 90, 1139–1146. [Google Scholar] [CrossRef]
- Van Dijk, J.M.; TerBrugge, K.G.; Willinsky, R.A.; Farb, R.I.; Wallace, M.C. Multidisciplinary Management of Spinal Dural Arteriovenous Fistulas: Clinical Presentation and Long-Term Follow-up in 49 Patients. Stroke 2002, 33, 1578–1583. [Google Scholar] [CrossRef]
- Steinmetz, M.P.; Chow, M.M.; Krishnaney, A.A.; Andrews-Hinders, D.; Benzel, E.C.; Masaryk, T.J.; Mayberg, M.R.; Rasmussen, P.A. Outcome after the Treatment of Spinal Dural Arteriovenous Fistulae: A Contemporary Single-Institution Series and Meta-Analysis. Neurosurgery 2004, 55, 77–87. [Google Scholar] [CrossRef]
- Niimi, Y.; Berenstein, A.; Setton, A.; Neophytides, A. Embolization of Spinal Dural Arteriovenous Fistulae: Results and Follow-Up. Neurosurgery 1997, 40, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Sherif, C.; Gruber, A.; Bavinzski, G.; Standhardt, H.; Widhalm, G.; Gibson, D.; Richling, B.; Knosp, E. Long-Term Outcome of a Multidisciplinary Concept of Spinal Dural Arteriovenous Fistulae Treatment. Neuroradiology 2008, 50, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bakker, N.A.; Uyttenboogaart, M.; Luijckx, G.J.; Eshghi, O.S.; Mazuri, A.; Metzemaekers, J.D.; Groen, R.J.; Van Dijk, J.M. Recurrence Rates after Surgical or Endovascular Treatment of Spinal Dural Arteriovenous Fistulas: A Meta-Analysis. Neurosurgery 2015, 77, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Fassett, D.R.; Rammos, S.K.; Patel, P.; Parikh, H.; Couldwell, W.T. Intracranial Subarachnoid Hemorrhage Resulting from Cervical Spine Dural Arteriovenous Fistulas: Literature Review and Case Presentation. Neurosurg. Focus 2009, 26, E4. [Google Scholar] [CrossRef]
- Aviv, R.I.; Shad, A.; Tomlinson, G.; Niemann, D.; Teddy, P.J.; Molyneux, A.J.; Byrne, J.V. Cervical Dural Arteriovenous Fistulae Manifesting as Subarachnoid Hemorrhage: Report of Two Cases and Literature Review. AJNR Am. J. Neuroradiol. 2004, 25, 854–858. [Google Scholar]
- Julia Wrenczycki.14. Deutscher Wirbelsäulenkongress. Eur. Spine J. 2019, 28, 2660–2758. [Google Scholar] [CrossRef]

| Variable | Overall Cohort |
|---|---|
| Age, mean (SD) | 61.8 (8.4 SD) |
| Sex, N (%) | |
| 16 (59.3%) |
| 11 (40.7) |
| Symptoms N (%) | |
| 24 (88.9) |
| 10/25 (40) |
| 13/25 (52) |
| 9/25 (36) |
| 11/25 (44) |
| 8/25 (32) |
| 6/25 (24) |
| 2/25 (8) |
| 1/25 (4) |
| 2 (7.4) |
| Past medical history, N (%) | |
| 9 (33.3) |
| 2 (7.4) |
| 3 (11.1) |
| 1 (3.7) |
| Location, N (%) | |
| 3 (11.1) |
| 15 (55.6) |
| 8 (29.6) |
| 1 (3.7) |
| Duration of hospitalization, mean (SD) | 10.1 (6.8) |
| Functional state | |
| Cohort for spinal scores | 25/27 |
| mJOA score on admission, mean (SD) | 14 (2.9) |
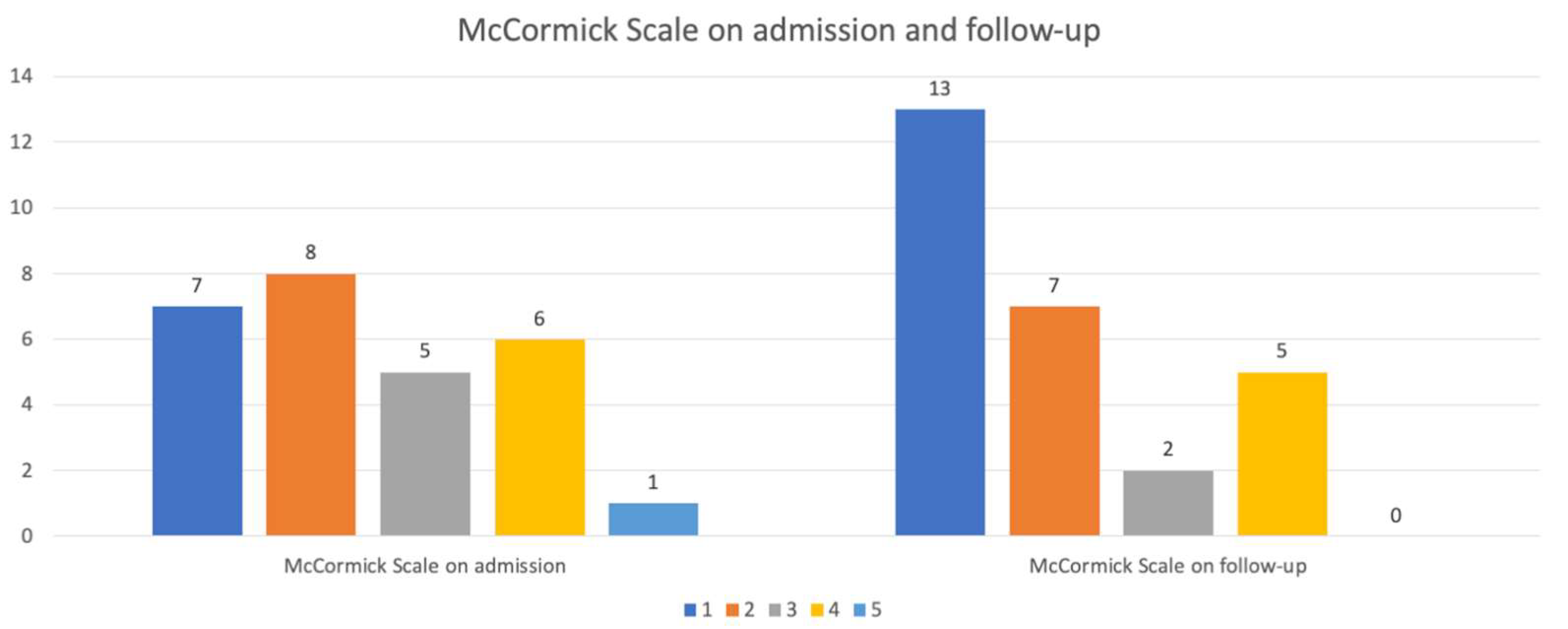
| McCormick Scale on admission, N (%) | |
| 6 (24) |
| 8 (32) |
| 5 (20) |
| 6 (24) |
| mJOA score on discharge, mean (SD) | 14.2 (2.8) |
| McCormick Scale on discharge, N (%) | |
| 8 (32) |
| 5 (20) |
| 7 (28) |
| 4 (16) |
| 1 (4) |
| mJOA score on FU, mean (SD) | 15.5 (2.2) |
| McCormick Scale on FU, N (%) | |
| 12 (48) |
| 7 (28) |
| 2 (8) |
| 4 (16) |
| Univariable Analysis | |||
|---|---|---|---|
| Coefficient | 95% CI | p-Value | |
| Age, mean (SD), N(%) | −0.1 | −0.19–0.01 | 0.08 |
| Female Sex, N(%) | −0.87 | −2.73–0.99 | 0.35 |
| Worsening after surgery | 0.85 | −1.3–3 | 0.42 |
| mJOA score on admission | 0.6 | 0.4–0.81 | <0.001 |
| Univariable Analysis | |||
|---|---|---|---|
| OR | 95%CI | p-Value | |
| Age, mean (SD), N(%) | 1.11 | 0.99–1.24 | 0.06 |
| Female Sex, N(%) | 0.4 | 0.09–1.85 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hostettler, I.C.; Butenschoen, V.M.; Meyer, B.; Wostrack, M. Functional Outcome in Patients with Dural Arteriovenous Fistulae after Surgical Treatment. Surgeries 2020, 1, 54-62. https://doi.org/10.3390/surgeries1020007
Hostettler IC, Butenschoen VM, Meyer B, Wostrack M. Functional Outcome in Patients with Dural Arteriovenous Fistulae after Surgical Treatment. Surgeries. 2020; 1(2):54-62. https://doi.org/10.3390/surgeries1020007
Chicago/Turabian StyleHostettler, Isabel C, Vicki M Butenschoen, Bernhard Meyer, and Maria Wostrack. 2020. "Functional Outcome in Patients with Dural Arteriovenous Fistulae after Surgical Treatment" Surgeries 1, no. 2: 54-62. https://doi.org/10.3390/surgeries1020007
APA StyleHostettler, I. C., Butenschoen, V. M., Meyer, B., & Wostrack, M. (2020). Functional Outcome in Patients with Dural Arteriovenous Fistulae after Surgical Treatment. Surgeries, 1(2), 54-62. https://doi.org/10.3390/surgeries1020007








