Frontal Regions and Executive Function Testing: A Doubted Association Shown by Brain-Injured Patients
Abstract
1. Introduction
1.1. Limits of Executive Function Tests
1.2. Anatomical Localization of Executive Functions
1.3. The Present Study
2. Materials and Methods
2.1. Participants
2.2. Materials and Procedure
2.3. Data Analysis
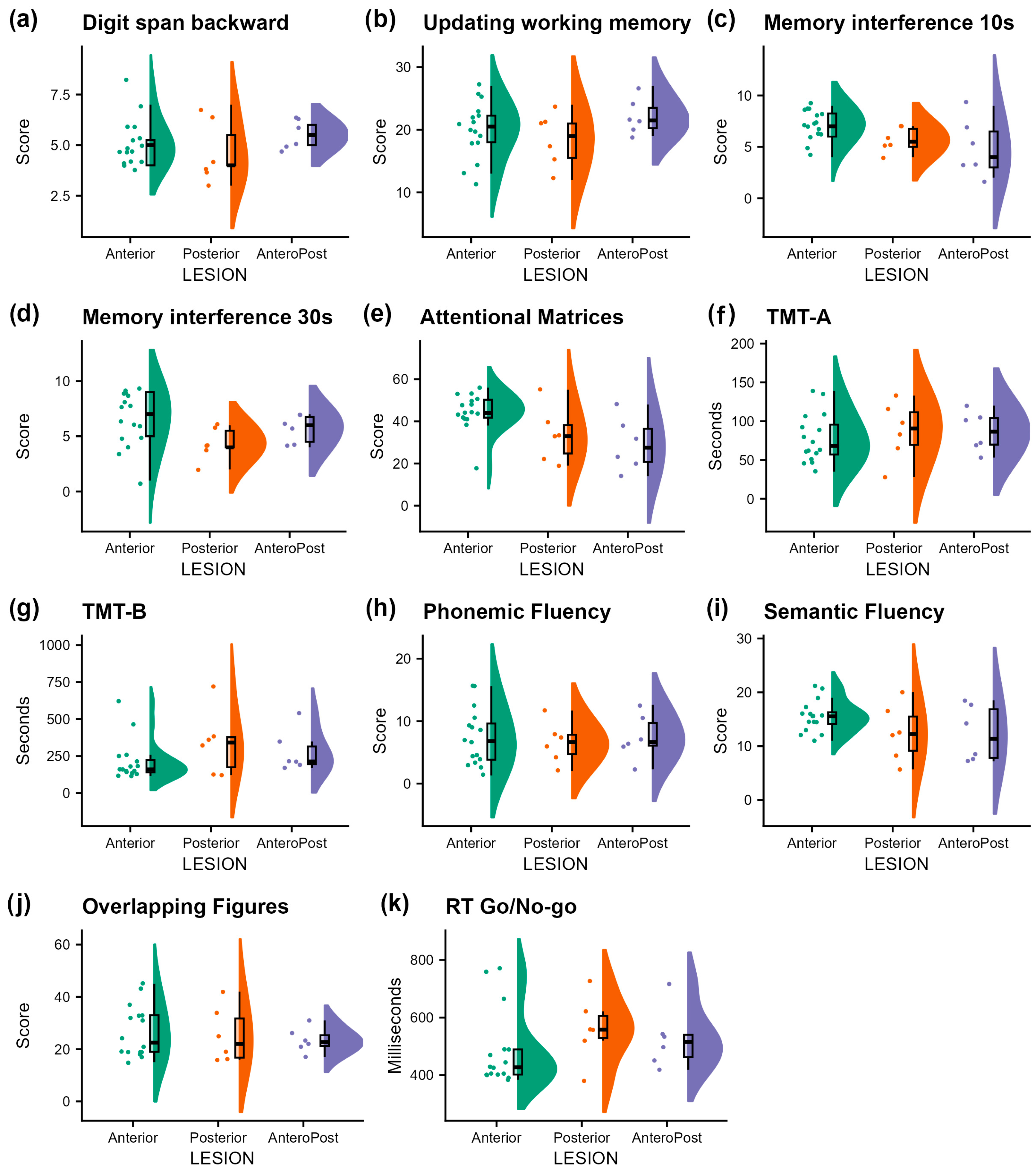
3. Results
| Executive Function | Test | Anterior vs. Posterior | Anterior vs. AnteroPost | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | Std. Error | Test Statistics | p-Value | p-Value Corrected | Coefficient | Std. Error | Test Statistics | p-Value | p-Value Corrected | ||
| Working Memory | Digit span backward | −0.46 | 0.55 | −0.84 | 0.41 | 0.70 | 0.38 | 0.55 | 0.69 | 0.50 | 0.75 |
| Updating working memory | −1.67 | 2.02 | −0.82 | 0.42 | 0.70 | 2.17 | 2.02 | 1.07 | 0.29 | 0.73 | |
| Memory with interference 10 s | −1.46 | 0.85 | −1.73 | 0.10 | 0.43 | −2.29 | 0.85 | −2.71 | 0.01 * | 0.10 | |
| Memory with interference 30 s | −2.23 | 1.02 | −2.19 | 0.04 * | 0.30 | −0.90 | 1.02 | −0.88 | 0.39 | 0.75 | |
| Attention | Attentional Matrices | −10.96 | 5.06 | −2.17 | 0.04 * | 0.30 | −15.46 | 5.06 | −3.06 | 0.01 * | 0.10 |
| TMT-A (sec.) | 9.23 | 15.18 | 0.61 | 0.55 | 0.75 | 8.73 | 15.18 | 0.58 | 0.57 | 0.81 | |
| Inhibition | Mean RT Go/No-go (msec.) | −0.83 | 1.98 | −0.42 | 0.68 | 0.82 | 0.09 | 1.98 | 0.04 | 0.97 | 1.00 |
| Switching and Flexibility | TMT-B (sec.) | 124.50 | 75.89 | 1.64 | 0.11 | 0.43 | 65.17 | 75.89 | 0.86 | 0.40 | 0.75 |
| Phonemic Fluency | −3.05 | 1.92 | −1.59 | 0.13 | 0.43 | −3.24 | 1.92 | −1.69 | 0.10 | 0.45 | |
| Semantic Fluency | −1.23 | 4.38 | −0.28 | 0.78 | 0.87 | −3.16 | 4.38 | −0.72 | 0.48 | 0.75 | |
| Overlapping Figures | 0.15 | 0.12 | 1.28 | 0.21 | 0.57 | 0.09 | 0.12 | 0.74 | 0.47 | 0.75 | |
| Planning | Tower of London | −1.42 | 2.83 | −0.50 | 0.62 | 0.78 | −1.02 | 2.83 | −0.36 | 0.72 | 0.90 |
| Elithorn’s Perceptual Maze Test | −6.53 | 2.18 | −3.00 | 0.01 * | 0.15 | −1.95 | 2.18 | −0.89 | 0.28 | 0.73 | |
| City Map test: | |||||||||||
| Errands/movements | −0.69 | 0.64 | −1.08 | 0.28 | 0.60 | 0.25 | 0.58 | 0.43 | 0.67 | 0.90 | |
| Total time | −2.53 | 2.18 | −1.16 | 0.26 | 0.60 | 3.50 | 2.18 | 1.60 | 0.12 | 0.45 | |
| Readings | −0.82 | 0.19 | −4.21 | <0.001 * | <0.001 * | 0.05 | 0.14 | 0.39 | 0.70 | 0.90 | |
| Constraints | 0.77 | 1.08 | 0.72 | 0.47 | 0.74 | −0.14 | 1.27 | −0.11 | 0.91 | 1.00 | |
| Omissions | 0.10 | 1.02 | 0.09 | 0.93 | 0.95 | 0.79 | 0.98 | 0.81 | 0.42 | 0.75 | |
| Perseverations | 0.69 | 1.00 | 0.69 | 0.49 | 0.74 | 0.00 | 0.96 | 0.00 | 1.00 | 1.00 | |
| Maps test: | |||||||||||
| Optimization index | 0.90 | 0.65 | 1.38 | 0.18 | 0.54 | 0.98 | 0.65 | 1.51 | 0.15 | 0.50 | |
| Initial Planning Time (msec.) | 1931 | 2926 | 0.66 | 0.52 | 0.74 | 6060 | 2926 | 2.07 | 0.05 | 0.38 | |
| Execution time (msec.) | 13,480 | 8654 | 1.56 | 0.13 | 0.43 | 7727 | 8654 | 0.89 | 0.38 | 0.75 | |
| Planning Index | 0.98 | 0.97 | 1.01 | 0.32 | 0.60 | 1.60 | 0.97 | 1.64 | 0.11 | 0.45 | |
| Planning Index Variance | 1.11 | 3.07 | 0.36 | 0.72 | 0.83 | 8.06 | 3.07 | 2.63 | 0.01 * | 0.10 | |
| Heuristic dispersion | 2.69 | 5.05 | 0.53 | 0.60 | 0.78 | 6.41 | 6.09 | 1.05 | 0.30 | 0.82 | |
| Other Cognitive Functions | Test | Anterior vs. Posterior | ||||
|---|---|---|---|---|---|---|
| Coefficient | Std. Error | T-Value | p-Value | p-Value Corrected | ||
| Speed of processing | Mean Simple RT (msec.) | 0.16 | 0.14 | 1.16 | 0.26 | 0.39 |
| Mean Choice RT (msec.) | 0.20 | 0.10 | 2.06 | 0.05 | 0.30 | |
| Short-term memory | Story recall (Immediate) | −3.97 | 2.29 | −1.73 | 0.10 | 0.30 |
| Story recall (Delayed) | −3.67 | 2.60 | −1.41 | 0.17 | 0.34 | |
| Visuo-spatial abilities | Clock Drawing | −0.91 | 1.15 | −0.79 | 0.44 | 0.53 |
| Rey’s Complex Fig. Copy | −0.38 | 0.90 | −0.42 | 0.68 | 0.68 | |
| Anterior vs. AnteroPost | ||||||
| Speed of processing | Mean Simple RT (msec.) | 0.11 | 0.14 | 0.82 | 0.42 | 0.50 |
| Mean Choice RT (msec.) | 0.09 | 0.10 | 0.98 | 0.34 | 0.50 | |
| Short-term memory | Story recall (Immediate) | 0.87 | 2.29 | 0.38 | 0.71 | 0.71 |
| Story recall (Delayed) | 3.33 | 2.60 | 1.28 | 0.21 | 0.42 | |
| Visuo-spatial abilities | Clock Drawing | −2.48 | 1.15 | −2.15 | 0.04 * | 0.24 |
| Rey’s Complex Fig. Copy | −1.61 | 0.90 | −1.79 | 0.09 | 0.27 | |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EFs | Executive Functions |
| CFA | Confirmatory Factor Analysis |
| WCST | Wisconsin Card Sorting test |
| VR | Virtual Reality |
| PFC | PreFrontal Cortex |
| DLPFC | DorsoLateral PreFrontal Cortex |
| dACC | dorsal Anterior Cingulate Cortex |
| GLM | Generalized Linear Model |
| IRR | Incidence Rate Ratio |
| FDR | False Discovery Rate |
| TMS | Transcranial Magnetic Stimulation |
| TDCS | Transcranial Direct Current Stimulation |
Appendix A


References
- Luria, A.R. The Working Brain; Penguin Books Ltd.: Baltimore, MD, USA, 1973. [Google Scholar]
- Norman, D.; Shallice, T. Attention to Action: Willed and Automatic Control of Behavior. In Consciousness and Self Regulation: Advances in Research and Theory; Davidson, R., Schwartz, G., Shapiro, D., Eds.; Plenum: New York, NY, USA, 1986; Volume 4, pp. 1–18. [Google Scholar]
- Duncan, J.; Burgess, P.; Emslie, H. Fluid Intelligence after Frontal Lobe Lesion. Neuropsychologia 1995, 33, 261–268. [Google Scholar] [CrossRef]
- Duncan, J.; Emslie, H.; Williams, P.; Johnson, R.; Freer, C. Intelligence and the Frontal Lobe: The Organization of Goal-Directed Behavior. Cogn. Psychol. 1996, 30, 257–303. [Google Scholar] [CrossRef]
- Kimberg, D.Y.; Farah, M.J. A Unified Account of Cognitive Impairments Following Frontal Lobe Damage: The Role of Working Memory in Complex, Organized Behavior. J. Exp. Psychol. Gen. 1993, 122, 411–428. [Google Scholar] [CrossRef]
- Jurado, M.B.; Rosselli, M. The Elusive Nature of Executive Functions: A Review of Our Current Understanding. Neuropsychol. Rev. 2007, 17, 213–233. [Google Scholar] [CrossRef]
- Chan, R.C.; Shum, D.; Toulopoulou, T.; Chen, E.Y. Assessment of Executive Functions: Review of Instruments and Identification of Critical Issues. Arch. Clin. Neuropsychol. 2008, 23, 201–216. [Google Scholar] [CrossRef]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Friedman, N.P.; Miyake, A. Unity and Diversity of Executive Functions: Individual Differences as a Window on Cognitive Structure. Cortex 2017, 86, 186–204. [Google Scholar] [CrossRef]
- Karr, J.E.; Areshenkoff, C.N.; Rast, P.; Hofer, S.M.; Iverson, G.L.; Garcia-Barrera, M.A. The Unity and Diversity of Executive Functions: A Systematic Review and Re-Analysis of Latent Variable Studies. Psychol. Bull. 2018, 144, 1147–1185. [Google Scholar] [CrossRef] [PubMed]
- Burgess, P.W. Theory and Methodology in Executive Function Research. In Methodology of Frontal and Executive Function; Rabbitt, P., Ed.; Psychology Press: Hove, UK, 1997; pp. 81–116. [Google Scholar]
- Lezak, M.D. Neuropsychological Assessment, 3rd ed.; Oxford Univ. Press: New York, NY, USA, 1995. [Google Scholar]
- Wechsler, D. A standardized memory scale for clinical use. J. Psychol. 1945, 19, 87–95. [Google Scholar] [CrossRef]
- Petrides, M.; Milner, B. Deficits on subject-ordered tasks after frontal-and temporal-lobe lesions in man. Neuropsychologia 1982, 20, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Gorenstein, E.E.; Mammato, C.A.; Sandy, J.M. Performance of inattentive-overactive children on selected measures of prefrontal-type function. J. Clin. Psychol. 1989, 45, 619–632. [Google Scholar] [CrossRef] [PubMed]
- McAndrews, M.P.; Milner, B. The frontal cortex and memory for temporal order. Neuropsychologia 1991, 29, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Schacter, D.L. Memory, amnesia, and frontal lobe dysfunction. Psychobiology 1987, 15, 21–36. [Google Scholar] [CrossRef]
- Delis, D.C.; Kramer, J.H.; Kaplan, E.; Ober, B.A. California Verbal Learning Test: Adult Version. Manual; Psychological Corporation: San Antonio, TX, USA, 1987. [Google Scholar]
- Corsi, P.M. Human Memory and the Medial Temporal Region of the Brain. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 1972. [Google Scholar]
- Gevins, A.S.; Cutillo, B.C. Neuroelectric evidence for distributed processing in human working memory. Electroencephalogr. Clin. Neurophysiol. 1993, 87, 128–143. [Google Scholar] [CrossRef]
- Spinnler, H.; Tognoni, G. Standardizzazione e Taratura Italiana di Test Neuropsicologici. Ital. J. Neurol. Sci. 1987, 6, 21–120. [Google Scholar]
- Morris, N.; Jones, D.M. Memory updating in working memory: The role of the central executive. Br. J. Psychol. 1990, 81, 111–121. [Google Scholar] [CrossRef]
- Belleville, S.; Peretz, I.; Malenfant, D. Examination of the working memory components in normal aging and in dementia of the Alzheimer type. Neuropsychologia 1996, 34, 195–207. [Google Scholar] [CrossRef]
- Osterrieth, P.A. Le test de copie d’une figure complexe; contribution à l’étude de la perception et de la mémoire. Arch. Psychol. 1944, 30, 206–356. [Google Scholar]
- Manly, T.; Anderson, V.; Nimmo-Smith, I.; Turner, A.; Watson, P.; Robertson, I.H. The differential assessment of children’s attention: The Test of Everyday Attention for Children (TEA-Ch), normative sample and ADHD performance. J. Child Psychol. Psychiatry 2001, 42, 1065–1081. [Google Scholar]
- Conners, C.K. Conners’ Continuous Performance Test User’s Manual; Multi-Health Systems: Toronto, ON, Canada, 1992. [Google Scholar]
- Toulouse, E.; Piéron, H. Technique de Psychologie Experimentale: Examen des Sujets; Paris Octave Doin, Editeur: Paris, France, 1904. [Google Scholar]
- Reitan, R.M. Validity of the Trail Making Test as an Indication of Organic Brain Damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Milner, B. Effects of Brain Lesions on Card Sorting. Arch. Neurol. 1963, 9, 90–100. [Google Scholar] [CrossRef]
- Burgess, P.W.; Shallice, T. Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia 1996, 34, 263–272. [Google Scholar] [CrossRef]
- Burgess, P.W.; Shallice, T. The Hayling and Brixton Tests; Thames Valley Test Company: Thurston, UK, 1997. [Google Scholar]
- Borkowsky, J.G.; Benton, A.L.; Spreen, O. Word Fluency and Brain Damage. Neuropsychologia 1967, 5, 135–140. [Google Scholar] [CrossRef]
- Jones-Gotman, M.; Milner, B. Design fluency: The invention of nonsense drawings after focal cortical lesions. Neuropsychologia 1977, 15, 653–674. [Google Scholar] [CrossRef]
- Poppelreuter, W. Die Psychischen Schaedungen Durch Kopfschuss in Kriege 1914–1916; Voss: Leipzig, Germany, 1917. [Google Scholar]
- Stroop, J.R. Studies of Interference in Serial Verbal Reactions. J. Exp. Psychol. 1935, 6, 643–661. [Google Scholar] [CrossRef]
- Luria, A.R.; Homskaya, E.D. Disturbance in the regulative role of speech with frontal lobe lesions. In The Frontal Granular Cortex and Behavior; Warren, J.M., Akert, K., Eds.; McGraw-Hill: New York, NY, USA, 1964; pp. 353–372. [Google Scholar]
- Logan, G.D. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In Inhibitory Processes in Attention, Memory, and Language; Dagenbach, D., Carr, T.H., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 189–239. [Google Scholar]
- Tipper, S.P. The negative priming effect: Inhibitory priming by ignored objects. Q. J. Exp. Psychol. 1985, 37, 571–590. [Google Scholar] [CrossRef] [PubMed]
- Guitton, D.; Buchtel, H.A.; Douglas, R.M. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp. Brain Res. 1985, 58, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.; Waterman, M.; Prescott, H.; Murdoch-Eaton, D. A new Stroop-like measure of inhibitory function development: Typical developmental trends. J. Child Psychol. Psychiatry 2003, 44, 561–575. [Google Scholar] [CrossRef]
- Porteus, S.D. Porteus Maze Test; Psychological Corporation: San Antonio, TX, USA, 1959. [Google Scholar]
- Elithorn, A. A Preliminary Report on a Perceptual Maze Test Sensitive to Brain Damage. J. Neurol. Neurosurg. Psychiatry 1955, 18, 287–292. [Google Scholar] [CrossRef]
- Borys, S.V.; Spitz, H.H.; Dorans, B.A. Tower of Hanoi performance of retarded young adults and nonretarded children as a function of solution length and goal state. J. Exp. Child. Psychol. 1982, 33, 87–110. [Google Scholar] [CrossRef]
- Shallice, T. Specific Impairments of Planning. Philos. Trans. R. Soc. Lond. B 1982, 298, 199–209. [Google Scholar]
- Kofler, M.J.; Irwin, L.N.; Soto, E.F.; Groves, N.B.; Harmon, S.L.; Sarver, D.E. Executive Functioning Heterogeneity in Pediatric ADHD. J. Abnorm. Child. Psychol. 2019, 47, 273–286. [Google Scholar] [CrossRef]
- Stuss, D.T.; Alexander, M.P. Executive Functions and the Frontal Lobes: A Conceptual View. Psychol. Res. 2000, 63, 289–298. [Google Scholar] [CrossRef]
- Nelson, H. A Modified Card Sorting Response Sensitive to Frontal Lobe Defects. Cortex 1976, 12, 313–324. [Google Scholar] [CrossRef]
- Heaton, R.K.; Chelune, G.J.; Talley, J.L.; Kay, G.G.; Curtiss, G. Wisconsin Card Sorting Test Manual Revised and Expanded; Psychological Assessment Resources: Odessa, FL, USA, 1993. [Google Scholar]
- Barceló, F.; Sanz, M.; Molina, V.; Rubia, F.J. The Wisconsin Card Sorting Test and the Assessment of Frontal Function: A Validation Study with Event-Related Potentials. Neuropsychologia 1997, 35, 399–408. [Google Scholar] [CrossRef]
- Konishi, S.; Kawazu, M.; Uchida, I.; Kikyo, H.; Asakura, I.; Miyashita, Y. Contribution of Working Memory to Transient Activation in Human Inferior Prefrontal Cortex during Performance of the Wisconsin Card Sorting Test. Cereb. Cortex 1999, 9, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Monchi, O.; Petrides, M.; Petre, V.; Worsley, K.; Dagher, A. Wisconsin Card Sorting Revisited: Distinct Neural Circuits Participating in Different Stages of the Task Identified by Event-Related Functional Magnetic Resonance Imaging. J. Neurosci. 2001, 21, 7733–7741. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Emory, E. Executive Function and the Frontal Lobes: A Meta-Analytic Review. Neuropsychol. Rev. 2006, 16, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Andrés, P. Frontal Cortex as the Central Executive of Working Memory: Time to Revise Our View. Cortex 2003, 39, 871–895. [Google Scholar] [CrossRef]
- Londei, A.; D’Ausilio, A.; Basso, D.; Sestieri, C.; Del Gratta, C.; Romani, G.L.; Olivetti Belardinelli, M. Sensory-Motor Brain Network Connectivity for Speech Comprehension. Hum. Brain Mapp. 2010, 31, 567–580. [Google Scholar] [CrossRef]
- Goel, V.; Grafman, J. Are the Frontal Lobes Implicated in “Planning” Functions? Interpreting Data from the Tower of Hanoi. Neuropsychologia 1995, 33, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Kafer, K.L.; Hunter, M. On Testing the Validity of Planning/Problem-Solving Tasks in a Normal Population. J. Int. Neuropsychol. Soc. 1997, 3, 108–119. [Google Scholar] [CrossRef]
- Archibald, Y.M. Time as a Variable in the Performance of Hemisphere-Damaged Patients on the Elithorn Perceptual Maze Test. Cortex 1978, 14, 22–31. [Google Scholar] [CrossRef]
- Lawler, E.L.; Lenstra, J.K.; Kan, A.H.G.R.; Shmoys, D.B. The Traveling Salesman Problem; Wiley: New York, NY, USA, 1985. [Google Scholar]
- Bisiacchi, P.S.; Sgaramella, T. Neuropsychological Evaluation of Prospective Memory in Old Age. Brain Cogn. 1995, 28, 82. [Google Scholar]
- Funke, J.; Krüger, T. “Plan-A-Day”: Konzeption Eines Modifizierbaren Instruments zur Führungskräfte-Auswahl sowie Erste Empirische Befunde. In Neue Konzepte und Instrumente zur Planungsdiagnostik; Funke, J., Fritz, A., Eds.; Deutscher Psychologen Verlag: Bonn, Germany, 1995; pp. 97–120. [Google Scholar]
- Basso, D.; Bisiacchi, P.S.; Cotelli, M.; Farinello, C. Planning Times during Travelling Salesman’s Problem: Differences between Closed Head Injury and Normal Subjects. Brain Cogn. 2001, 46, 38–42. [Google Scholar] [CrossRef]
- Cipresso, P.; Albani, G.; Serino, S.; Pedroli, E.; Pallavicini, F.; Mauro, A.; Riva, G. Virtual Multiple Errands Test (VMET): A Virtual Reality-Based Tool to Detect Early Executive Functions Deficit in Parkinson’s Disease. Front. Behav. Neurosci. 2014, 8, 405. [Google Scholar] [CrossRef]
- Borgnis, F.; Baglio, F.; Pedroli, E.; Rossetto, F.; Uccellatore, L.; Oliveira, J.A.G.; Riva, G.; Cipresso, P. Available Virtual Reality-Based Tools for Executive Functions: A Systematic Review. Front. Psychol. 2022, 13, 833136. [Google Scholar]
- Wen, T.; Liu, D.C.; Hsieh, S. Connectivity patterns in cognitive control networks predict naturalistic multitasking ability. Neuropsychologia 2018, 1, 195–202. [Google Scholar] [CrossRef]
- Baddeley, A.D. Working Memory; Oxford University Press: Oxford, UK, 1986. [Google Scholar]
- Shallice, T. From Neuropsychology to Mental Structure; Cambridge University Press: New York, NY, USA, 1988. [Google Scholar]
- Ruiz-Gutiérrez, J.; Arias-Sánchez, S.; Martín-Monzón, I. Neuropsychology of executive functions in patients with focal lesion in the prefrontal cortex: A systematic review. Brain Cogn. 2020, 146, 105633. [Google Scholar] [CrossRef] [PubMed]
- Karr, J.E.; Rodriguez, J.E.; Goh, P.K.; Martel, M.M.; Rast, P. The Unity and Diversity of Executive Functions: A Network Approach to Life Span Development. Dev. Psychol. 2022, 58, 751–767. [Google Scholar] [CrossRef]
- Menon, V.; D’Esposito, M. The Role of PFC Networks in Cognitive Control and Executive Function. Neuropsychopharmacology 2022, 47, 90–103. [Google Scholar] [CrossRef]
- Salehinejad, M.A.; Ghanavati, E.; Rashid, M.H.A.; Nitsche, M.A. Hot and Cold Executive Functions in the Brain: A Prefrontal-Cingular Network. Brain Neurosci. Adv. 2021, 5, 1–19. [Google Scholar] [CrossRef]
- Duncan, J. An Adaptive Coding Model of Neural Function in Prefrontal Cortex. Nat. Rev. Neurosci. 2001, 2, 820–829. [Google Scholar] [CrossRef]
- Burgess, P.W.; Stuss, D.T. Fifty Years of Prefrontal Cortex Research: Impact on Assessment. J. Int. Neuropsychol. Soc. 2017, 23, 755–767. [Google Scholar] [CrossRef]
- Phillips, L.H. Do “Frontal Tests” Measure Executive Function? Issue of Assessment and Evidence from Fluency Tests. In Methodology of Frontal and Executive Functions; Rabbitt, P., Ed.; Psychology Press: East Sussex, UK, 1997; pp. 191–213. [Google Scholar]
- Anderson, S.W.; Damasio, H.; Jones, R.D.; Tranel, D. Wisconsin Card Sorting Test Performance as a Measure of Frontal Lobe Damage. J. Clin. Exp. Neuropsychol. 1991, 13, 909–922. [Google Scholar] [CrossRef]
- Mountain, M.A.; Snow, W.G. Wisconsin Card Sorting Test as a Measure of Frontal Pathology. A Review. Clin. Neuropsychol. 1993, 7, 108–118. [Google Scholar] [CrossRef]
- Andrés, P.; Van der Linden, M. Are Central Executive Functions Working in Patients with Focal Frontal Lesions? Neuropsychologia 2002, 40, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Parkin, A.J. The Central Executive Does Not Exist. J. Int. Neuropsychol. Soc. 1998, 4, 518–552. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.C.; Court, J.H.; Raven, J. Manual for Raven’s Progressive Matrices and Vocabulary Scales: Section 3. Standard Progressive Matrices; Oxford Psychologists Press: Oxford, UK, 1996. [Google Scholar]
- Basso, A.; Capitani, E.; Laiacona, M. Raven’s Coloured Progressive Matrices: Normative Values on 305 Adult Normal Controls. Funct. Neurol. 1987, 2, 189–194. [Google Scholar]
- Damasio, A.R.; Damasio, H. Brain and language. Sci. Am. 1992, 267, 88–109. [Google Scholar] [CrossRef]
- Palladino, P.; Jarrold, C. Do Updating Tasks Involve Updating? Evidence from Comparisons with Immediate Serial Recall. Q. J. Exp. Psychol. 2008, 61, 392–399. [Google Scholar] [CrossRef]
- Peterson, L.R.; Peterson, M.J. Short-Term Retention of Individual Verbal Items. J. Exp. Psychol. 1959, 58, 193–198. [Google Scholar] [CrossRef]
- Rey, A. Les Troubles de la Mémoire et Leur Examen Psychométrique; Dessart: Bruxelles, Belgium, 1966. [Google Scholar]
- O’Rourke, N.; Tuokko, H.; Hayden, S.; Beattie, B.L. Early Identification of Dementia: Predictive Validity of the Clock Test. Arch. Clin. Neuropsychol. 1997, 12, 257–267. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 26 July 2025).
- Raaijmakers, Q. Effectiveness of Different Missing Data Treatments in Survey with Likert-Type Data: Introducing the Relative Mean Substitution Approach. Educ. Psychol. Meas. 1999, 59, 725–748. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Azouvi, P.; Vallat-Azouvi, C.; Joseph, P.A.; Meulemans, T.; Bertola, C.; Le Gall, D.; Bellmann, A.; Roussel, M.; Coyette, F.; Krier, M.; et al. Executive functions deficits after severe traumatic brain injury: The GREFEX study. J. Head Trauma Rehabil. 2016, 31, E10–E20. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, V.; Visalli, A.; Facchini, S.; Rossato, C.; Bertoldo, A.; Silvestri, E.; Cecchin, D.; Capizzi, M.; Anglani, M.; Baro, V.; et al. Impaired cognitive control in patients with brain tumors. Neuropsychologia 2022, 169, 108187. [Google Scholar] [CrossRef] [PubMed]
- Eslinger, P.J.; Damasio, A.R. Severe disturbance of higher cognition after bilateral frontal lobe ablation: Patient EVR. Neurology 1985, 35, 1731–1741. [Google Scholar] [CrossRef]
- Menon, V.; Adleman, N.E.; White, C.D.; Glover, G.H.; Reiss, A.L. Error-related brain activation during a Go/NoGo response inhibition task. Hum. Brain Mapp. 2001, 12, 131–143. [Google Scholar] [CrossRef]
- van Holk, M.; Mejias, J.F. Biologically plausible models of cognitive flexibility: Merging recurrent neural networks with full-brain dynamics. Curr. Opin. Behav. Sci. 2024, 56, 101351. [Google Scholar] [CrossRef]
- Lezak, M.D. The problem of assessing executive functions. Int. J. Psychol. 1982, 17, 281–297. [Google Scholar] [CrossRef]
- Chaytor, N.; Schmitter-Edgecombe, M.; Burr, R. Improving the ecological validity of executive functioning assessment. Arch. Clin. Neuropsychol. 2006, 21, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Packwood, S.; Hodgetts, H.M.; Tremblay, S. A multiperspective approach to the conceptualization of executive functions. J. Clin. Exp. Neuropsychol. 2011, 33, 456–470. [Google Scholar] [CrossRef] [PubMed]

| Processes | Measures | Source |
|---|---|---|
| Learning and Memory | - Digit Span | Wechsler (1945) [14] |
| - Self-Ordered Pointing | Petrides & Milner (1982) [15] | |
| - Sequential Matching Memory test | Gorestein et al. (1989) [16] | |
| - Temporal Order Memory | McAndrews & Milner (1991) [17] | |
| - Source Memory | Schacter (1987) [18] | |
| - California Verbal Learning Test | Delis et al. (1987) [19] | |
| - Corsi Block Tapping | Corsi (1971) [20] | |
| - N-back paradigm | Gevins and Cutillo (1993) [21] | |
| - Story recall | Spinnler & Tognoni (1987) [22] | |
| - Updating working memory | Morris & Jones (1990) [23] | |
| - Memory with interference | Belleville, Peretz & Malefant (1996) [24] | |
| - Rey-Osterrieth’s complex figure | Osterrieth (1944) [25] | |
| Attention | - Test of Everyday Attention for Children | Manly et al. (2001) [26] |
| - Continuous Performance Test | Conners (1992) [27] | |
| - Attentional Matrices | Spinnler & Tognoni (1987) [22] | |
| - Cancellation test | Toulouse & Pieròn (1904) [28] | |
| - Trail Making test, part A | Reitan (1958) [29] | |
| Switching and Flexibility | - Wisconsin Card Sorting test | Milner (1963) [30] |
| - Trail Making test, part B | Reitan (1958) [29] | |
| - Hayling Sentence Completion | Burgess & Shallice (1996) [31] | |
| - Brixton Spatial Anticipation test | Burgess & Shallice (1997) [32] | |
| - Word Fluency | Borkowsky et al. (1967) [33] | |
| - Design Fluency | Jones-Gotman & Milner (1977) [34] | |
| - Overlapping Figures | Poppelreuter (1917) [35] | |
| Inhibition | - Stroop test | Stroop (1935) [36] |
| - Go/No-go | Luria & Homskaya (1964) [37] | |
| - Stop signal | Logan (1994) [38] | |
| - Negative priming | Tipper (1985) [39] | |
| - Anti-saccade task | Guitton et al. (1985) [40] | |
| - Conflicting Motor Response | Wright et al. (2003) [41] | |
| Planning | - Porteus Mazes | Porteus (1959) [42] |
| - Elithorn’s Perceptual Maze | Elithorn (1955) [43] | |
| - Tower of Hanoi | Borys et al. (1982) [44] | |
| - Tower of London | Shallice (1982) [45] |
| Left Hemisphere | Right Hemisphere | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | F01 | F02 | F03 | F04 | F05 | F06 | F07 | F08 | F09 | F10 | F11 | F12 | T | P | F0 | F02 | F03 | F04 | F05 | F06 | F07 | F08 | F09 | F10 | F11 | F12 | T | P |
| 1- M.S. | X | X | X | X | X | X | X | X | X | |||||||||||||||||||
| 2- L.M. | X | X | X | X | X | |||||||||||||||||||||||
| 3- O.A. | X | X | X | X | ||||||||||||||||||||||||
| 4- B.D. | X | X | X | X | X | X | X | |||||||||||||||||||||
| 5- C.S. | X | X | X | |||||||||||||||||||||||||
| 6- P.P. | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||
| 7- S.F. | X | X | X | X | X | |||||||||||||||||||||||
| 8- S.G. | X | X | X | X | X | X | X | X | ||||||||||||||||||||
| 9- G.D. | X | X | X | |||||||||||||||||||||||||
| 10- T.D. | X | X | ||||||||||||||||||||||||||
| 11- Z.F. | X | X | X | |||||||||||||||||||||||||
| 12- S.M. | X | X | X | X | ||||||||||||||||||||||||
| 13- C.D. | X | X | X | X | ||||||||||||||||||||||||
| 14- G.A. | X | X | X | X | X | X | X | |||||||||||||||||||||
| 15- G.S. | X | X | X | |||||||||||||||||||||||||
| 16- B.G. | X | X | X | |||||||||||||||||||||||||
| 17- A.B. | X | X | ||||||||||||||||||||||||||
| 18- C.G. | X | X | X | |||||||||||||||||||||||||
| 19- S.G. | X | X | X | X | X | X | X | |||||||||||||||||||||
| 20- B.B. | X | X | X | |||||||||||||||||||||||||
| 21- M.M. | X | X | X | |||||||||||||||||||||||||
| 22- G.N. | X | X | X | |||||||||||||||||||||||||
| 23- S.A. | X | X | X | |||||||||||||||||||||||||
| 24- S.M. | X | X | X | X | ||||||||||||||||||||||||
| 25- F.M. | X | X | ||||||||||||||||||||||||||
| 26- R.I. | X | |||||||||||||||||||||||||||
| 27- F.O. | X | X | X | |||||||||||||||||||||||||
| 28- D.A. | X | X | X | |||||||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso, D.; Bosio, I.; Tarantino, V.; Carabba, F. Frontal Regions and Executive Function Testing: A Doubted Association Shown by Brain-Injured Patients. NeuroSci 2025, 6, 105. https://doi.org/10.3390/neurosci6040105
Basso D, Bosio I, Tarantino V, Carabba F. Frontal Regions and Executive Function Testing: A Doubted Association Shown by Brain-Injured Patients. NeuroSci. 2025; 6(4):105. https://doi.org/10.3390/neurosci6040105
Chicago/Turabian StyleBasso, Demis, Ida Bosio, Vincenza Tarantino, and Francesco Carabba. 2025. "Frontal Regions and Executive Function Testing: A Doubted Association Shown by Brain-Injured Patients" NeuroSci 6, no. 4: 105. https://doi.org/10.3390/neurosci6040105
APA StyleBasso, D., Bosio, I., Tarantino, V., & Carabba, F. (2025). Frontal Regions and Executive Function Testing: A Doubted Association Shown by Brain-Injured Patients. NeuroSci, 6(4), 105. https://doi.org/10.3390/neurosci6040105







