The Pathobiological Underpinnings of Psychosis: From the Stress-Related Hypothesis to a Multisystemic Approach
Abstract
1. Introduction
2. Immunity
3. Redox
4. Metabolism
5. Neuroendocrine/Stress System
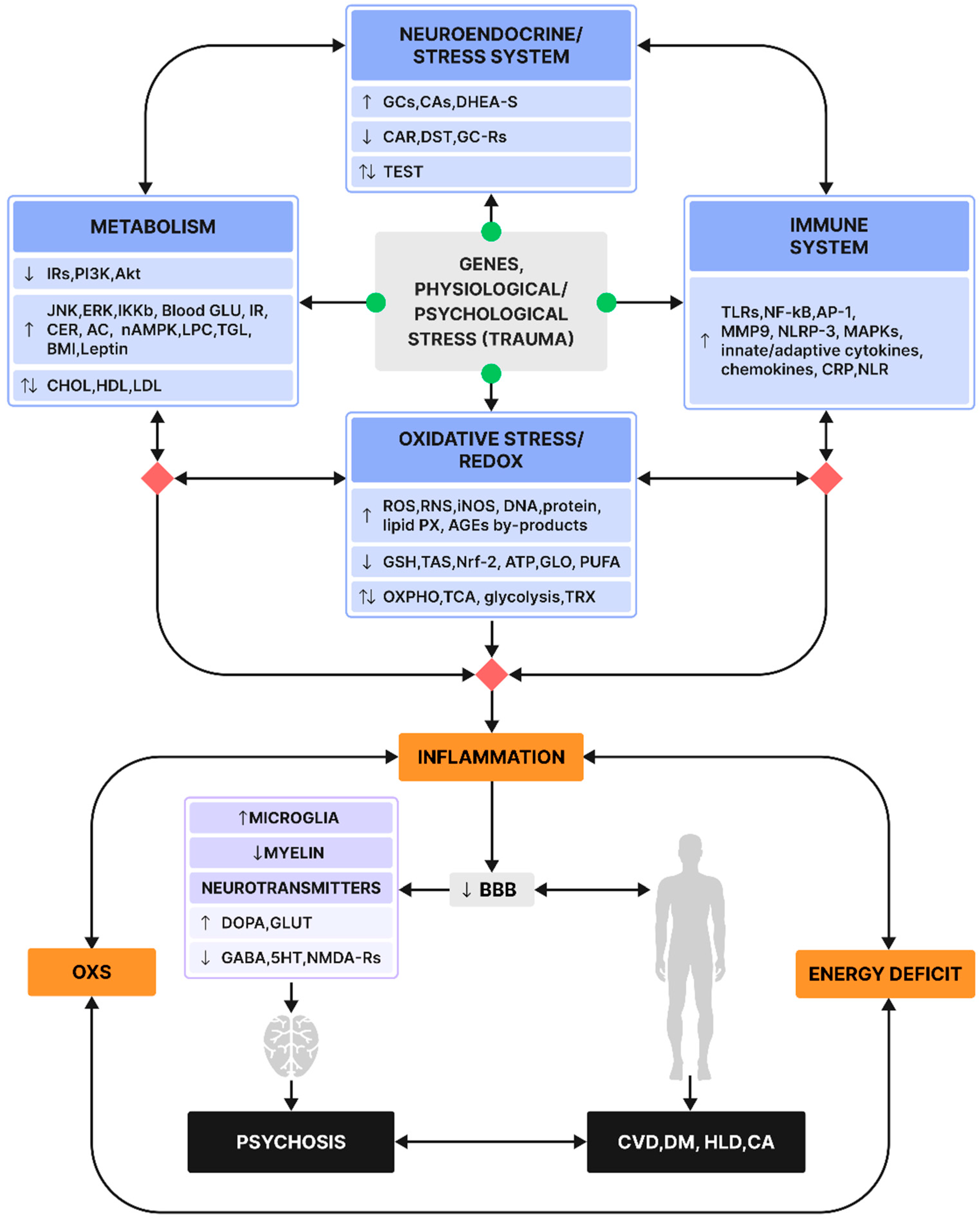
6. Multisystemic Interactions
7. Discussion
8. Future Directions
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Moreno-Küstner, B.; Martín, C.; Pastor, L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS ONE 2018, 13, e0195687. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The Dopamine Hypothesis of Schizophrenia: Version III--The Final Common Pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C.; Zukin, S.R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 1991, 148, 1301–1308. [Google Scholar] [CrossRef]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Schwieler, L.; Nilsson, L.; Linderholm, K.; Engberg, G. The kynurenic acid hypothesis of schizophrenia. Physiol. Behav. 2007, 92, 203–209. [Google Scholar] [CrossRef]
- Pralong, D.; Tomaskovic-Crook, E.; Opeskin, K.; Copolov, D.; Dean, B. Serotonin2A receptors are reduced in the planum temporale from subjects with schizophrenia. Schizophr. Res. 2000, 44, 35–45. [Google Scholar] [CrossRef]
- Karanikas, E. The immune-stress/endocrine-redox-metabolic nature of psychosis’ etiopathology; focus on the intersystemic pathways interactions. Neurosci. Lett. 2022, 794, 137011. [Google Scholar] [CrossRef]
- A Landek-Salgado, M.; E Faust, T.; Sawa, A. Molecular substrates of schizophrenia: Homeostatic signaling to connectivity. Mol. Psychiatry 2015, 21, 10–28. [Google Scholar] [CrossRef]
- Agorastos, A.; Pervanidou, P.; Chrousos, G.P.; Baker, D.G. Developmental Trajectories of Early Life Stress and Trauma: A Narrative Review on Neurobiological Aspects Beyond Stress System Dysregulation. Front. Psychiatry 2019, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Simoes, L.R.; Quevedo, J.; Zhang, X.Y. Microglial Activation and Psychotic Disorders: Evidence from Pre-clinical and Clinical Studies. Curr. Top. Behav. Neurosci. 2020, 44, 161–205. [Google Scholar]
- Frank, M.G.; Weber, M.D.; Watkins, L.R.; Maier, S.F. Stress-induced neuroinflammatory priming: A liability factor in the etiology of psychiatric disorders. Neurobiol. Stress 2016, 4, 62–70. [Google Scholar] [CrossRef]
- Karanikas, E. Psychologically traumatic oxidative stress; a comprehensive review of redox mechanisms and related inflammatory implications. Psychopharmacol. Bull. 2021, 51, 65. [Google Scholar]
- Karanikas, E. The Gordian knot of the immune-redox systems’ interactions in psychosis. Int. Clin. Psychopharmacol. 2023, 38, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Flatow, J.; Buckley, P.; Miller, B.J. Meta-Analysis of Oxidative Stress in Schizophrenia. Biol. Psychiatry 2013, 74, 400–409. [Google Scholar] [CrossRef]
- Fraguas, D.; Díaz-Caneja, C.M.; Rodríguez-Quiroga, A.; Arango, C. Oxidative Stress and Inflammation in Early Onset First Episode Psychosis: A Systematic Review and Meta-Analysis. Int. J. Neuropsychopharmacol. 2017, 20, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-Analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef]
- Postolache, T.T.; del Bosque-Plata, L.; Jabbour, S.; Vergare, M.; Wu, R.; Gragnoli, C. Co-shared genetics and possible risk gene pathway partially explain the comorbidity of schizophrenia, major depressive disorder, type 2 diabetes, and metabolic syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2019, 180, 186–203. [Google Scholar] [CrossRef]
- Perpiñán, E.; Sanchez-Fueyo, A.; Safinia, N. Immunoregulation: The interplay between metabolism and redox homeostasis. Front. Transplant. 2023, 2, 1283275. [Google Scholar] [CrossRef]
- Hoogland, I.C.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflammation 2015, 12, 114. [Google Scholar] [CrossRef]
- Wilkins, H.M.; Weidling, I.W.; Ji, Y.; Swerdlow, R.H. Mitochondria-Derived Damage-Associated Molecular Patterns in Neurodegeneration. Front. Immunol. 2017, 8, 508. [Google Scholar] [CrossRef]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal Immune Activation Alters Fetal Brain Development through Interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Meyer, U.; Feldon, J.; Schedlowski, M.; Yee, B.K. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci. Biobehav. Rev. 2005, 29, 913–947. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Feldon, J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav. Brain Res. 2009, 204, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Oskvig, D.B.; Elkahloun, A.G.; Johnson, K.R.; Phillips, T.M.; Herkenham, M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav. Immun. 2012, 26, 623–634. [Google Scholar] [CrossRef]
- Winter, C.; Djodari-Irani, A.; Sohr, R.; Morgenstern, R.; Feldon, J.; Juckel, G.; Meyer, U. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: Implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int. J. Neuropsychopharmacol. 2008, 12, 513–524. [Google Scholar] [CrossRef]
- Upthegrove, R.; Manzanares-Teson, N.; Barnes, N.M. Cytokine function in medication-naive first episode psychosis: A systematic review and meta-analysis. Schizophr. Res. 2014, 155, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, C.; Elsworthy, R.J.; Upthegrove, R.; Wood, S.J.; Aldred, S. Inflammation in first-episode psychosis: The contribution of inflammatory biomarkers to the emergence of negative symptoms, a systematic review and meta-analysis. Acta Psychiatr. Scand. 2022, 146, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, T.; Osimo, E.F.; Brugger, S.; Mondelli, V.; A McCutcheon, R.; Howes, O.D. A Meta-analysis of Immune Parameters, Variability, and Assessment of Modal Distribution in Psychosis and Test of the Immune Subgroup Hypothesis. Schizophr. Bull. 2018, 45, 1120–1133. [Google Scholar] [CrossRef]
- Parksepp, M.; Haring, L.; Kilk, K.; Taalberg, E.; Kangro, R.; Zilmer, M.; Vasar, E. A Marked Low-Grade Inflammation and a Significant Deterioration in Metabolic Status in First-Episode Schizophrenia: A Five-Year Follow-Up Study. Metabolites 2022, 12, 983. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Noto, C.; Kanchanatawan, B.; Anderson, G.; Kubera, M.; Carvalho, A.F.; Maes, M. The Role of Aberrations in the Immune-Inflammatory Response System (IRS) and the Compensatory Immune-Regulatory Reflex System (CIRS) in Different Phenotypes of Schizophrenia: The IRS-CIRS Theory of Schizophrenia. Mol. Neurobiol. 2019, 57, 778–797. [Google Scholar] [CrossRef]
- Kelsven, S.; de la Fuente-Sandoval, C.; Achim, C.L.; Reyes-Madrigal, F.; Mirzakhanian, H.; Domingues, I.; Cadenhead, K. Immuno-inflammatory changes across phases of early psychosis: The impact of antipsychotic medication and stage of illness. Schizophr. Res. 2020, 226, 13–23. [Google Scholar] [CrossRef]
- Föcking, M.; Dicker, P.; Lopez, L.M.; Cannon, M.; Schäfer, M.R.; McGorry, P.D.; Smesny, S.; Cotter, D.R.; Amminger, G.P. Differential expression of the inflammation marker IL12p40 in the at-risk mental state for psychosis: A predictor of transition to psychotic disorder? BMC Psychiatry 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Perkins, D.O.; Jeffries, C.D.; Addington, J.; Bearden, C.E.; Cadenhead, K.S.; Cannon, T.D.; Cornblatt, B.A.; Mathalon, D.H.; McGlashan, T.H.; Seidman, L.J.; et al. Towards a Psychosis Risk Blood Diagnostic for Persons Experiencing High-Risk Symptoms: Preliminary Results From the NAPLS Project. Schizophr. Bull. 2014, 41, 419–428. [Google Scholar] [CrossRef]
- Kappelmann, N.; Khandaker, G.M.; Dal, H.; Stochl, J.; Kosidou, K.; Jones, P.B.; Dalman, C.; Karlsson, H. Systemic inflammation and intelligence in early adulthood and subsequent risk of schizophrenia and other non-affective psychoses: A longitudinal cohort and co-relative study. Psychol. Med. 2018, 49, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Pearson, R.M.; Zammit, S.; Lewis, G.; Jones, P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: A population-based longitudinal study. JAMA Psychiatry 2014, 71, 1121–1128. [Google Scholar] [CrossRef]
- Metcalf, S.A.; Jones, P.B.; Nordstrom, T.; Timonen, M.; Mäki, P.; Miettunen, J.; Jääskeläinen, E.; Järvelin, M.-R.; Stochl, J.; Murray, G.K.; et al. Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: A prospective birth cohort study. Brain Behav. Immun. 2017, 59, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Osimo, E.F.; Baxter, L.; Stochl, J.; Perry, B.I.; Metcalf, S.A.; Kunutsor, S.K.; Laukkanen, J.A.; Wium-Andersen, M.K.; Jones, P.B.; Khandaker, G.M. Longitudinal association between CRP levels and risk of psychosis: A meta-analysis of population-based cohort studies. NPJ Schizophr. 2021, 7, 31. [Google Scholar] [CrossRef]
- Al-Amin, M.M.; Nasir Uddin, M.M.; Mahmud Reza, H. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin. Psychopharmacol. Neurosci. 2013, 11, 144–151. [Google Scholar] [CrossRef]
- Capuzzi, E.; Bartoli, F.; Crocamo, C.; Clerici, M.; Carrà, G. Acute variations of cytokine levels after antipsychotic treatment in drug-naïve subjects with a first-episode psychosis: A meta-analysis. Neurosci. Biobehav. Rev. 2017, 77, 122–128. [Google Scholar] [CrossRef]
- Drzyzga, Ł.; Obuchowicz, E.; Marcinowska, A.; Herman, Z.S. Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav. Immun. 2006, 20, 532–545. [Google Scholar] [CrossRef]
- Tourjman, V.; Kouassi, É.; Koué, M.-È.; Rocchetti, M.; Fortin-Fournier, S.; Fusar-Poli, P.; Potvin, S. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: A meta-analysis. Schizophr. Res. 2013, 151, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Amerio, A.; Magnani, L.; Arduino, G.; Fesce, F.; de Filippis, R.; Parise, A.; Costanza, A.; Nguyen, K.D.; Saverino, D.; De Berardis, D.; et al. Immunomodulatory Effects of Clozapine: More Than Just a Side Effect in Schizophrenia. Curr. Neuropharmacol. 2024, 22, 1233–1247. [Google Scholar] [CrossRef]
- Makola, R.T.; Kgaladi, J.; More, G.K.; van Vuren, P.J.; Paweska, J.T.; Matsebatlela, T.M. Lithium inhibits NF-κB nuclear translocation and modulate inflammation profiles in Rift valley fever virus-infected Raw 264.7 macrophages. Virol. J. 2021, 18, 1–15. [Google Scholar] [CrossRef]
- Troib, A.; Azab, A.N. Effects of psychotropic drugs on Nuclear Factor kappa B. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1198–1208. [Google Scholar]
- Fond, G.; Lançon, C.; Korchia, T.; Auquier, P.; Boyer, L. The Role of Inflammation in the Treatment of Schizophrenia. Front. Psychiatry 2020, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Çakici, N.; Van Beveren, N.J.M.; Judge-Hundal, G.; Koola, M.M.; Sommer, I.E.C. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: A meta-analysis. Psychol. Med. 2019, 49, 2307–2319. [Google Scholar] [CrossRef]
- Szota, A.M.; Radajewska, I.; Ćwiklińska-Jurkowska, M.; Lis, K.; Grudzka, P.; Dróżdż, W. Changes in IL-6, IL-12, IL-5, IL-10 and TGF-β1 Concentration in Patients with Treatment-Resistant Schizophrenia (TRS) Following Electroconvulsive Therapy (ECT)—A Pilot Study. Biomedicines 2024, 12, 2637. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Khalifa, C.; Defour, J.-P.; Latinne, D.; Van Pel, M.-C.; De Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Milas, G.P.; Michopoulos, I. Neutrophil-to-lymphocyte ratio in schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2019, 206, 4–12. [Google Scholar] [CrossRef]
- Mazza, M.G.; Lucchi, S.; Rossetti, A.; Clerici, M. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio and platelet-lymphocyte ratio in non-affective psychosis: A meta-analysis and systematic review. World J. Biol. Psychiatry 2019, 21, 326–338. [Google Scholar] [CrossRef]
- Jackson, A.J.; Miller, B.J. Meta-analysis of total and differential white blood cell counts in schizophrenia. Acta Psychiatr. Scand. 2019, 142, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Engl, E.; Attwell, D. Non-signalling energy use in the brain. J. Physiol. 2015, 593, 3417–3429. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. The oxidative environment and protein damage. Biochim. et Biophys. Acta (BBA)-Proteins Proteom. 2005, 1703, 93–109. [Google Scholar] [CrossRef]
- Smesny, S.; Milleit, B.; Hipler, U.-C.; Milleit, C.; Schäfer, M.R.; Klier, C.M.; Holub, M.; Holzer, I.; E Berger, G.; Otto, M.; et al. Omega-3 fatty acid supplementation changes intracellular phospholipase A2 activity and membrane fatty acid profiles in individuals at ultra-high risk for psychosis. Mol. Psychiatry 2013, 19, 317–324. [Google Scholar] [CrossRef]
- Zamaria, N. Alteration of polyunsaturated fatty acid status and metabolism in health and disease. Reprod. Nutr. Dev. 2004, 44, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kamsler, A.; Segal, M. Hydrogen Peroxide Modulation of Synaptic Plasticity. J. Neurosci. 2003, 23, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.T.; Pao, M.; Holland, S.M.; Klann, E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J. Neurochem. 2005, 94, 299–306. [Google Scholar] [CrossRef]
- Reyes, R.C.; Brennan, A.M.; Shen, Y.; Baldwin, Y.; Swanson, R.A. Activation of Neuronal NMDA Receptors Induces Superoxide-Mediated Oxidative Stress in Neighboring Neurons and Astrocytes. J. Neurosci. 2012, 32, 12973–12978. [Google Scholar] [CrossRef] [PubMed]
- Barron, H.; Hafizi, S.; Andreazza, A.C.; Mizrahi, R. Neuroinflammation and Oxidative Stress in Psychosis and Psychosis Risk. Int. J. Mol. Sci. 2017, 18, 651. [Google Scholar] [CrossRef] [PubMed]
- Basma, A.N.; Morris, E.J.; Nicklas, W.J.; Geller, H.M. l-DOPA Cytotoxicity to PC12 Cells in Culture Is via Its Autoxidation. J. Neurochem. 1995, 64, 825–832. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta Med. Okayama 2008, 62, 141–150. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Dmitrieva, E.M.; Parshukova, D.A.; Kazantseva, D.V.; Vasilieva, A.R.; Smirnova, L.P. Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxidative Med. Cell. Longev. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Homayoun, H.; Moghaddam, B. NMDA Receptor Hypofunction Produces Opposite Effects on Prefrontal Cortex Interneurons and Pyramidal Neurons. J. Neurosci. 2007, 27, 11496–11500. [Google Scholar] [CrossRef]
- Sorce, S.; Schiavone, S.; Tucci, P.; Colaianna, M.; Jaquet, V.; Cuomo, V.; Dubois-Dauphin, M.; Trabace, L.; Krause, K.-H. The NADPH Oxidase NOX2 Controls Glutamate Release: A Novel Mechanism Involved in Psychosis-Like Ketamine Responses. J. Neurosci. 2010, 30, 11317–11325. [Google Scholar] [CrossRef]
- Do, K.Q.; Cabungcal, J.H.; Frank, A.; Steullet, P.; Cuenod, M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 2009, 19, 220–230. [Google Scholar] [CrossRef]
- Bryll, A.; Skrzypek, J.; Krzyściak, W.; Szelągowska, M.; Śmierciak, N.; Kozicz, T.; Popiela, T. Oxidative-Antioxidant Imbalance and Impaired Glucose Metabolism in Schizophrenia. Biomolecules 2020, 10, 384. [Google Scholar] [CrossRef]
- Chien, Y.-L.; Hwu, H.-G.; Hwang, T.-J.; Hsieh, M.H.; Liu, C.-C.; Lin-Shiau, S.-Y.; Liu, C.-M. Clinical implications of oxidative stress in schizophrenia: Acute relapse and chronic stable phase. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 99, 109868. [Google Scholar] [CrossRef]
- Yao, J.K.; Keshavan, M.S. Antioxidants, Redox Signaling, and Pathophysiology in Schizophrenia: An Integrative View. Antioxidants Redox Signal. 2011, 15, 2011–2035. [Google Scholar] [CrossRef]
- Mahadik, S.P.; Mukherjee, S.; Scheffer, R.; E Correnti, E.; Mahadik, J.S. Elevated Plasma Lipid Peroxides at the Onset of Nonaffective Psychosis. Biol. Psychiatry 1998, 43, 674–679. [Google Scholar] [CrossRef]
- Ruiz-Litago, F.; Seco, J.; Echevarría, E.; Martínez-Cengotitabengoa, M.; Gil, J.; Irazusta, J.; González-Pinto, A.M. Adaptive response in the antioxidant defence system in the course and outcome in first-episode schizophrenia patients: A 12-months follow-up study. Psychiatry Res. 2012, 200, 218–222. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Solmi, M.; Sanches, M.; Machado, M.O.; Stubbs, B.; Ajnakina, O.; Sherman, C.; Sun, Y.R.; Liu, C.S.; Brunoni, A.R.; et al. Evidence-based umbrella review of 162 peripheral biomarkers for major mental disorders. Transl. Psychiatry 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Steullet, P.; Cabungcal, J.H.; Monin, A.; Dwir, D.; O’Donnell, P.; Cuenod, M.; Do, K.Q. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A "central hub" in schizophrenia pathophysiology? Schizophr. Res. 2016, 176, 41–51. [Google Scholar] [CrossRef]
- Koga, M.; Serritella, A.V.; Sawa, A.; Sedlak, T.W. Implications for reactive oxygen species in schizophrenia pathogenesis. Schizophr. Res. 2016, 176, 52–71. [Google Scholar] [CrossRef]
- Yao, J.K.; Leonard, S.; Reddy, R. Altered Glutathione Redox State in Schizophrenia. Dis. Markers 2006, 22, 83–93. [Google Scholar] [CrossRef]
- Li, X.F.; Zheng, Y.L.; Xiu, M.H.; Chen, D.C.; Kosten, T.R.; Zhang, X.Y. Reduced plasma total antioxidant status in first-episode drug-naive patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1064–1067. [Google Scholar] [CrossRef]
- Bai, Z.-L.; Li, X.-S.; Chen, G.-Y.; Du, Y.; Wei, Z.-X.; Chen, X.; Zheng, G.-E.; Deng, W.; Cheng, Y. Serum Oxidative Stress Marker Levels in Unmedicated and Medicated Patients with Schizophrenia. J. Mol. Neurosci. 2018, 66, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Gawryluk, J.W.; Wang, J.-F.; Andreazza, A.C.; Shao, L.; Young, L.T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 2011, 14, 123–130. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Olas, B.; Głowacki, R.; Bald, E. Oxidative/Nitrative Modifications of Plasma Proteins and Thiols from Patients with Schizophrenia. Neuropsychobiology 2009, 59, 1–7. [Google Scholar] [CrossRef]
- Altuntas, I.; Aksoy, H.; Coskun, I.; Çayköylü, A.; Akçay, F. Erythrocyte Superoxide Dismutase and Glutathione Peroxidase Activities, and Malondialdehyde and Reduced Glutathione Levels in Schizophrenic Patients. cclm 2000, 38, 1277–1281. [Google Scholar] [CrossRef]
- Do, K.Q.; Trabesinger, A.H.; Kirsten-Krüger, M.; Lauer, C.J.; Dydak, U.; Hell, D.; Holsboer, F.; Boesiger, P.; Cuénod, M. Schizophrenia: Glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur. J. Neurosci. 2000, 12, 3721–3728. [Google Scholar] [CrossRef]
- Kumar, J.; Liddle, E.B.; Fernandes, C.C.; Palaniyappan, L.; Hall, E.L.; Robson, S.E.; Simmonite, M.; Fiesal, J.; Katshu, M.Z.; Qureshi, A.; et al. Glutathione and glutamate in schizophrenia: A 7T MRS study. Mol. Psychiatry 2018, 25, 873–882. [Google Scholar] [CrossRef]
- Micó, J.A.; Rojas-Corrales, M.O.; Gibert-Rahola, J.; Parellada, M.; Moreno, D.; Fraguas, D.; Graell, M.; Gil, J.; Irazusta, J.; Castro-Fornieles, J.; et al. Reduced antioxidant defense in early onset first-episode psychosis: A case-control study. BMC Psychiatry 2011, 11, 26. [Google Scholar] [CrossRef]
- Raffa, M.; Atig, F.; Mhalla, A.; Kerkeni, A.; Mechri, A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry 2011, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.; Keshavan, M.; Yao, J.K. Reduced plasma antioxidants in first-episode patients with schizophrenia. Schizophr. Res. 2003, 62, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.M.; Pradhan, S.; Coughlin, J.M.; Trivedi, A.; DuBois, S.L.; Crawford, J.L.; Sedlak, T.W.; Nucifora, F.C.; Nestadt, G.; Nucifora, L.G.; et al. Assessing Brain Metabolism With 7-T Proton Magnetic Resonance Spectroscopy in Patients With First-Episode Psychosis. JAMA Psychiatry 2019, 76, 314–323. [Google Scholar] [CrossRef]
- Savio, L.E.B.; Leite-Aguiar, R.; Alves, V.S.; Coutinho-Silva, R.; Wyse, A.T. Purinergic signaling in the modulation of redox biology. Redox Biol. 2021, 47, 102137. [Google Scholar] [CrossRef]
- Jiang, J.; Peng, C.; Sun, L.; Li, J.; Qing, Y.; Hu, X.; Yang, X.; Li, Y.; Xu, C.; Zhang, J.; et al. Leukocyte Proteomic Profiling in First-Episode Schizophrenia Patients: Does Oxidative Stress Play Central Roles in the Pathophysiology Network of Schizophrenia? Antioxidants Redox Signal. 2019, 31, 579–588. [Google Scholar] [CrossRef]
- Ben Othmen, L.; Mechri, A.; Fendri, C.; Bost, M.; Chazot, G.; Gaha, L.; Kerkeni, A. Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 155–159. [Google Scholar] [CrossRef]
- Zeni-Graiff, M.; Rios, A.C.; Maurya, P.K.; Rizzo, L.B.; Sethi, S.; Yamagata, A.S.; Mansur, R.B.; Pan, P.M.; Asevedo, E.; Cunha, G.R.; et al. Peripheral levels of superoxide dismutase and glutathione peroxidase in youths in ultra-high risk for psychosis: A pilot study. CNS Spectrums 2017, 24, 333–337. [Google Scholar] [CrossRef]
- Lavoie, S.; Berger, M.; Schlögelhofer, M.; Schäfer, M.R.; Rice, S.; Kim, S.-W.; Hesse, J.; McGorry, P.D.; Smesny, S.; Amminger, G.P. Erythrocyte glutathione levels as long-term predictor of transition to psychosis. Transl. Psychiatry 2017, 7, e1064. [Google Scholar] [CrossRef] [PubMed]
- Mössner, R.; Schuhmacher, A.; Wagner, M.; Quednow, B.B.; Frommann, I.; Kühn, K.-U.; Schwab, S.G.; Rietschel, M.; Falkai, P.; Wölwer, W.; et al. DAOA/G72 predicts the progression of prodromal syndromes to first episode psychosis. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 260, 209–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Magistretti, P.J.; Pellerin, L.; Martin, J.L. Brain energy metabolism: An integrated cellular perspective. In Psychopharmacology: The Fourth Generation of Progress; Bloom, F.E., Kupfer, D.J., Eds.; Raven Press: New York, NY, USA, 1995; pp. 657–670. [Google Scholar][Green Version]
- Matafome, P.; Rodrigues, T.; Sena, C.; Seiça, R. Methylglyoxal in Metabolic Disorders: Facts, Myths, and Promises. Med. Res. Rev. 2017, 37, 368–403. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; de la Monte, S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease – is this type 3 diabetes? J. Alzheimer’s Dis. 2005, 7, 63–80. [Google Scholar] [CrossRef]
- Hardie, D.G.; Salt, I.P.; Hawley, S.A.; Davies, S.P. AMP-activated protein kinase: An ultrasensitive system for monitoring cellular energy charge. Biochem. J. 1999, 338, 717–722. [Google Scholar] [CrossRef]
- Hawley, S.A.; Selbert, M.A.; Goldstein, E.G.; Edelman, A.M.; Carling, D.; Hardie, D.G. 5′-AMP Activates the AMP-activated Protein Kinase Cascade, and Ca2+/Calmodulin Activates the Calmodulin-dependent Protein Kinase I Cascade, via Three Independent Mechanisms. J. Biol. Chem. 1995, 270, 27186–27191. [Google Scholar] [CrossRef]
- Escartin, C.; Pierre, K.; Colin, A.; Brouillet, E.; Delzescaux, T.; Guillermier, M.; Dhenain, M.; Déglon, N.; Hantraye, P.; Pellerin, L.; et al. Activation of Astrocytes by CNTF Induces Metabolic Plasticity and Increases Resistance to Metabolic Insults. J. Neurosci. 2007, 27, 7094–7104. [Google Scholar] [CrossRef]
- Steiner, J.; Bernstein, H.-G.; Schiltz, K.; Müller, U.J.; Westphal, S.; Drexhage, H.A.; Bogerts, B. Immune system and glucose metabolism interaction in schizophrenia: A chicken–egg dilemma. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 287–294. [Google Scholar] [CrossRef]
- Subramanian, S.; Chait, A. Hypertriglyceridemia secondary to obesity and diabetes. Biochim. et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 819–825. [Google Scholar] [CrossRef]
- Khan, A.H.; Pessin, J.E. Insulin regulation of glucose uptake: A complex interplay of intracellular signalling pathways. Diabetologia 2002, 45, 1475–1483. [Google Scholar] [CrossRef]
- Tanti, J.-F.; Jager, J. Cellular mechanisms of insulin resistance: Role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr. Opin. Pharmacol. 2009, 9, 753–762. [Google Scholar] [CrossRef]
- Zick, Y. Ser/Thr Phosphorylation of IRS Proteins: A Molecular Basis for Insulin Resistance. Sci. STKE 2005, 2005, pe4. [Google Scholar] [CrossRef] [PubMed]
- Regazzetti, C.; Peraldi, P.; GrémEaux, T.; Najem-Lendom, R.; Ben-Sahra, I.; Cormont, M.; Bost, F.; Le Marchand-Brustel, Y.; Tanti, J.-F.; Giorgetti-Peraldi, S. Hypoxia Decreases Insulin Signaling Pathways in Adipocytes. Diabetes 2009, 58, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed]
- Dahoun, T.; Trossbach, S.V.; Brandon, N.J.; Korth, C.; Howes, O.D. The impact of Disrupted-in-Schizophrenia 1 (DISC1) on the dopaminergic system: A systematic review. Transl. Psychiatry 2017, 7, e1015. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, A.; Nowosielska, A.; Przewozniak, N.; Aryee, K.E.; DiIorio, P.; Blodgett, D.; Yang, C.; Campbell-Thompson, M.; Atkinson, M.; Shultz, L.; et al. Beyond the brain: Disrupted in schizophrenia 1 regulates pancreatic β-cell function via glycogen synthase kinase-3β. FASEB J. 2016, 30, 983–993. [Google Scholar] [CrossRef]
- Kooy, F.H. Hyperglycemia in mental disorders. Brain 1919, 42, 214–289. [Google Scholar] [CrossRef]
- Mensvoort, F.A.P.M.V.; Blok, G.; Blom, J.D. Insulin shock treatment in The Hague from 1937 to the end of the 1950s. Tijdschr. Psychiatr. 2012, 54, 869–877. [Google Scholar]
- Appel, K.E.; Farr, C.B. The Blood Sugar Reaction to Insulin in Psychoses. Arch. Neurol. Psychiatry 1929, 21, 145–148. [Google Scholar] [CrossRef]
- Schoepf, D.; Potluri, R.; Uppal, H.; Natalwala, A.; Narendran, P.; Heun, R. Type-2 diabetes mellitus in schizophrenia: Increased prevalence and major risk factor of excess mortality in a naturalistic 7-year follow-up. Eur. Psychiatry 2012, 27, 33–42. [Google Scholar] [CrossRef]
- Laursen, T.M.; Nordentoft, M.; Mortensen, P.B. Excess Early Mortality in Schizophrenia. Annu. Rev. Clin. Psychol. 2014, 10, 425–448. [Google Scholar] [CrossRef]
- Dasgupta, A.; Singh, O.P.; Rout, J.K.; Saha, T.; Mandal, S. Insulin resistance and metabolic profile in antipsychotic naïve schizophrenia patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 1202–1207. [Google Scholar] [CrossRef]
- Fernandez-Egea, E.; Bernardo, M.; Donner, T.; Conget, I.; Parellada, E.; Justicia, A.; Esmatjes, E.; Garcia-Rizo, C.; Kirkpatrick, B. Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. Br. J. Psychiatry 2009, 194, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Tsang, T.M.; Huang, J.T.-J.; Leweke, F.M.; Koethe, D.; Gerth, C.W.; Nolden, B.M.; Gross, S.; Schreiber, D.; Nicholson, J.K.; et al. Metabolic Profiling of CSF: Evidence That Early Intervention May Impact on Disease Progression and Outcome in Schizophrenia. PLOS Med. 2006, 3, e327. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Fernandez-Egea, E.; Garcia-Rizo, C.; Bernardo, M. Differences in glucose tolerance between deficit and nondeficit schizophrenia. Schizophr. Res. 2009, 107, 122–127. [Google Scholar] [CrossRef][Green Version]
- Ryan, M.C.; Collins, P.; Thakore, J.H. Impaired Fasting Glucose Tolerance in First-Episode, Drug-Naive Patients With Schizophrenia. Am. J. Psychiatry 2003, 160, 284–289. [Google Scholar] [CrossRef]
- van Nimwegen, L.J.M.; Storosum, J.G.; Blumer, R.M.E.; Allick, G.; Venema, H.W.; de Haan, L.; Becker, H.; van Amelsvoort, T.; Ackermans, M.T.; Fliers, E.; et al. Hepatic Insulin Resistance in Antipsychotic Naive Schizophrenic Patients: Stable Isotope Studies of Glucose Metabolism. J. Clin. Endocrinol. Metab. 2008, 93, 572–577. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Z.; Wu, R.; Zhong, Z.; Wei, Q.; Wang, H.; Diao, F.; Wang, J.; Zheng, L.; Zhao, J.; et al. The comparison of glycometabolism parameters and lipid profiles between drug-naïve, first-episode schizophrenia patients and healthy controls. Schizophr. Res. 2013, 150, 157–162. [Google Scholar] [CrossRef]
- Arranz, B.; Rosel, P.; Ramírez, N.; Dueñas, R.; Fernández, P.; Sanchez, J.M.; Navarro, M.A.; San, L. Insulin Resistance and Increased Leptin Concentrations in Noncompliant Schizophrenia Patients but Not in Antipsychotic-Naive First-Episode Schizophrenia Patients. J. Clin. Psychiatry 2004, 65, 1335–1342. [Google Scholar] [CrossRef]
- Sengupta, S.; Parrillaescobar, M.; Klink, R.; Fathalli, F.; Yingkinng; Stip, E.; Baptista, T.; Malla, A.; Joober, R. Are metabolic indices different between drug-naïve first-episode psychosis patients and healthy controls? Schizophr. Res. 2008, 102, 329–336. [Google Scholar] [CrossRef]
- Perry, B.I.; McIntosh, G.; Weich, S.; Singh, S.; Rees, K. The association between first-episode psychosis and abnormal glycaemic control: Systematic review and meta-analysis. Lancet Psychiatry 2016, 3, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, T.; Beck, K.; Gobjila, C.; Donocik, J.G.; Jauhar, S.; Howes, O.D. Impaired Glucose Homeostasis in First-Episode Schizophrenia: A Systematic Review and Meta-analysis. JAMA Psychiatry 2017, 74, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.-G.; Ernst, T.; Lendeckel, U.; Bukowska, A.; Ansorge, S.; Stauch, R.; Have, S.T.; Steiner, J.; Dobrowolny, H.; Bogerts, B. Reduced neuronal expression of insulin-degrading enzyme in the dorsolateral prefrontal cortex of patients with haloperidol-treated, chronic schizophrenia. J. Psychiatr. Res. 2009, 43, 1095–1105. [Google Scholar] [CrossRef]
- Emamian, E.S.; Hall, D.; Birnbaum, M.J.; Karayiorgou, M.; Gogos, J.A. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat. Genet. 2004, 36, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ksiezak-Reding, H.; Riggio, S.; Haroutunian, V.; Pasinetti, G.M. Insulin receptor deficits in schizophrenia and in cellular and animal models of insulin receptor dysfunction. Schizophr. Res. 2006, 84, 1–14. [Google Scholar] [CrossRef]
- Buchsbaum, M.S.; Buchsbaum, B.R.; Hazlett, E.A.; Haznedar, M.M.; Newmark, R.; Tang, C.Y.; Hof, P.R. Relative glucose metabolic rate higher in white matter in patients with schizophrenia. Am. J. Psychiatry 2007, 164, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Mitelman, S.A.; Byne, W.; Kemether, E.M.; Hazlett, E.A.; Buchsbaum, M.S. Metabolic Disconnection Between the Mediodorsal Nucleus of the Thalamus and Cortical Brodmann’s Areas of the Left Hemisphere in Schizophrenia. Am. J. Psychiatry 2005, 162, 1733–1735. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, C.A.; Thaker, G.K.; Buchanan, R.; Kirkpatrick, B.; Alphs, L.D.; Chase, T.N.; Carpenter, W.T. Limbic System Abnormalities Identified in Schizophrenia Using Positron Emission Tomography With Fluorodeoxyglucose and Neocortical Alterations With Deficit Syndrome. Arch. Gen. Psychiatry 1992, 49, 522–530. [Google Scholar] [CrossRef]
- Anthony, K.; Reed, L.J.; Dunn, J.T.; Bingham, E.; Hopkins, D.; Marsden, P.K.; Amiel, S.A. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: The cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes 2006, 55, 2986–2992. [Google Scholar] [CrossRef]
- Bingham, E.M.; Hopkins, D.; Smith, D.; Pernet, A.; Hallett, W.; Reed, L.; Marsden, P.K.; Amiel, S.A. The role of insulin in human brain glucose metabolism: An 18fluorodeoxyglucose positron emission tomography study. Diabetes 2002, 51, 3384–3390. [Google Scholar] [CrossRef]
- Wijtenburg, S.A.; Kapogiannis, D.; Korenic, S.A.; Mullins, R.J.; Tran, J.; Gaston, F.E.; Chen, S.; Mustapic, M.; Hong, L.E.; Rowland, L.M. Brain insulin resistance and altered brain glucose are related to memory impairments in schizophrenia. Schizophr. Res. 2019, 208, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Chouinard, V.-A.; Henderson, D.C.; Man, C.D.; Valeri, L.; Gray, B.E.; Ryan, K.P.; Cypess, A.M.; Cobelli, C.; Cohen, B.M.; Öngür, D. Impaired insulin signaling in unaffected siblings and patients with first-episode psychosis. Mol. Psychiatry 2018, 24, 1513–1522. [Google Scholar] [CrossRef]
- Spelman, L.M.; Walsh, P.I.; Sharifi, N.; Collins, P.; Thakore, J.H. Impaired glucose tolerance in firstepisode drug-naive patients with schizophrenia. Diabet. Med. 2007, 24, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Prestwood, T.R.; Asgariroozbehani, R.; Wu, S.; Agarwal, S.M.; Logan, R.W.; Ballon, J.S.; Hahn, M.K.; Freyberg, Z. Roles of inflammation in intrinsic pathophysiology and antipsychotic drug-induced metabolic disturbances of schizophrenia. Behav. Brain Res. 2021, 402, 113101. [Google Scholar] [CrossRef]
- Perry, B.I.; Upthegrove, R.; Thompson, A.; Marwaha, S.; Zammit, S.; Singh, S.P.; Khandaker, G. Dysglycaemia, Inflammation and Psychosis: Findings From the UK ALSPAC Birth Cohort. Schizophr. Bull. 2018, 45, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Vancampfort, D.; Wampers, M.; Mitchell, A.J.; Correll, C.U.; De Herdt, A.; Probst, M.; De Hert, M. A meta-analysis of cardio-metabolic abnormalities in drug naïve, first-episode and multi-episode patients with schizophrenia versus general population controls. World Psychiatry 2013, 12, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Coentre, R.; Levy, P.; Góis, C.; Figueira, M.L. Metabolic syndrome following a first episode of psychosis: Results of a 1-year longitudinal study conducted in metropolitan Lisbon, Portugal. J. Int. Med. Res. 2022, 50. [Google Scholar] [CrossRef]
- Doknic, M.; Maric, N.P.; Britvic, D.; Pekic, S.; Damjanovic, A.; Miljic, D.; Stojanovic, M.; Radojicic, Z.; Gasic, M.J.; Popovic, V. Bone Remodeling, Bone Mass and Weight Gain in Patients with Stabilized Schizophrenia in Real-Life Conditions Treated with Long-Acting Injectable Risperidone. Neuroendocrinology 2011, 94, 246–254. [Google Scholar] [CrossRef]
- Popovic, V.; Doknic, M.; Maric, N.; Pekic, S.; Damjanovic, A.; Miljic, D.; Popovic, S.; Miljic, N.; Djurovic, M.; Jasovic-Gasic, M.; et al. Changes in Neuroendocrine and Metabolic Hormones Induced by Atypical Antipsychotics in Normal-Weight Patients with Schizophrenia. Neuroendocrinology 2007, 85, 249–256. [Google Scholar] [CrossRef]
- Pillinger, T.; A McCutcheon, R.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Chen, J.-D.; Zheng, X.-Y. Potential mechanisms of atypical antipsychotic-induced hypertriglyceridemia. Psychopharmacology 2013, 229, 1–7. [Google Scholar] [CrossRef]
- Misiak, B.; Stańczykiewicz, B.; Łaczmański, Ł.; Frydecka, D. Lipid profile disturbances in antipsychotic-naive patients with first-episode non-affective psychosis: A systematic review and meta-analysis. Schizophr. Res. 2017, 190, 18–27. [Google Scholar] [CrossRef]
- Enez Darcin, A.; Yalcin Cavus, S.; Dilbaz, N.; Kaya, H.; Dogan, E. Metabolic syndrome in drug-naïve and drug-free patients with schizophrenia and in their siblings. Schizophr. Res. 2015, 166, 201–206. [Google Scholar] [CrossRef]
- Kriisa, K. Profile of Acylcarnitines, Inflammation and Oxidative Stress in First-Episode Psychosis Before and After Antipsychotic Treatment. Ph.D. Thesis, Estonia-University of Tartu, Tartu, Estonia, 2018. [Google Scholar]
- Su, Q.; Bi, F.; Yang, S.; Yan, H.; Sun, X.; Wang, J.; Qiu, Y.; Li, M.; Li, S.; Li, J. Identification of Plasma Biomarkers in Drug-Naïve Schizophrenia Using Targeted Metabolomics. Psychiatry Investig. 2023, 20, 818–825. [Google Scholar] [CrossRef]
- Orešič, M.; Tang, J.; Seppänen-Laakso, T.; Mattila, I.; E Saarni, S.; I Saarni, S.; Lönnqvist, J.; Sysi-Aho, M.; Hyötyläinen, T.; Perälä, J.; et al. Metabolome in schizophrenia and other psychotic disorders: A general population-based study. Genome Med. 2011, 3, 1–14. [Google Scholar] [CrossRef]
- Khan, M.M.; Evans, D.R.; Gunna, V.; Scheffer, R.E.; Parikh, V.V.; Mahadik, S.P. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr. Res. 2002, 58, 1–10. [Google Scholar] [CrossRef]
- Suvitaival, T.; Mantere, O.; Kieseppä, T.; Mattila, I.; Pöhö, P.; Hyötyläinen, T.; Suvisaari, J.; Orešič, M. Serum metabolite profile associates with the development of metabolic co-morbidities in first-episode psychosis. Transl. Psychiatry 2016, 6, e951. [Google Scholar] [CrossRef]
- Dickens, A.M.; Sen, P.; Kempton, M.J.; Barrantes-Vidal, N.; Iyegbe, C.; Nordentoft, M.; Pollak, T.; Riecher-Rössler, A.; Ruhrmann, S.; Sachs, G.; et al. Dysregulated Lipid Metabolism Precedes Onset of Psychosis. Biol. Psychiatry 2021, 89, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. Stress, chronic inflammation, and emotional and physical well-being: Concurrent effects and chronic sequelae. J. Allergy Clin. Immunol. 2000, 106, S275–S291. [Google Scholar] [CrossRef]
- Selye, H. Stress and the General Adaptation Syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef]
- Dubrovsky, B.O. Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 169–192. [Google Scholar] [CrossRef]
- Bellavance, M.; Rivest, S. The HPA–immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 2014, 5, 136. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: A reconceptualization. Transl. Psychiatry 2017, 7, e1024. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.F.; Diforio, D. Schizophrenia: A neural diathesis-stress model. Psychol. Rev. 1997, 104, 667–685. [Google Scholar] [CrossRef]
- Shao, X.; Li, J.; Wang, S.; Chen, G.; Xu, J.; Ji, X.; Li, L.; Lu, W.; Zhou, T. Exogenous dopamine induces dehydroepiandrosterone sulfotransferase (rSULT2A1) in rat liver and changes the pharmacokinetic profile of moxifloxacin in rats. Drug Metab. Pharmacokinet. 2015, 30, 97–104. [Google Scholar] [CrossRef]
- Pruessner, M.; Cullen, A.E.; Aas, M.; Walker, E.F. The neural diathesis-stress model of schizophrenia revisited: An update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci. Biobehav. Rev. 2017, 73, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Karanikas, E.; Antoniadis, D.; Garyfallos, G.D. The role of cortisol in first episode of psychosis: A systematic review. Curr. Psychiatry Rep. 2014, 16, 503. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.J.; Dinan, T.G. Review: A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: Implications for mortality. J. Psychopharmacol. 2010, 24, 91–118. [Google Scholar] [CrossRef]
- Girshkin, L.; Matheson, S.L.; Shepherd, A.M.; Green, M.J. Morning cortisol levels in schizophrenia and bipolar disorder: A meta-analysis. Psychoneuroendocrinology 2014, 49, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Ciufolini, S.; Dazzan, P.; Kempton, M.J.; Pariante, C.; Mondelli, V. HPA axis response to social stress is attenuated in schizophrenia but normal in depression: Evidence from a meta-analysis of existing studies. Neurosci. Biobehav. Rev. 2014, 47, 359–368. [Google Scholar] [CrossRef]
- Zorn, J.V.; Schür, R.R.; Boks, M.P.; Kahn, R.S.; Joëls, M.; Vinkers, C.H. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 77, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Kraeuter, A.K.; Romanik, D.; Malouf, P.; Amminger, G.P.; Sarnyai, Z. Cortisol awakening response in patients with psychosis: Systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 68, 157–166. [Google Scholar] [CrossRef]
- Borges, S.; Gayer-Anderson, C.; Mondelli, V. A systematic review of the activity of the hypothalamic–pituitary–adrenal axis in first episode psychosis. Psychoneuroendocrinology 2013, 38, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, D.B.; Miller, B.J. Meta-analysis of blood cortisol levels in individuals with first-episode psychosis. Psychoneuroendocrinology 2019, 104, 269–275. [Google Scholar] [CrossRef]
- Misiak, B.; Pruessner, M.; Samochowiec, J.; Wiśniewski, M.; Reginia, A.; Stańczykiewicz, B. A meta-analysis of blood and salivary cortisol levels in first-episode psychosis and high-risk individuals. Front. Neuroendocr. 2021, 62, 100930. [Google Scholar] [CrossRef]
- Andrade, E.H.; Rizzo, L.B.; Noto, C.; Ota, V.K.; Gadelha, A.; Daruy-Filho, L.; Tasso, B.d.e.C.; Mansur, R.B.; Cordeiro, Q.; Belangero, S.I.; et al. Hair cortisol in drug-naïve first-episode individuals with psychosis. Braz. J. Psychiatry 2016, 38, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Heuvel, L.L.v.D.; Smit, A.M.; Stalder, T.; Kirschbaum, C.; Seedat, S.; Emsley, R. Hair cortisol levels in schizophrenia and metabolic syndrome. Early Interv. Psychiatry 2022, 16, 902–911. [Google Scholar] [CrossRef]
- Ryan, M.C.; Flanagan, S.; Kinsella, U.; Keeling, F.; Thakore, J.H. The effects of atypical antipsychotics on visceral fat distribution in first episode, drug-naive patients with schizophrenia. Life Sci. 2004, 74, 1999–2008. [Google Scholar] [CrossRef]
- Venkatasubramanian, G.; Chittiprol, S.; Neelakantachar, N.; Shetty, T.; Gangadhar, B.N. Effect of antipsychotic treatment on Insulin-like Growth Factor-1 and cortisol in schizophrenia: A longitudinal study. Schizophr. Res. 2010, 119, 131–137. [Google Scholar] [CrossRef]
- Cesková, E.; Kaspárek, T.; Zourková, A.; Prikryl, R. Dexamethasone suppression test in first-episode schizophrenia. Neuro Endocrinol. Lett. 2006, 27, 433–437. [Google Scholar]
- Chaumette, B.; Kebir, O.; Mam-Lam-Fook, C.; Morvan, Y.; Bourgin, J.; Godsil, B.P.; Plaze, M.; Gaillard, R.; Jay, T.M.; Krebs, M.-O. Salivary cortisol in early psychosis: New findings and meta-analysis. Psychoneuroendocrinology 2016, 63, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Collip, D.; Nicolson, N.A.; Lardinois, M.; Lataster, T.; van Os, J.; Myin-Germeys, I. Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychol. Med. 2011, 41, 2305–2315. [Google Scholar] [CrossRef]
- Walker, E.F.; Trotman, H.D.; Pearce, B.D.; Addington, J.; Cadenhead, K.S.; Cornblatt, B.A.; Heinssen, R.; Mathalon, D.H.; Perkins, D.O.; Seidman, L.J.; et al. Cortisol Levels and Risk for Psychosis: Initial Findings from the North American Prodrome Longitudinal Study. Biol. Psychiatry 2013, 74, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Labad, J. The role of cortisol and prolactin in the pathogenesis and clinical expression of psychotic disorders. Psychoneuroendocrinology 2019, 102, 24–36. [Google Scholar] [CrossRef]
- Handa, R.J.; Weiser, M.J. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front. Neuroendocrinol. 2014, 35, 197–220. [Google Scholar] [CrossRef]
- Xu, J.-J.; Wang, S.-Y.; Chen, Y.; Chen, G.-P.; Li, Z.-Q.; Shao, X.-Y.; Li, L.; Lu, W.; Zhou, T.-Y. Dopamine D1 receptor activation induces dehydroepiandrosterone sulfotransferase (SULT2A1) in HepG2 cells. Acta Pharmacol. Sin. 2014, 35, 889–898. [Google Scholar] [CrossRef][Green Version]
- Misiak, B.; Frydecka, D.; Loska, O.; Moustafa, A.A.; Samochowiec, J.; Kasznia, J.; Stańczykiewicz, B. Testosterone, DHEA and DHEA-S in patients with schizophrenia: A systematic review and meta-analysis. Psychoneuroendocrinology 2018, 89, 92–102. [Google Scholar] [CrossRef]
- Heringa, S.M.; Begemann, M.J.; Goverde, A.J.; Sommer, I.E. Sex hormones and oxytocin augmentation strategies in schizophrenia: A quantitative review. Schizophr. Res. 2015, 168, 603–613. [Google Scholar] [CrossRef]
- Fernandez-Egea, E.; Garcia-Rizo, C.; Miller, B.; Justicia, A.; Bernardo, M.; Kirkpatrick, B. Testosterone in Newly Diagnosed, Antipsychotic-Naïve Men with Nonaffective Psychosis: A Test of the Accelerated Aging Hypothesis. Schizophr. Res. 2010, 117, 294. [Google Scholar] [CrossRef]
- Huber, T.J.; Tettenborn, C.; Leifke, E.; Emrich, H.M. Sex hormones in psychotic men. Psychoneuroendocrinology 2005, 30, 111–114. [Google Scholar] [CrossRef]
- Taherianfard, M.; Shariaty, M. Evaluation of serum steroid hormones in schizophrenic patients. Indian J. Med. Sci. 2004, 58, 3–9. [Google Scholar] [PubMed]
- Petrikis, P.; Tigas, S.; Tzallas, A.T.; Karampas, A.; Papadopoulos, I.; Skapinakis, P. Sex hormone levels in drug-naïve, first-episode patients with psychosis. Int. J. Psychiatry Clin. Pract. 2020, 24, 20–24. [Google Scholar] [CrossRef]
- Hayes, L.N.; Severance, E.G.; Leek, J.T.; Gressitt, K.L.; Rohleder, C.; Coughlin, J.M.; Leweke, F.M.; Yolken, R.H.; Sawa, A. Inflammatory Molecular Signature Associated With Infectious Agents in Psychosis. Schizophr. Bull. 2014, 40, 963–972. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protective and Damaging Effects of Stress Mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef]
- Billack, B. Macrophage activation: Role of toll-like receptors, nitric oxide, and nuclear factor kappa B. Am. J. Pharm. Educ. 2006, 70, 102. [Google Scholar] [CrossRef]
- Karanikas, E.; Daskalakis, N.P.; Agorastos, A. Oxidative Dysregulation in Early Life Stress and Posttraumatic Stress Disorder: A Comprehensive Review. Brain Sci. 2021, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Wu, F.; Tyml, K.; Wilson, J.X. iNOS expression requires NADPH oxidase-dependent redox signaling in microvascular endothelial cells. J. Cell. Physiol. 2008, 217, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Haslund-Vinding, J.; McBean, G.; Jaquet, V.; Vilhardt, F. NADPH oxidases in oxidant production by microglia: Activating receptors, pharmacology and association with disease. Br. J. Pharmacol. 2017, 174, 1733–1749. [Google Scholar] [CrossRef] [PubMed]
- Pawate, S.; Shen, Q.; Fan, F.; Bhat, N.R. Redox regulation of glial inflammatory response to lipopolysaccharide and interferon? J. Neurosci. Res. 2004, 77, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Vilhardt, F.; Haslund-Vinding, J.; Jaquet, V.; McBean, G. Microglia antioxidant systems and redox signalling. Br. J. Pharmacol. 2016, 174, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Cuenod, M.; Steullet, P.; Cabungcal, J.-H.; Dwir, D.; Khadimallah, I.; Klauser, P.; Conus, P.; Do, K.Q. Caught in vicious circles: A perspective on dynamic feed-forward loops driving oxidative stress in schizophrenia. Mol. Psychiatry 2021, 27, 1886–1897. [Google Scholar] [CrossRef]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80 Pt C, 309–321. [Google Scholar] [CrossRef]
- Tan, K.S.; Nackley, A.G.; Satterfield, K.; Maixner, W.; Diatchenko, L.; Flood, P.M. β2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-κB-independent mechanisms. Cell. Signal. 2007, 19, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Wolf, J.; Andrassy, M.; Rohleder, N.; Humpert, P.M.; Petrov, D.; Ferstl, R.; von Eynatten, M.; Wendt, T.; Rudofsky, G.; et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. USA 2003, 100, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pr. 2014, 105, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-López, G.; Giovanoli, S.; Langhans, W.; Meyer, U. Priming of Metabolic Dysfunctions by Prenatal Immune Activation in Mice: Relevance to Schizophrenia. Schizophr. Bull. 2011, 39, 319–329. [Google Scholar] [CrossRef] [PubMed]
- García, M.C.; Wernstedt, I.; Berndtsson, A.; Enge, M.; Bell, M.; Hultgren, O.; Horn, M.; Ahrén, B.; Enerback, S.; Ohlsson, C.; et al. Mature-Onset Obesity in Interleukin-1 Receptor I Knockout Mice. Diabetes 2006, 55, 1205–1213. [Google Scholar] [CrossRef]
- Uysal, K.T.; Wiesbrock, S.M.; Marino, M.W.; Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 1997, 389, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.-W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 2005, 11, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Hers, I.; Vincent, E.E.; Tavaré, J.M. Akt signalling in health and disease. Cell. Signal. 2011, 23, 1515–1527. [Google Scholar] [CrossRef]
- Freyberg, Z.; Ferrando, S.J.; Javitch, J.A. Roles of the Akt/GSK-3 and Wnt Signaling Pathways in Schizophrenia and Antipsychotic Drug Action. Am. J. Psychiatry 2010, 167, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Dwir, D.; Giangreco, B.; Xin, L.; Tenenbaum, L.; Cabungcal, J.-H.; Steullet, P.; Goupil, A.; Cleusix, M.; Jenni, R.; Chtarto, A.; et al. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: A reverse translation study in schizophrenia patients. Mol. Psychiatry 2019, 25, 2889–2904. [Google Scholar] [CrossRef]
- Devanarayanan, S.; Nandeesha, H.; Kattimani, S.; Sarkar, S. Relationship between matrix metalloproteinase-9 and oxidative stress in drug-free male schizophrenia: A case control study. cclm 2016, 54, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Rog, J.; Dzikowski, M.; Juchnowicz, D.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M.; Karakula-Juchnowicz, H. The importance of blood count and oxidative stress in the drug-naïve first episode schizophrenia. Eur. Psychiatry 2022, 65, S793. [Google Scholar] [CrossRef]
- Koido, K.; Innos, J.; Haring, L.; Zilmer, M.; Ottas, A.; Vasar, E. Taurine and Epidermal Growth Factor Belong to the Signature of First-Episode Psychosis. Front. Neurosci. 2016, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhao, L.; Fan, Y.; Lv, Q.; Wu, K.; Lang, X.; Li, Z.; Yi, Z.; Geng, D. Interaction between TNF-α and oxidative stress status in first-episode drug-naïve schizophrenia. Psychoneuroendocrinology 2020, 114, 104595. [Google Scholar] [CrossRef]
- Goff, D.C.; Zeng, B.; Ardekani, B.A.; Diminich, E.D.; Tang, Y.; Fan, X.; Galatzer-Levy, I.; Li, C.; Troxel, A.B.; Wang, J. Association of Hippocampal Atrophy With Duration of Untreated Psychosis and Molecular Biomarkers During Initial Antipsychotic Treatment of First-Episode Psychosis. JAMA Psychiatry 2018, 75, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.; Wood, S.J.; Elsworthy, R.J.; Upthegrove, R.; Aldred, S. Exercise as a protective mechanism against the negative effects of oxidative stress in first-episode psychosis: A biomarker-led study. Transl. Psychiatry 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Parellada, M.; MacDowell, K.S.; García-Bueno, B.; Cabrera, B.; González-Pinto, A.; Saiz, P.; Lobo, A.; Rodriguez-Jimenez, R.; Berrocoso, E.; et al. Differences in the regulation of inflammatory pathways in adolescent- and adult-onset first-episode psychosis. Eur. Child Adolesc. Psychiatry 2019, 28, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Sarnyai, Z. Allostatic load is associated with psychotic symptoms and decreases with antipsychotic treatment in patients with schizophrenia and first-episode psychosis. Psychoneuroendocrinology 2018, 90, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Nettis, M.A.; Pariante, C.M.; Mondelli, V. Early-Life Adversity, Systemic Inflammation and Comorbid Physical and Psychiatric Illnesses of Adult Life. Curr. Top Behav. Neurosci. 2020, 44, 207–225. [Google Scholar]
- Russell, A.; Ciufolini, S.; Gardner-Sood, P.; Bonaccorso, S.; Gaughran, F.; Dazzan, P.; Pariante, C.M.; Mondelli, V. Inflammation and metabolic changes in first episode psychosis: Preliminary results from a longitudinal study. Brain Behav. Immun. 2015, 49, 25–29. [Google Scholar] [CrossRef]
- Bocchio-Chiavetto, L.; Zanardini, R.; Tosato, S.; Ventriglia, M.; Ferrari, C.; Bonetto, C.; Lasalvia, A.; Giubilini, F.; Fioritti, A.; Pileggi, F.; et al. Immune and metabolic alterations in first episode psychosis (FEP) patients. Brain Behav. Immun. 2018, 70, 315–324. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Zhang, M.; Deng, Y.; Yi, Z.; Shi, T. Exploring the pathogenetic association between schizophrenia and type 2 diabetes mellitus diseases based on pathway analysis. BMC Med Genom. 2013, 6, S17. [Google Scholar] [CrossRef]
- Noto, C.; Ota, V.K.; Santoro, M.L.; Gouvea, E.S.; Silva, P.N.; Spindola, L.M.; Cordeiro, Q.; Bressan, R.A.; Gadelha, A.; Brietzke, E.; et al. Depression, Cytokine, and Cytokine by Treatment Interactions Modulate Gene Expression in Antipsychotic Naïve First Episode Psychosis. Mol. Neurobiol. 2015, 53, 5701–5709. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K. Mitochondrial Involvement in Mental Disorders: Energy Metabolism and Genetic and Environmental Factors. Adv. Exp. Med. Biol. 2019, 1118, 63–70. [Google Scholar]
- Uranova, N.A.; Orlovskaia, D.D.; Vikhreva, O.V.; Zimina, I.S.; Rakhmanova, V.I. Morfometricheskaia otsenka ul’trastrukturnykh plasticheskikh perestroek v mozge pri éndogennykh psikhozakh (reaktsii oligodendroglii) [Morphometric study of ultrastructural changes in oligodendroglial cells in the postmortem brain in endogenous psychoses]. Vestn. Ross. Akad. Meditsinskikh Nauk. 2001, 7, 42–48. [Google Scholar]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Cooper, A.J.; Thida, T.; Sehovic, S.; Lukas, S.E.; Cohen, B.M.; Zhang, X.; Öngür, D. In Vivo Evidence for Cerebral Bioenergetic Abnormalities in Schizophrenia Measured Using31P Magnetization Transfer Spectroscopy. JAMA Psychiatry 2014, 71, 19–27. [Google Scholar] [CrossRef]
- Kolar, D.; Kleteckova, L.; Skalova, K.; Brozka, H.; Kalous, M.; Vales, K. Glycolytic and Krebs cycle enzymes activity in rat prefrontal cortex, hippocampus, and striatum after single and repeated NMDA inhibition by MK-801. NeuroToxicology 2022, 90, 35–47. [Google Scholar] [CrossRef]
- Gao, Z.; Xiu, M.; Liu, J.; Wu, F.; Zhang, X.-Y. Obesity, antioxidants and negative symptom improvement in first-episode schizophrenia patients treated with risperidone. Schizophrenia 2023, 9, 1–6. [Google Scholar] [CrossRef]
- Tao, Q.; Miao, Y.; Li, H.; Yuan, X.; Huang, X.; Wang, Y.; Andreassen, O.A.; Fan, X.; Yang, Y.; Song, X. Insulin Resistance and Oxidative Stress: In Relation to Cognitive Function and Psychopathology in Drug-Naïve, First-Episode Drug-Free Schizophrenia. Front. Psychiatry 2020, 11, 537280. [Google Scholar] [CrossRef]
- Prabakaran, S.; Swatton, J.E.; Ryan, M.M.; Huffaker, S.J.; Huang, J.T.-J.; Griffin, J.L.; Wayland, M.; Freeman, T.; Dudbridge, F.; Lilley, K.S.; et al. Mitochondrial dysfunction in schizophrenia: Evidence for compromised brain metabolism and oxidative stress. Mol. Psychiatry 2004, 9, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Brzustowicz, L.M.; Simone, J.; Mohseni, P.; Hayter, J.E.; Hodgkinson, K.A.; Chow, E.W.; Bassett, A.S. Linkage Disequilibrium Mapping of Schizophrenia Susceptibility to the CAPON Region of Chromosome 1q22. Am. J. Hum. Genet. 2004, 74, 1057–1063. [Google Scholar] [CrossRef][Green Version]
- Gough, S.C.L.; O’dOnovan, M.C. Clustering of metabolic comorbidity in schizophrenia: A genetic contribution? J. Psychopharmacol. 2005, 19, 47–55. [Google Scholar] [CrossRef]
- Belbasis, L.; Köhler, C.A.; Stefanis, N.; Stubbs, B.; van Os, J.; Vieta, E.; Seeman, M.V.; Arango, C.; Carvalho, A.F.; Evangelou, E. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: An umbrella review of meta-analyses. Acta Psychiatr. Scand. 2017, 137, 88–97. [Google Scholar] [CrossRef]
- Eren, F.; Schwieler, L.; Orhan, F.; Malmqvist, A.; Piehl, F.; Cervenka, S.; Sellgren, C.M.; Fatouros-Bergman, H.; Engberg, G.; Erhardt, S. Immunological protein profiling of first-episode psychosis patients identifies CSF and blood biomarkers correlating with disease severity. Brain Behav. Immun. 2023, 111, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, T.; D’aMbrosio, E.; McCutcheon, R.; Howes, O.D. Correction to: Is psychosis a multisystem disorder? A meta-review of central nervous system, immune, cardiometabolic, and endocrine alterations in first-episode psychosis and perspective on potential models. Mol. Psychiatry 2018, 24, 928. [Google Scholar] [CrossRef]
- van Beveren, N.J.M.; Schwarz, E.; Noll, R.; Guest, P.C.; Meijer, C.; de Haan, L.; Bahn, S. Evidence for disturbed insulin and growth hormone signaling as potential risk factors in the development of schizophrenia. Transl. Psychiatry 2014, 4, e430. [Google Scholar] [CrossRef]
- Krzyściak, W.; Szwajca, M.; Śmierciak, N.; Chrzan, R.; Turek, A.; Karcz, P.; Bryll, A.; Pilecki, M.; Morava, E.; Ligęzka, A.; et al. From periphery immunity to central domain through clinical interview as a new insight on schizophrenia. Sci. Rep. 2024, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ji, H.; Niu, Z.; Liu, H.; Wu, X.; Yang, L.; Wang, Z.; Chen, J.; Fang, Y. Biochemical and Endocrine Parameters for the Discrimination and Calibration of Bipolar Disorder or Major Depressive Disorder. Front. Psychiatry 2022, 13, 875141. [Google Scholar] [CrossRef]
- de Witte, L.; Tomasik, J.; Schwarz, E.; Guest, P.C.; Rahmoune, H.; Kahn, R.S.; Bahn, S. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr. Res. 2014, 154, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Rambaud, V.; Marzo, A.; Chaumette, B. Oxidative Stress and Emergence of Psychosis. Antioxidants 2022, 11, 1870. [Google Scholar] [CrossRef]
- Li, H.-C.; Chen, Q.-Z.; Ma, Y.; Zhou, J.-F. Imbalanced free radicals and antioxidant defense systems in schizophrenia: A comparative study. J. Zhejiang Univ. B 2006, 7, 981–986. [Google Scholar] [CrossRef][Green Version]
- Cullen, A.E.; Labad, J.; Oliver, D.; Al-Diwani, A.; Minichino, A.; Fusar-Poli, P. The Translational Future of Stress Neurobiology and Psychosis Vulnerability: A Review of the Evidence. Curr. Neuropharmacol. 2024, 22, 350–377. [Google Scholar] [CrossRef]
- Karanikas, E. Cortisol and cytokines in early psychosis, do they correlate? A scoping review. Neurol. Psychiatry Brain Res. 2019, 32, 91–98. [Google Scholar] [CrossRef]
- Plitman, E.; Iwata, Y.; Caravaggio, F.; Nakajima, S.; Chung, J.K.; Gerretsen, P.; Kim, J.; Takeuchi, H.; Chakravarty, M.M.; Remington, G.; et al. Kynurenic Acid in Schizophrenia: A Systematic Review and Meta-analysis. Schizophr. Bull. 2017, 43, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Sominsky, L.; Walder, K.R.; Berk, M.; Marx, W.; Carvalho, A.F.; Bortolasci, C.C.; Maes, M.; Puri, B.K. Inflammation and Nitro-oxidative Stress as Drivers of Endocannabinoid System Aberrations in Mood Disorders and Schizophrenia. Mol. Neurobiol. 2022, 59, 3485–3503. [Google Scholar] [CrossRef]
- Parkar, S.R.; Ramanathan, S.; Nair, N.; Batra, S.; Adarkar, S.; Kund, P.; Baghel, N.S.; Moghe, S. Are the effects of cannabis dependence on glucose metabolism similar to schizophrenia? An FDG PET understanding. Indian J. Psychiatry 2011, 53, 13–20. [Google Scholar] [CrossRef]
- Labad, J.; Ortega, L.; Cabezas, Á.; Montalvo, I.; Arranz, S.; Algora, M.J.; Solé, M.; Martorell, L.; Vilella, E.; Sánchez-Gistau, V. Hypothalamic-pituitary-adrenal axis function and exposure to stress factors and cannabis use in recent-onset psychosis. World J. Biol. Psychiatry 2019, 21, 564–571. [Google Scholar] [CrossRef]
- Kucukgoncu, S.; Guloksuz, S.; Tek, C. Effects of curcumin on cognitive functioning and inflammatory state in schizophrenia: A double-blind, placebo-controlled pilot trial. J. Clin. Psychopharmacol. 2019, 39, 182–184. [Google Scholar] [CrossRef]
- Mitra, S.; Natarajan, R.; Ziedonis, D.; Fan, X. Antioxidant and anti-inflammatory nutrient status, supplementation, and mechanisms in patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 78, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bachu, A.K.; Singal, P.; Griffin, B.; Harbaugh, L.; Prasad, S.; Jain, L.; Mohiuddin, S.; Papudesi, B.N.; Nagi, T.; Youssef, N.A.; et al. Kratom use and mental health: A systematic literature review and case example. J. Addict. Dis. 2023, 42, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Cao, T.; Cai, H. Peripheral biomarkers of treatment-resistant schizophrenia: Genetic, inflammation and stress perspectives. Front. Pharmacol. 2022, 13, 1005702. [Google Scholar] [CrossRef]
- Sanada, K.; Montero-Marin, J.; Barceló-Soler, A.; Ikuse, D.; Ota, M.; Hirata, A.; Yoshizawa, A.; Hatanaka, R.; Valero, M.S.; Demarzo, M.; et al. Effects of Mindfulness-Based Interventions on Biomarkers and Low-Grade Inflammation in Patients with Psychiatric Disorders: A Meta-Analytic Review. Int. J. Mol. Sci. 2020, 21, 2484. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karanikas, E. The Pathobiological Underpinnings of Psychosis: From the Stress-Related Hypothesis to a Multisystemic Approach. NeuroSci 2025, 6, 99. https://doi.org/10.3390/neurosci6040099
Karanikas E. The Pathobiological Underpinnings of Psychosis: From the Stress-Related Hypothesis to a Multisystemic Approach. NeuroSci. 2025; 6(4):99. https://doi.org/10.3390/neurosci6040099
Chicago/Turabian StyleKaranikas, Evangelos. 2025. "The Pathobiological Underpinnings of Psychosis: From the Stress-Related Hypothesis to a Multisystemic Approach" NeuroSci 6, no. 4: 99. https://doi.org/10.3390/neurosci6040099
APA StyleKaranikas, E. (2025). The Pathobiological Underpinnings of Psychosis: From the Stress-Related Hypothesis to a Multisystemic Approach. NeuroSci, 6(4), 99. https://doi.org/10.3390/neurosci6040099





