The Long and Winding Road to Understanding Autism
Abstract
1. Introduction
2. Yesterday
3. Dig It
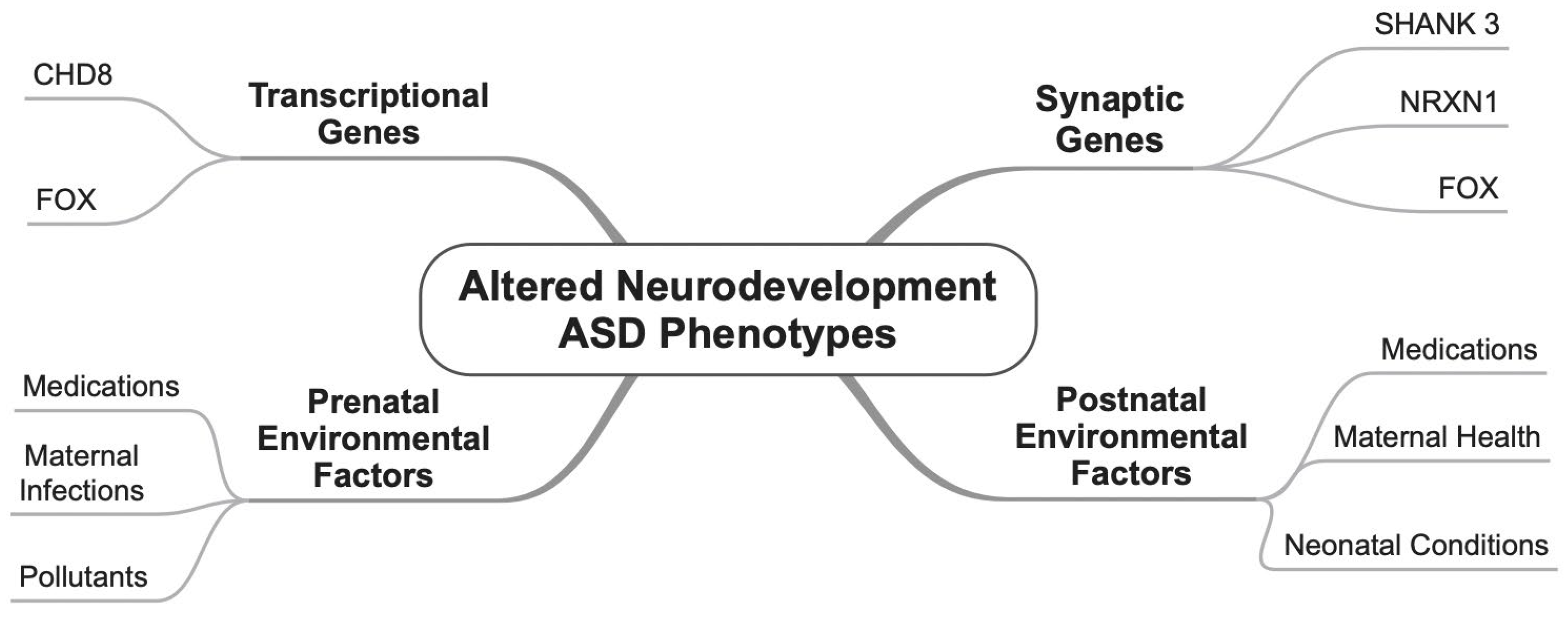
3.1. Genes
3.2. Environmental Factors
4. Boys
5. Girl
6. What Goes on
6.1. Head Circumference
6.2. Cerebral Cortex
6.3. Basal Ganglia
6.4. Amygdala
6.5. Hippocampus
6.6. Thalamus
6.7. Hypothalamus
6.8. Brain Stem
6.9. Cerebellum
7. I’m Looking Through You
8. I Should Have Known Better
9. With a Little Help from My Friends
10. Savoy Truffle
11. Golden Slumbers
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moskowitz, A.; Heim, G. Eugen Bleuler’s Dementia Praecox or the Group of Schizophrenias (1911): A Centenary Appreciation and Reconsideration. Schizophr. Bull. 2011, 37, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Manouilenko, I.; Bejerot, S. Sukhareva—Prior to Asperger and Kanner. Nord. J. Psychiat. 2015, 69, 1761–1764. [Google Scholar] [CrossRef]
- Kanner, L. Autistic Disturbances of Affective Contact. Nerv. Child 1943, 2, 217–250. [Google Scholar]
- Harris, J. Leo Kanner and Autism: A 75-Year Perspective. Int. Rev. Psychiatry 2018, 30, 3–17. [Google Scholar] [CrossRef]
- McAlonan, G.M.; Daly, E.; Kumari, V.; Critchley, H.D.; van Amelsvoort, T.; Suckling, J.; Simmons, A.; Sigmundsson, T.; Greenwood, K.; Russell, A.; et al. Brain Anatomy and Sensorimotor Gating in Asperger’s Syndrome. Brain 2002, 125, 1594–1606. [Google Scholar] [CrossRef] [PubMed]
- Tryon, P.A.; Mayes, S.D.; Rhodes, R.L.; Waldo, M. Can Asperger’s Disorder Be Differentiated from Autism Using DSM-IV Criteria? Focus Autism Other Dev. Disabil. 2006, 21, 2–6. [Google Scholar] [CrossRef]
- Miller, J.N.; Ozonoff, S. Did Asperger’s Cases Have Asperger Disorder? A Research Note. J. Child Psychol. Psychiatry 1997, 38, 247–251. [Google Scholar] [CrossRef]
- Barahona-Corrêa, J.B.; Filipe, C.N. A Concise History of Asperger Syndrome: The Short Reign of a Troublesome Diagnosis. Front. Psychol. 2016, 6, 2024. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Luche, R.D.; Gesi, C.; Moroni, I.; Carmassi, C.; Maj, M. From Asperger’s to DSM-5 Autism Spectrum Disorder and Beyond: A Subthreshold Autism Spectrum Model. Clin. Pract. Epidemiol. Ment. Health 2016, 12, 120–131. [Google Scholar] [CrossRef]
- Gabis, L.V.; Pomeroy, J. An Etiologic Classification of Autism Spectrum Disorders. Isr. Med. Assoc. J. IMAJ 2014, 16, 295–298. [Google Scholar]
- Velmeshev, D.; Magistri, M.; Mazza, E.M.C.; Lally, P.; Khoury, N.; D’Elia, E.R.; Bicciato, S.; Faghihi, M.A. Cell-Type-Specific Analysis of Molecular Pathology in Autism Identifies Common Genes and Pathways Affected Across Neocortical Regions. Mol. Neurobiol. 2020, 57, 2279–2289. [Google Scholar] [CrossRef]
- Hollestein, V.; Poelmans, G.; Forde, N.J.; Beckmann, C.F.; Ecker, C.; Mann, C.; Schäfer, T.; Moessnang, C.; Baumeister, S.; Banaschewski, T.; et al. Excitatory/Inhibitory Imbalance in Autism: The Role of Glutamate and GABA Gene-Sets in Symptoms and Cortical Brain Structure. Transl. Psychiatry 2023, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Brueggeman, L.; Koomar, T.; Michaelson, J.J. Forecasting Risk Gene Discovery in Autism with Machine Learning and Genome-Scale Data. Sci. Rep. 2020, 10, 4569. [Google Scholar] [CrossRef] [PubMed]
- Yenkoyan, K.; Mkhitaryan, M.; Bjørklund, G. Environmental Risk Factors in Autism Spectrum Disorder: A Narrative Review. Curr. Med. Chem. 2024, 31, 2345–2360. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M.; Rutter, M. Neurodevelopmental Disorders. Lancet Psychiatry 2017, 4, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, R.; Hu, Z.-M.; Wang, X.-H.; Gao, X.; Wang, T.; Tang, M.-X. Key Synaptic Pathology in Autism Spectrum Disorder: Genetic Mechanisms and Recent Advances. J. Integr. Neurosci. 2024, 23, 184. [Google Scholar] [CrossRef]
- Shiraishi, T.; Katayama, Y.; Nishiyama, M.; Shoji, H.; Miyakawa, T.; Mizoo, T.; Matsumoto, A.; Hijikata, A.; Shirai, T.; Mayanagi, K.; et al. The Complex Etiology of Autism Spectrum Disorder Due to Missense Mutations of CHD8. Mol. Psychiatry 2024, 29, 2145–2160. [Google Scholar] [CrossRef]
- Basson, M.A. Neurodevelopmental Functions of CHD8: New Insights and Questions. Biochem. Soc. Trans. 2024, 52, 15–27. [Google Scholar] [CrossRef]
- Parfenenko, M.A.; Dantsev, I.S.; Bochenkov, S.V.; Kuramagomedova, R.G.; Vinogradova, N.V.; Afanaseva, M.P.; Groznova, O.S.; Voinova, V.I. Expansion of Phenotypic and Genotypic Data in Autism Spectrum Disorders Due to Variants in the CHD8 Gene. Neurogenetics 2024, 26, 4. [Google Scholar] [CrossRef]
- Phelan, M.C.; Rogers, R.C.; Saul, R.A.; Stapleton, G.A.; Sweet, K.; McDermid, H.; Shaw, S.R.; Claytor, J.; Willis, J.; Kelly, D.P. 22q13 Deletion Syndrome. Am. J. Med. Genet. 2001, 101, 91–99. [Google Scholar] [CrossRef]
- Jung, S.; Park, M. Shank Postsynaptic Scaffolding Proteins in Autism Spectrum Disorder: Mouse Models and Their Dysfunctions in Behaviors, Synapses, and Molecules. Pharmacol. Res. 2022, 182, 106340. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S.; Waga, C. SHANK3 as an Autism Spectrum Disorder-Associated Gene. Brain Dev. 2013, 35, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.; Feng, G. SHANK Proteins: Roles at the Synapse and in Autism Spectrum Disorder. Nat. Rev. Neurosci. 2017, 18, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Tromp, A.; Mowry, B.; Giacomotto, J. Neurexins in Autism and Schizophrenia—A Review of Patient Mutations, Mouse Models and Potential Future Directions. Mol. Psychiatry 2021, 26, 747–760. [Google Scholar] [CrossRef]
- Cooper, J.N.; Mittal, J.; Sangadi, A.; Klassen, D.L.; King, A.M.; Zalta, M.; Mittal, R.; Eshraghi, A.A. Landscape of NRXN1 Gene Variants in Phenotypic Manifestations of Autism Spectrum Disorder: A Systematic Review. J. Clin. Med. 2024, 13, 2067. [Google Scholar] [CrossRef]
- Onay, H.; Kacamak, D.; Kavasoglu, A.; Akgun, B.; Yalcinli, M.; Kose, S.; Ozbaran, B. Mutation Analysis of the NRXN1 Gene in Autism Spectrum Disorders. Balk. J. Med. Genet. 2016, 19, 17–22. [Google Scholar] [CrossRef]
- Armstrong, E.C.; Caruso, A.; Servadio, M.; Andreae, L.C.; Trezza, V.; Scattoni, M.L.; Fernandes, C. Assessing the Developmental Trajectory of Mouse Models of Neurodevelopmental Disorders: Social and Communication Deficits in Mice with Neurexin 1α Deletion. Genes Brain Behav. 2020, 19, e12630. [Google Scholar] [CrossRef]
- Xu, B.; Ho, Y.; Fasolino, M.; Medina, J.; O’Brien, W.T.; Lamonica, J.M.; Nugent, E.; Brodkin, E.S.; Fuccillo, M.V.; Bucan, M.; et al. Allelic Contribution of Nrxn1α to Autism-Relevant Behavioral Phenotypes in Mice. PLoS Genet. 2023, 19, e1010659. [Google Scholar] [CrossRef]
- Trabzuni, D.; Ramasamy, A.; Imran, S.; Walker, R.; Smith, C.; Weale, M.E.; Hardy, J.; Ryten, M.; Consortium, N.A.B.E. Widespread Sex Differences in Gene Expression and Splicing in the Adult Human Brain. Nat. Commun. 2013, 4, 2771. [Google Scholar] [CrossRef]
- Laarakker, M.C.; Reinders, N.R.; Bruining, H.; Ophoff, R.A.; Kas, M.J.H. Sex-Dependent Novelty Response in Neurexin-1α Mutant Mice. PLoS ONE 2012, 7, e31503. [Google Scholar] [CrossRef][Green Version]
- Molloy, C.J.; Cooke, J.; Gatford, N.J.F.; Rivera-Olvera, A.; Avazzadeh, S.; Homberg, J.R.; Grandjean, J.; Fernandes, C.; Shen, S.; Loth, E.; et al. Bridging the Translational Gap: What Can Synaptopathies Tell Us about Autism? Front. Mol. Neurosci. 2023, 16, 1191323. [Google Scholar] [CrossRef]
- Hannenhalli, S.; Kaestner, K.H. The Evolution of Fox Genes and Their Role in Development and Disease. Nat. Rev. Genet. 2009, 10, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Fevre, A.K.L.; Taylor, S.; Malek, N.H.; Horn, D.; Carr, C.W.; Abdul-Rahman, O.A.; O’Donnell, S.; Burgess, T.; Shaw, M.; Gecz, J.; et al. FOXP1 Mutations Cause Intellectual Disability and a Recognizable Phenotype. Am. J. Med. Genet. Part A 2013, 161, 3166–3175. [Google Scholar] [CrossRef] [PubMed]
- Bowers, J.M.; Konopka, G. ASD-Relevant Animal Models of the Foxp Family of Transcription Factors. Autism-Open Access 2012, 10 (Suppl. 1), 10082. [Google Scholar] [CrossRef]
- Hamdan, F.F.; Daoud, H.; Rochefort, D.; Piton, A.; Gauthier, J.; Langlois, M.; Foomani, G.; Dobrzeniecka, S.; Krebs, M.-O.; Joober, R.; et al. De Novo Mutations in FOXP1 in Cases with Intellectual Disability, Autism, and Language Impairment. Am. J. Hum. Genet. 2010, 87, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.E.; Scharff, C. FOXP2 as a Molecular Window into Speech and Language. Trends Genet. 2009, 25, 166–177. [Google Scholar] [CrossRef]
- Vernes, S.C.; Fisher, S.E. Unravelling Neurogenetic Networks Implicated in Developmental Language Disorders. Biochem. Soc. Trans. 2009, 37, 1263–1269. [Google Scholar] [CrossRef]
- Bowers, J.M.; Konopka, G. The Role of the FOXP Family of Transcription Factors in ASD. Dis. Markers 2012, 33, 251–260. [Google Scholar] [CrossRef]
- Cheroni, C.; Caporale, N.; Testa, G. Autism Spectrum Disorder at the Crossroad between Genes and Environment: Contributions, Convergences, and Interactions in ASD Developmental Pathophysiology. Mol. Autism 2020, 11, 69. [Google Scholar] [CrossRef]
- Li, Y.-M.; Ou, J.-J.; Liu, L.; Zhang, D.; Zhao, J.-P.; Tang, S.-Y. Association Between Maternal Obesity and Autism Spectrum Disorder in Offspring: A Meta-Analysis. J. Autism Dev. Disord. 2016, 46, 95–102. [Google Scholar] [CrossRef]
- Krakowiak, P.; Walker, C.K.; Bremer, A.A.; Baker, A.S.; Ozonoff, S.; Hansen, R.L.; Hertz-Picciotto, I. Maternal Metabolic Conditions and Risk for Autism and Other Neurodevelopmental Disorders. Pediatrics 2012, 129, e1121–e1128. [Google Scholar] [CrossRef]
- Tioleco, N.; Silberman, A.E.; Stratigos, K.; Banerjee-Basu, S.; Spann, M.N.; Whitaker, A.H.; Turner, J.B. Prenatal Maternal Infection and Risk for Autism in Offspring: A Meta-analysis. Autism Res. 2021, 14, 1296–1316. [Google Scholar] [CrossRef]
- Al-Beltagi, M.; Saeed, N.K.; Elbeltagi, R.; Bediwy, A.S.; Aftab, S.A.S.; Alhawamdeh, R. Viruses and Autism: A Bi-Mutual Cause and Effect. World J. Virol. 2023, 12, 172–192. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal Immune Activation: Implications for Neuropsychiatric Disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Volk, H.E.; Lurmann, F.; Penfold, B.; Hertz-Picciotto, I.; McConnell, R. Traffic-Related Air Pollution, Particulate Matter, and Autism. JAMA Psychiatry 2013, 70, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.F.; Hertz-Picciotto, I.; Pessah, I.N. Tipping the Balance of Autism Risk: Potential Mechanisms Linking Pesticides and Autism. Environ. Health Perspect. 2012, 120, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Román, P.; Ruiz-González, C.; Rueda-Ruzafa, L.; Cardona, D.; Requena, M.; Alarcón, R. Exposure to Environmental Pesticides and the Risk of Autism Spectrum Disorders: A Population-Based Case-Control Study. Medicina 2024, 60, 479. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Tu, Y.; Song, Y.; Yang, G.; You, M. The Relationship between Pesticide Exposure during Critical Neurodevelopment and Autism Spectrum Disorder: A Narrative Review. Environ. Res. 2022, 203, 111902. [Google Scholar] [CrossRef]
- Gorini, F.; Muratori, F.; Morales, M.A. The Role of Heavy Metal Pollution in Neurobehavioral Disorders: A Focus on Autism. Rev. J. Autism Dev. Disord. 2014, 1, 354–372. [Google Scholar] [CrossRef]
- Christensen, J.; Grønborg, T.K.; Sørensen, M.J.; Schendel, D.; Parner, E.T.; Pedersen, L.H.; Vestergaard, M. Prenatal Valproate Exposure and Risk of Autism Spectrum Disorders and Childhood Autism. JAMA 2013, 309, 1696–1703. [Google Scholar] [CrossRef]
- Harrington, R.A.; Lee, L.; Crum, R.M.; Zimmerman, A.W.; Hertz-Picciotto, I. Serotonin Hypothesis of Autism: Implications for Selective Serotonin Reuptake Inhibitor Use during Pregnancy. Autism Res. 2013, 6, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zou, M.; Sun, C.; Wu, L.; Chen, W.-X. Prenatal Folic Acid Supplements and Offspring’s Autism Spectrum Disorder: A Meta-Analysis and Meta-Regression. J. Autism Dev. Disord. 2022, 52, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Sourander, A.; Upadhyaya, S.; Surcel, H.-M.; Hinkka-Yli-Salomäki, S.; Cheslack-Postava, K.; Silwal, S.; Sucksdorff, M.; McKeague, I.W.; Brown, A.S. Maternal Vitamin D Levels During Pregnancy and Offspring Autism Spectrum Disorder. Biol. Psychiatry 2021, 90, 790–797. [Google Scholar] [CrossRef]
- Wang, C.; Geng, H.; Liu, W.; Zhang, G. Prenatal, Perinatal, and Postnatal Factors Associated with Autism. Medicine 2017, 96, e6696. [Google Scholar] [CrossRef] [PubMed]
- Bittker, S.S.; Bell, K.R. Acetaminophen, Antibiotics, Ear Infection, Breastfeeding, Vitamin D Drops, and Autism: An Epidemiological Study. Neuropsychiatr. Dis. Treat. 2018, 14, 1399–1414. [Google Scholar] [CrossRef]
- Peltier, M.R.; Fassett, M.J.; Mensah, N.A.; Khadka, N.; Yeh, M.Y.; Chiu, V.Y.; Oyelese, Y.; Getahun, D. Postpartum Depression Increases the Risk of Autism Diagnosis in the Offspring. JAACAP Open 2024, 3, 232–244. [Google Scholar] [CrossRef]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global Prevalence of Autism: A Systematic Review Update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Mendes, M.; Chen, D.Z.; Engchuan, W.; Leal, T.P.; Thiruvahindrapuram, B.; Trost, B.; Howe, J.L.; Pellecchia, G.; Nalpathamkalam, T.; Alexandrova, R.; et al. Chromosome X-Wide Common Variant Association Study in Autism Spectrum Disorder. Am. J. Hum. Genet. 2025, 112, 135–153. [Google Scholar] [CrossRef]
- LaSalle, J.M. X Chromosome Inactivation Timing Is Not EXACT: Implications for Autism Spectrum Disorders. Front. Genet. 2022, 13, 864848. [Google Scholar] [CrossRef]
- Berry, A.S.F.; Finucane, B.M.; Myers, S.M.; Walsh, L.K.; Seibert, J.M.; Martin, C.L.; Ledbetter, D.H.; Oetjens, M.T. A Genome-First Study of Sex Chromosome Aneuploidies Provides Evidence of Y Chromosome Dosage Effects on Autism Risk. Nat. Commun. 2024, 15, 8897. [Google Scholar] [CrossRef]
- Ross, J.L.; Bloy, L.; Roberts, T.P.L.; Miller, J.; Xing, C.; Silverman, L.A.; Zinn, A.R. Y Chromosome Gene Copy Number and Lack of Autism Phenotype in a Male with an Isodicentric Y Chromosome and Absent NLGN4Y Expression. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2019, 180, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S. Empathizing, Systemizing, and the Extreme Male Brain Theory of Autism. Prog. Brain Res. 2010, 186, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S. The Extreme Male Brain Theory of Autism. Trends Cogn. Sci. 2002, 6, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Lombardo, M.V.; Auyeung, B.; Ashwin, E.; Chakrabarti, B.; Knickmeyer, R. Why Are Autism Spectrum Conditions More Prevalent in Males? PLoS Biol. 2011, 9, e1001081. [Google Scholar] [CrossRef]
- Xiong, H.; Peterson, J.B.; Scott, S. Amniotic Testosterone and Psychological Sex Differences: A Systematic Review of the Extreme Male Brain Theory. Dev. Rev. 2020, 57, 100922. [Google Scholar] [CrossRef]
- Van Eijk, L.; Zietsch, B.P. Testing the Extreme Male Brain Hypothesis: Is Autism Spectrum Disorder Associated with a More Male-typical Brain? Autism Res. 2021, 14, 1597–1608. [Google Scholar] [CrossRef]
- Schumann, C.M.; Barnes, C.C.; Lord, C.; Courchesne, E. Amygdala Enlargement in Toddlers with Autism Related to Severity of Social and Communication Impairments. Biol. Psychiatry 2009, 66, 942–949. [Google Scholar] [CrossRef]
- Nordahl, C.W.; Scholz, R.; Yang, X.; Buonocore, M.H.; Simon, T.; Rogers, S.; Amaral, D.G. Increased Rate of Amygdala Growth in Children Aged 2 to 4 Years with Autism Spectrum Disorders: A Longitudinal Study. Arch. Gen. Psychiatry 2012, 69, 53–61. [Google Scholar] [CrossRef]
- Worsham, W.; Dalton, S.; Bilder, D.A. The Prenatal Hormone Milieu in Autism Spectrum Disorder. Front. Psychiatry 2021, 12, 655438. [Google Scholar] [CrossRef]
- Iqbal, J.; Huang, G.-D.; Xue, Y.-X.; Yang, M.; Jia, X.-J. Role of Estrogen in Sex Differences in Memory, Emotion and Neuropsychiatric Disorders. Mol. Biol. Rep. 2024, 51, 415. [Google Scholar] [CrossRef]
- Dumais, K.M.; Veenema, A.H. Vasopressin and Oxytocin Receptor Systems in the Brain: Sex Differences and Sex-Specific Regulation of Social Behavior. Front. Neuroendocr. 2016, 40, 1–23. [Google Scholar] [CrossRef]
- Quintana, D.S.; Glaser, B.D.; Kang, H.; Kildal, E.S.M.; Audunsdottir, K.; Sartorius, A.M.; Barth, C. The Interplay of Oxytocin and Sex Hormones. Neurosci. Biobehav. Rev. 2024, 163, 105765. [Google Scholar] [CrossRef] [PubMed]
- Moerkerke, M.; Peeters, M.; de Vries, L.; Daniels, N.; Steyaert, J.; Alaerts, K.; Boets, B. Endogenous Oxytocin Levels in Autism—A Meta-Analysis. Brain Sci. 2021, 11, 1545. [Google Scholar] [CrossRef] [PubMed]
- Josselsohn, A.; Zhao, Y.; Espinoza, D.; Hollander, E. Oxytocin in Neurodevelopmental Disorders: Autism Spectrum Disorder and Prader-Willi Syndrome. Pharmacol. Ther. 2024, 264, 108734. [Google Scholar] [CrossRef]
- Alamoudi, R.A.; Al-Jabri, B.A.; Alsulami, M.A.; Sabbagh, H.J. Prenatal Maternal Stress and the Severity of Autism Spectrum Disorder: A Cross-sectional Study. Dev. Psychobiol. 2023, 65, e22369. [Google Scholar] [CrossRef]
- Ram, S.; Howland, M.A.; Sandman, C.A.; Davis, E.P.; Glynn, L.M. Prenatal Risk for Autism Spectrum Disorder (ASD): Fetal Cortisol Exposure Predicts Child ASD Symptoms. Clin. Psychol. Sci. 2018, 7, 349–361. [Google Scholar] [CrossRef]
- Román, G.C.; Ghassabian, A.; Bongers-Schokking, J.J.; Jaddoe, V.W.V.; Hofman, A.; de Rijke, Y.B.; Verhulst, F.C.; Tiemeier, H. Association of Gestational Maternal Hypothyroxinemia and Increased Autism Risk. Ann. Neurol. 2013, 74, 733–742. [Google Scholar] [CrossRef]
- Mediane, D.H.; Basu, S.; Cahill, E.N.; Anastasiades, P.G. Medial Prefrontal Cortex Circuitry and Social Behaviour in Autism. Neuropharmacology 2024, 260, 110101. [Google Scholar] [CrossRef]
- Prigge, M.B.D.; Lange, N.; Bigler, E.D.; Merkley, T.L.; Neeley, E.S.; Abildskov, T.J.; Froehlich, A.L.; Nielsen, J.A.; Cooperrider, J.R.; Cariello, A.N.; et al. Corpus Callosum Area in Children and Adults with Autism. Res. Autism Spectr. Disord. 2013, 7, 221–234. [Google Scholar] [CrossRef]
- Zikopoulos, B.; Barbas, H. Altered Neural Connectivity in Excitatory and Inhibitory Cortical Circuits in Autism. Front. Hum. Neurosci. 2013, 7, 609. [Google Scholar] [CrossRef]
- Tsang, T.; Green, S.A.; Liu, J.; Lawrence, K.; Jeste, S.; Bookheimer, S.Y.; Dapretto, M. Salience Network Connectivity Is Altered in 6-Week-Old Infants at Heightened Likelihood for Developing Autism. Commun. Biol. 2024, 7, 485. [Google Scholar] [CrossRef]
- Wigdor, E.M.; Weiner, D.J.; Grove, J.; Fu, J.M.; Thompson, W.K.; Carey, C.E.; Baya, N.; van der Merwe, C.; Walters, R.K.; Satterstrom, F.K.; et al. The Female Protective Effect against Autism Spectrum Disorder. Cell Genom. 2022, 2, 100134. [Google Scholar] [CrossRef]
- Werling, D.M.; Parikshak, N.N.; Geschwind, D.H. Gene Expression in Human Brain Implicates Sexually Dimorphic Pathways in Autism Spectrum Disorders. Nat. Commun. 2016, 7, 10717. [Google Scholar] [CrossRef]
- Tubío-Fungueiriño, M.; Cruz, S.; Sampaio, A.; Carracedo, A.; Fernández-Prieto, M. Social Camouflaging in Females with Autism Spectrum Disorder: A Systematic Review. J. Autism Dev. Disord. 2021, 51, 2190–2199. [Google Scholar] [CrossRef]
- Cook, J.; Hull, L.; Crane, L.; Mandy, W. Camouflaging in Autism: A Systematic Review. Clin. Psychol. Rev. 2021, 89, 102080. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.S.; Lundwall, R.A.; Gabrielsen, T.; Cox, J.C.; South, M. Looking Good but Feeling Bad: “Camouflaging” Behaviors and Mental Health in Women with Autistic Traits. Autism 2020, 24, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Rea, H.M.; Øien, R.A.; Shic, F.; Webb, S.J.; Ratto, A.B. Sex Differences on the ADOS-2. J. Autism Dev. Disord. 2023, 53, 2878–2890. [Google Scholar] [CrossRef] [PubMed]
- Rynkiewicz, A.; Schuller, B.; Marchi, E.; Piana, S.; Camurri, A.; Lassalle, A.; Baron-Cohen, S. An Investigation of the ‘Female Camouflage Effect’ in Autism Using a Computerized ADOS-2 and a Test of Sex/Gender Differences. Mol. Autism 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Cirnigliaro, L.; Clericò, L.; Russo, L.C.; Prato, A.; Caruso, M.; Rizzo, R.; Barone, R. Head Circumference Growth in Children with Autism Spectrum Disorder: Trend and Clinical Correlates in the First Five Years of Life. Front. Psychiatry 2024, 15, 1431693. [Google Scholar] [CrossRef]
- Courchesne, E. Brain Development in Autism: Early Overgrowth Followed by Premature Arrest of Growth. Ment. Retard. Dev. Disabil. Res. Rev. 2004, 10, 106–111. [Google Scholar] [CrossRef]
- Fombonne, E.; Rogé, B.; Claverie, J.; Courty, S.; Frémolle, J. Microcephaly and Macrocephaly in Autism. J. Autism Dev. Disord. 1999, 29, 113–119. [Google Scholar] [CrossRef]
- Molnár, Z.; Clowry, G.J.; Šestan, N.; Alzu’bi, A.; Bakken, T.; Hevner, R.F.; Hüppi, P.S.; Kostović, I.; Rakic, P.; Anton, E.S.; et al. New Insights into the Development of the Human Cerebral Cortex. J. Anat. 2019, 235, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.J.; Martin, K.A.C. Neuronal Circuits of the Neocortex. Neuroscience 2004, 27, 419–451. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuys, R. The Insular Cortex: A Review. Prog. Brain Res. 2012, 195, 123–163. [Google Scholar] [CrossRef] [PubMed]
- Nordahl, C.W.; Dierker, D.; Mostafavi, I.; Schumann, C.M.; Rivera, S.M.; Amaral, D.G.; Essen, D.C.V. Cortical Folding Abnormalities in Autism Revealed by Surface-Based Morphometry. J. Neurosci. 2007, 27, 11725–11735. [Google Scholar] [CrossRef]
- Nair, A.; Treiber, J.M.; Shukla, D.K.; Shih, P.; Müller, R.-A. Impaired Thalamocortical Connectivity in Autism Spectrum Disorder: A Study of Functional and Anatomical Connectivity. Brain 2013, 136, 1942–1955. [Google Scholar] [CrossRef]
- Ning, M.; Li, C.; Gao, L.; Fan, J. Core-Symptom-Defined Cortical Gyrification Differences in Autism Spectrum Disorder. Front. Psychiatry 2021, 12, 619367. [Google Scholar] [CrossRef]
- Kohli, J.S.; Kinnear, M.K.; Fong, C.H.; Fishman, I.; Carper, R.A.; Müller, R.-A. Local Cortical Gyrification Is Increased in Children with Autism Spectrum Disorders, but Decreases Rapidly in Adolescents. Cereb. Cortex 2019, 29, 2412–2423. [Google Scholar] [CrossRef]
- Herbert, M.R.; Harris, G.J.; Adrien, K.T.; Ziegler, D.A.; Makris, N.; Kennedy, D.N.; Lange, N.T.; Chabris, C.F.; Bakardjiev, A.; Hodgson, J.; et al. Abnormal Asymmetry in Language Association Cortex in Autism. Ann. Neurol. 2002, 52, 588–596. [Google Scholar] [CrossRef]
- Ni, H.-C.; Lin, H.-Y.; Chen, Y.-C.; Tseng, W.-Y.I.; Gau, S.S.-F. Boys with Autism Spectrum Disorder Have Distinct Cortical Folding Patterns Underpinning Impaired Self-Regulation: A Surface-Based Morphometry Study. Brain Imaging Behav. 2020, 14, 2464–2476. [Google Scholar] [CrossRef]
- Otaru, S.; Lawrence, D.A. Autism: Genetics, Environmental Stressors, Maternal Immune Activation, and the Male Bias in Autism. Explor. Neuroprotective Ther. 2022, 2, 141–161. [Google Scholar] [CrossRef]
- Casanova, M.F.; Buxhoeveden, D.P.; Switala, A.E.; Roy, E. Minicolumnar Pathology in Autism. Neurology 2002, 58, 428–432. [Google Scholar] [CrossRef]
- McKavanagh, R.; Buckley, E.; Chance, S.A. Wider Minicolumns in Autism: A Neural Basis for Altered Processing? Brain 2015, 138, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, B.A.; Prigge, M.B.D.; Nielsen, J.A.; Froehlich, A.L.; Abildskov, T.J.; Anderson, J.S.; Fletcher, P.T.; Zygmunt, K.M.; Travers, B.G.; Lange, N.; et al. Longitudinal Changes in Cortical Thickness in Autism and Typical Development. Brain 2014, 137, 1799–1812. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-H.; Wu, N.; Yuan, X.-B. Toward a Better Understanding of Neuronal Migration Deficits in Autism Spectrum Disorders. Front. Cell Dev. Biol. 2019, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Alberts, I.; Li, X. The Apoptotic Perspective of Autism. Int. J. Dev. Neurosci. 2014, 36, 13–18. [Google Scholar] [CrossRef]
- Unnisa, A.; Greig, N.H.; Kamal, M.A. Modelling the Interplay Between Neuron-Glia Cell Dysfunction and Glial Therapy in Autism Spectrum Disorder. Curr. Neuropharmacol. 2023, 21, 547–559. [Google Scholar] [CrossRef]
- Iidaka, T.; Kogata, T.; Mano, Y.; Komeda, H. Thalamocortical Hyperconnectivity and Amygdala-Cortical Hypoconnectivity in Male Patients with Autism Spectrum Disorder. Front. Psychiatry 2019, 10, 252. [Google Scholar] [CrossRef]
- Subramanian, K.; Brandenburg, C.; Orsati, F.; Soghomonian, J.; Hussman, J.P.; Blatt, G.J. Basal Ganglia and Autism—A Translational Perspective. Autism Res. 2017, 10, 1751–1775. [Google Scholar] [CrossRef]
- Marcos, G.V.T.d.M.; Feitosa, D.D.M.; Paiva, K.M.; Oliveira, R.F.; da Rocha, G.S.; Guerra, L.M. de M.; Araújo, D.P. de; Goes, H.M.; Costa, S.; Oliveira, L.C. de; et al. Volumetric Alterations in the Basal Ganglia in Autism Spectrum Disorder: A Systematic Review. Int. J. Dev. Neurosci. 2024, 84, 163–176. [Google Scholar] [CrossRef]
- Brambilla, P.; Hardan, A.; di Nemi, S.U.; Perez, J.; Soares, J.C.; Barale, F. Brain Anatomy and Development in Autism: Review of Structural MRI Studies. Brain Res. Bull. 2003, 61, 557–569. [Google Scholar] [CrossRef]
- Hollander, E.; Anagnostou, E.; Chaplin, W.; Esposito, K.; Haznedar, M.M.; Licalzi, E.; Wasserman, S.; Soorya, L.; Buchsbaum, M. Striatal Volume on Magnetic Resonance Imaging and Repetitive Behaviors in Autism. Biol. Psychiatry 2005, 58, 226–232. [Google Scholar] [CrossRef]
- Qiu, T.; Chang, C.; Li, Y.; Qian, L.; Xiao, C.Y.; Xiao, T.; Xiao, X.; Xiao, Y.H.; Chu, K.K.; Lewis, M.H.; et al. Two Years Changes in the Development of Caudate Nucleus Are Involved in Restricted Repetitive Behaviors in 2–5-Year-Old Children with Autism Spectrum Disorder. Dev. Cogn. Neurosci. 2016, 19, 137–143. [Google Scholar] [CrossRef]
- Nickl-Jockschat, T.; Habel, U.; Michel, T.M.; Manning, J.; Laird, A.R.; Fox, P.T.; Schneider, F.; Eickhoff, S.B. Brain Structure Anomalies in Autism Spectrum Disorder—A Meta-analysis of VBM Studies Using Anatomic Likelihood Estimation. Hum. Brain Mapp. 2012, 33, 1470–1489. [Google Scholar] [CrossRef]
- Dichter, G.S. Functional Magnetic Resonance Imaging of Autism Spectrum Disorders. Dialogues Clin. Neurosci. 2012, 14, 319–351. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Ring, H.A.; Bullmore, E.T.; Wheelwright, S.; Ashwin, C.; Williams, S.C.R. The Amygdala Theory of Autism. Neurosci. Biobehav. Rev. 2000, 24, 355–364. [Google Scholar] [CrossRef]
- Wang, S.; Li, X. A Revisit of the Amygdala Theory of Autism: Twenty Years After. Neuropsychologia 2023, 183, 108519. [Google Scholar] [CrossRef]
- Lee, J.K.; Andrews, D.S.; Ozturk, A.; Solomon, M.; Rogers, S.; Amaral, D.G.; Nordahl, C.W. Altered Development of Amygdala-Connected Brain Regions in Males and Females with Autism. J. Neurosci. 2022, 42, 6145–6155. [Google Scholar] [CrossRef]
- Corbett, B.A.; Carmean, V.; Ravizza, S.; Wendelken, C.; Henry, M.L.; Carter, C.; Rivera, S.M. A Functional and Structural Study of Emotion and Face Processing in Children with Autism. Psychiatry Res. Neuroimaging 2009, 173, 196–205. [Google Scholar] [CrossRef]
- Schulkin, J. Autism and the Amygdala: An Endocrine Hypothesis. Brain Cogn. 2007, 65, 87–99. [Google Scholar] [CrossRef]
- Sumadevi, K.T. The Hippocampus: Anatomy, Function and Clinical Correlation. Sri Lanka Anat. J. 2024, 8, 6–20. [Google Scholar] [CrossRef]
- Banker, S.M.; Gu, X.; Schiller, D.; Foss-Feig, J.H. Hippocampal Contributions to Social and Cognitive Deficits in Autism Spectrum Disorder. Trends Neurosci. 2021, 44, 793–807. [Google Scholar] [CrossRef]
- Nicolson, R.; DeVito, T.J.; Vidal, C.N.; Sui, Y.; Hayashi, K.M.; Drost, D.J.; Williamson, P.C.; Rajakumar, N.; Toga, A.W.; Thompson, P.M. Detection and Mapping of Hippocampal Abnormalities in Autism. Psychiatry Res. Neuroimaging 2006, 148, 11–21. [Google Scholar] [CrossRef]
- Groen, W.; Teluij, M.; Buitelaar, J.; Tendolkar, I. Amygdala and Hippocampus Enlargement During Adolescence in Autism. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 552–560. [Google Scholar] [CrossRef]
- Richards, R.; Greimel, E.; Kliemann, D.; Koerte, I.K.; Schulte-Körne, G.; Reuter, M.; Wachinger, C. Increased Hippocampal Shape Asymmetry and Volumetric Ventricular Asymmetry in Autism Spectrum Disorder. NeuroImage Clin. 2020, 26, 102207. [Google Scholar] [CrossRef]
- Schumann, C.M.; Hamstra, J.; Goodlin-Jones, B.L.; Lotspeich, L.J.; Kwon, H.; Buonocore, M.H.; Lammers, C.R.; Reiss, A.L.; Amaral, D.G. The Amygdala Is Enlarged in Children But Not Adolescents with Autism; the Hippocampus Is Enlarged at All Ages. J. Neurosci. 2004, 24, 6392–6401. [Google Scholar] [CrossRef]
- Lawrence, Y.A.; Kemper, T.L.; Bauman, M.L.; Blatt, G.J. Parvalbumin-, Calbindin-, and Calretinin-immunoreactive Hippocampal Interneuron Density in Autism. Acta Neurol. Scand. 2010, 121, 99–108. [Google Scholar] [CrossRef]
- Long, J.; Li, H.; Liu, Y.; Liao, X.; Tang, Z.; Han, K.; Chen, J.; Zhang, H. Insights into the Structure and Function of the Hippocampus: Implications for the Pathophysiology and Treatment of Autism Spectrum Disorder. Front. Psychiatry 2024, 15, 1364858. [Google Scholar] [CrossRef]
- Kember, J.; Patenaude, P.; Sweatman, H.; Schaik, L.V.; Tabuenca, Z.; Chai, X.J. Specialization of Anterior and Posterior Hippocampal Functional Connectivity Differs in Autism. Autism Res. 2024, 17, 1126–1139. [Google Scholar] [CrossRef]
- Liu, J.; Okada, N.J.; Cummings, K.K.; Jung, J.; Patterson, G.; Bookheimer, S.Y.; Jeste, S.S.; Dapretto, M. Emerging Atypicalities in Functional Connectivity of Language-Related Networks in Young Infants at High Familial Risk for ASD. Dev. Cogn. Neurosci. 2020, 45, 100814. [Google Scholar] [CrossRef]
- Saalmann, Y.B.; Kastner, S. The Cognitive Thalamus. Front. Syst. Neurosci. 2015, 9, 39. [Google Scholar] [CrossRef]
- Tsatsanis, K.D.; Rourke, B.P.; Klin, A.; Volkmar, F.R.; Cicchetti, D.; Schultz, R.T. Reduced Thalamic Volume in High-Functioning Individuals with Autism. Biol. Psychiatry 2003, 53, 121–129. [Google Scholar] [CrossRef]
- Hardan, A.Y.; Girgis, R.R.; Adams, J.; Gilbert, A.R.; Keshavan, M.S.; Minshew, N.J. Abnormal Brain Size Effect on the Thalamus in Autism. Psychiatry Res. Neuroimaging 2006, 147, 145–151. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Reduced Local and Increased Long-Range Functional Connectivity of the Thalamus in Autism Spectrum Disorder. Cereb. Cortex 2019, 29, 573–585. [Google Scholar] [CrossRef]
- Caria, A.; Ciringione, L.; de Falco, S. Morphofunctional Alterations of the Hypothalamus and Social Behavior in Autism Spectrum Disorders. Brain Sci. 2020, 10, 435. [Google Scholar] [CrossRef]
- Kurth, F.; Narr, K.L.; Woods, R.P.; O’Neill, J.; Alger, J.R.; Caplan, R.; McCracken, J.T.; Toga, A.W.; Levitt, J.G. Diminished Gray Matter Within the Hypothalamus in Autism Disorder: A Potential Link to Hormonal Effects? Biol. Psychiatry 2011, 70, 278–282. [Google Scholar] [CrossRef]
- Romano, A.; Tempesta, B.; Bonaventura, M.V.M.D.; Gaetani, S. From Autism to Eating Disorders and More: The Role of Oxytocin in Neuropsychiatric Disorders. Front. Neurosci. 2016, 9, 497. [Google Scholar] [CrossRef]
- Courchesne, E. Brainstem, Cerebellar and Limbic Neuroanatomical Abnormalities in Autism. Curr. Opin. Neurobiol. 1997, 7, 269–278. [Google Scholar] [CrossRef]
- Jou, R.J.; Frazier, T.W.; Keshavan, M.S.; Minshew, N.J.; Hardan, A.Y. A Two-Year Longitudinal Pilot MRI Study of the Brainstem in Autism. Behav. Brain Res. 2013, 251, 163–167. [Google Scholar] [CrossRef]
- Smith, A.; Storti, S.; Lukose, R.; Kulesza, R.J., Jr. Structural and Functional Aberrations of the Auditory Brainstem in Autism Spectrum Disorder. J. Osteopat. Med. 2019, 119, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Seif, A.; Shea, C.; Schmid, S.; Stevenson, R.A. A Systematic Review of Brainstem Contributions to Autism Spectrum Disorder. Front. Integr. Neurosci. 2021, 15, 760116. [Google Scholar] [CrossRef] [PubMed]
- Pillion, J.P.; Boatman-Reich, D.; Gordon, B. Auditory Brainstem Pathology in Autism Spectrum Disorder: A Review. Cogn. Behav. Neurol. Off. J. Soc. Behav. Cogn. Neurol. 2018, 31, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Dadalko, O.I.; Travers, B.G. Evidence for Brainstem Contributions to Autism Spectrum Disorders. Front. Integr. Neurosci. 2018, 12, 47. [Google Scholar] [CrossRef]
- Baizer, J.S. Functional and Neuropathological Evidence for a Role of the Brainstem in Autism. Front. Integr. Neurosci. 2021, 15, 748977. [Google Scholar] [CrossRef]
- Courchesne, E.; Yeung-Courchesne, R.; Press, G.A.; Hesselink, J.R.; Jernigan, T.L. Hypoplasia of Cerebellar Vermal Lobules VI and VII in Autism. N. Engl. J. Med. 1988, 318, 1349–1354. [Google Scholar] [CrossRef]
- Stanfield, A.C.; McIntosh, A.M.; Spencer, M.D.; Philip, R.; Gaur, S.; Lawrie, S.M. Towards a Neuroanatomy of Autism: A Systematic Review and Meta-Analysis of Structural Magnetic Resonance Imaging Studies. Eur. Psychiatry 2008, 23, 289–299. [Google Scholar] [CrossRef]
- Courchesne, E.; Saitoh, O.; Yeung-Courchesne, R.; Press, G.A.; Lincoln, A.J.; Haas, R.H.; Schreibman, L. Abnormality of Cerebellar Vermian Lobules VI and VII in Patients with Infantile Autism: Identification of Hypoplastic and Hyperplastic Subgroups with MR Imaging. AJR Am. J. Roentgenol. 1994, 162, 123–130. [Google Scholar] [CrossRef]
- Lin, C.-W.; Lin, H.-Y.; Lo, Y.-C.; Chen, Y.-J.; Hsu, Y.-C.; Chen, Y.-L.; Tseng, W.-Y.I.; Gau, S.S.-F. Alterations in White Matter Microstructure and Regional Volume Are Related to Motor Functions in Boys with Autism Spectrum Disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 90, 76–83. [Google Scholar] [CrossRef]
- Zhang, F.; Savadjiev, P.; Cai, W.; Song, Y.; Rathi, Y.; Tunç, B.; Parker, D.; Kapur, T.; Schultz, R.T.; Makris, N.; et al. Whole Brain White Matter Connectivity Analysis Using Machine Learning: An Application to Autism. NeuroImage 2018, 172, 826–837. [Google Scholar] [CrossRef]
- Mostofsky, S.H.; Powell, S.K.; Simmonds, D.J.; Goldberg, M.C.; Caffo, B.; Pekar, J.J. Decreased Connectivity and Cerebellar Activity in Autism during Motor Task Performance. Brain 2009, 132, 2413–2425. [Google Scholar] [CrossRef]
- Wang, S.S.-H.; Kloth, A.D.; Badura, A. The Cerebellum, Sensitive Periods, and Autism. Neuron 2014, 83, 518–532. [Google Scholar] [CrossRef]
- Buckner, R.L.; Krienen, F.M.; Castellanos, A.; Diaz, J.C.; Yeo, B.T.T. The Organization of the Human Cerebellum Estimated by Intrinsic Functional Connectivity. J. Neurophysiol. 2011, 106, 2322–2345. [Google Scholar] [CrossRef] [PubMed]
- Hampson, D.R.; Blatt, G.J. Autism Spectrum Disorders and Neuropathology of the Cerebellum. Front. Neurosci. 2015, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Aldinger, K.A.; Ashwood, P.; Bauman, M.L.; Blaha, C.D.; Blatt, G.J.; Chauhan, A.; Chauhan, V.; Dager, S.R.; Dickson, P.E.; et al. Consensus Paper: Pathological Role of the Cerebellum in Autism. Cerebellum 2012, 11, 777–807. [Google Scholar] [CrossRef] [PubMed]
- Bauman, M.L.; Kemper, T.L. Neuroanatomic Observations of the Brain in Autism: A Review and Future Directions. Int. J. Dev. Neurosci. 2005, 23, 183–187. [Google Scholar] [CrossRef]
- di Pellegrino, G.; Fadiga, L.; Fogassi, L.; Gallese, V.; Rizzolatti, G. Understanding Motor Events: A Neurophysiological Study. Exp. Brain Res. 1992, 91, 176–180. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Fabbri-Destro, M. Mirror Neurons: From Discovery to Autism. Exp. Brain Res. 2010, 200, 223–237. [Google Scholar] [CrossRef]
- Iacoboni, M.; Dapretto, M. The Mirror Neuron System and the Consequences of Its Dysfunction. Nat. Rev. Neurosci. 2006, 7, nrn2024. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Sinigaglia, C. The Mirror Mechanism: A Basic Principle of Brain Function. Nat. Rev. Neurosci. 2016, 17, 757–765. [Google Scholar] [CrossRef]
- Fogassi, L.; Ferrari, P.F.; Gesierich, B.; Rozzi, S.; Chersi, F.; Rizzolatti, G. Parietal Lobe: From Action Organization to Intention Understanding. Science 2005, 308, 662–667. [Google Scholar] [CrossRef]
- Arbib, M.A. From Monkey-like Action Recognition to Human Language: An Evolutionary Framework for Neurolinguistics. Behav. Brain Sci. 2005, 28, 105–124. [Google Scholar] [CrossRef]
- Lepage, J.; Théoret, H. EEG Evidence for the Presence of an Action Observation–Execution Matching System in Children. Eur. J. Neurosci. 2006, 23, 2505–2510. [Google Scholar] [CrossRef]
- Iacoboni, M.; Mazziotta, J.C. Mirror Neuron System: Basic Findings and Clinical Applications. Ann. Neurol. 2007, 62, 213–218. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Oberman, L.M. Broken Mirrors: A Theory of Autism. Sci. Am. 2007, 17, 20–29. [Google Scholar] [CrossRef]
- Oberman, L.M.; McCleery, J.P.; Hubbard, E.M.; Bernier, R.; Wiersema, J.R.; Raymaekers, R.; Pineda, J.A. Developmental Changes in Mu Suppression to Observed and Executed Actions in Autism Spectrum Disorders. Soc. Cogn. Affect. Neurosci. 2013, 8, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Dapretto, M.; Davies, M.S.; Pfeifer, J.H.; Scott, A.A.; Sigman, M.; Bookheimer, S.Y.; Iacoboni, M. Understanding Emotions in Others: Mirror Neuron Dysfunction in Children with Autism Spectrum Disorders. Nat. Neurosci. 2006, 9, 28–30. [Google Scholar] [CrossRef]
- Enticott, P.G.; Kennedy, H.A.; Rinehart, N.J.; Tonge, B.J.; Bradshaw, J.L.; Taffe, J.R.; Daskalakis, Z.J.; Fitzgerald, P.B. Mirror Neuron Activity Associated with Social Impairments but Not Age in Autism Spectrum Disorder. Biol. Psychiatry 2012, 71, 427–433. [Google Scholar] [CrossRef]
- Hamilton, A.F. de C. Emulation and Mimicry for Social Interaction: A Theoretical Approach to Imitation in Autism. Q. J. Exp. Psychol. 2008, 61, 101–115. [Google Scholar] [CrossRef]
- Wang, Y.; Hamilton, A.F. de C. Social Top-down Response Modulation (STORM): A Model of the Control of Mimicry in Social Interaction. Front. Hum. Neurosci. 2012, 6, 153. [Google Scholar] [CrossRef]
- Ingersoll, B.; Lalonde, K. The Impact of Object and Gesture Imitation Training on Language Use in Children With Autism Spectrum Disorder. J. Speech Lang. Hear. Res. 2010, 53, 1040–1051. [Google Scholar] [CrossRef]
- Pineda, J.A.; Carrasco, K.; Datko, M.; Pillen, S.; Schalles, M. Neurofeedback Training Produces Normalization in Behavioural and Electrophysiological Measures of High-Functioning Autism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130183. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; Rubeis, S.D.; Drapeau, E.; Buxbaum, J.D.; et al. Autism Spectrum Disorder: Neuropathology and Animal Models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Yang, M.; Lord, C.; Crawley, J.N. Behavioural Phenotyping Assays for Mouse Models of Autism. Nat. Rev. Neurosci. 2010, 11, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, H.Y. MeCP2 Dysfunction in Humans and Mice. J. Child Neurol. 2005, 20, 736–740. [Google Scholar] [CrossRef]
- Manzo, J.; Hernández-Aguilar, M.E.; Toledo-Cárdenas, M.R.; Herrera-Covarrubias, D.; Coria-Avila, G.A. Dysregulation of Neural Tube Vascular Development as an Aetiological Factor in Autism Spectrum Disorder: Insights from Valproic Acid Exposure. J. Physiol. 2025, ahead of print. [Google Scholar] [CrossRef]
- Zhou, Y.; Sharma, J.; Ke, Q.; Landman, R.; Yuan, J.; Chen, H.; Hayden, D.S.; Fisher, J.W.; Jiang, M.; Menegas, W.; et al. Atypical Behaviour and Connectivity in SHANK3-Mutant Macaques. Nature 2019, 570, 326–331. [Google Scholar] [CrossRef]
- Wu, S.-H.; Li, X.; Qin, D.-D.; Zhang, L.-H.; Cheng, T.-L.; Chen, Z.-F.; Nie, B.-B.; Ren, X.-F.; Wu, J.; Wang, W.-C.; et al. Induction of Core Symptoms of Autism Spectrum Disorder by in Vivo CRISPR/Cas9-Based Gene Editing in the Brain of Adolescent Rhesus Monkeys. Sci. Bull. 2021, 66, 937–946. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Zhang, J.-T.; Cai, Y.-J.; Cheng, T.-L.; Cheng, C.; Wang, Y.; Zhang, C.-C.; Nie, Y.-H.; Chen, Z.-F.; et al. Autism-like Behaviours and Germline Transmission in Transgenic Monkeys Overexpressing MeCP2. Nature 2016, 530, 98–102. [Google Scholar] [CrossRef]
- Velázquez-Landa, X.; Carrillo, P.; Coria-Avila, G.A.; Herrera-Covarrubias, D.; García, L.I.; Toledo-Cárdenas, M.R.; Hernández-Aguilar, M.E.; Manzo, J. Zebrafish Sexual Behavior in Plain and Enriched Environments: Parameters in the Valproate Model of Autism. Fishes 2023, 8, 156. [Google Scholar] [CrossRef]
- Sakai, C.; Ijaz, S.; Hoffman, E.J. Zebrafish Models of Neurodevelopmental Disorders: Past, Present, and Future. Front. Mol. Neurosci. 2018, 11, 294. [Google Scholar] [CrossRef]
- Van Alphen, B.; van Swinderen, B. Drosophila Strategies to Study Psychiatric Disorders. Brain Res. Bull. 2013, 92, 1–11. [Google Scholar] [CrossRef]
- Panaitof, S.C. A Songbird Animal Model for Dissecting the Genetic Bases of Autism Spectrum Disorder. Dis. Markers 2012, 33, 241–249. [Google Scholar] [CrossRef]
- Cruz-Magos, O.E.; Herrera-Meza, G.; García, L.I.; Coria-Avila, G.A.; Herrera-Covarrubias, D.; Toledo-Cárdenas, M.R.; Hernández-Aguilar, M.E.; Manzo, J. Multiunit Recording of Cerebellar Cortex in Autistic Male Rats during Social Interaction in Enriched Environments. NeuroSci 2023, 4, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Sale, A.; Berardi, N.; Maffei, L. Environment and Brain Plasticity: Towards an Endogenous Pharmacotherapy. Physiol. Rev. 2014, 94, 189–234. [Google Scholar] [CrossRef] [PubMed]
- Lessmann, V.; Gottmann, K.; Malcangio, M. Neurotropin Secretion: Current Facts and Future Prospects. Prog. Neurobiol. 2003, 69, 341–374. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Ann. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Spoto, G.; Butera, A.; Albertini, M.L.; Consoli, C.; Ceraolo, G.; Nicotera, A.G.; Di Rosa, G. The Ambiguous Role of Growth Factors in Autism: What Do We Really Know? Int. J. Mol. Sci. 2025, 26, 1607. [Google Scholar] [CrossRef]
- Liu, S.-H.; Shi, X.-J.; Fan, F.-C.; Cheng, Y. Peripheral Blood Neurotrophic Factor Levels in Children with Autism Spectrum Disorder: A Meta-Analysis. Sci. Rep. 2021, 11, 15. [Google Scholar] [CrossRef]
- Ma, K.; Taylor, C.; Williamson, M.; Newton, S.S.; Qin, L. Diminished Activity-Dependent BDNF Signaling Differentially Causes Autism-Like Behavioral Deficits in Male and Female Mice. Front. Psychiatry 2023, 14, 1182472. [Google Scholar] [CrossRef]
- Almeida, L.E.F.; Roby, C.D.; Krueger, B.K. Increased BDNF Expression in Fetal Brain in the Valproic Acid Model of Autism. Mol. Cell. Neurosci. 2014, 59, 57–62. [Google Scholar] [CrossRef]
- Mlynarska, E.; Barszcz, E.; Budny, E.; Gajewska, A.; Kopec, K.; Wasiak, J.; Rysz, J.; Franczyk, B. The Gut-Brain-Microbiota Connection and Its Role in Autism Spectrum Disorders. Nutrients 2025, 17, 1135. [Google Scholar] [CrossRef]
- Takyi, E.; Nirmalkar, K.; Adams, J.; Krajmalnik-Brown, R. Interventions Targeting the Gut Microbiota and Their Possible Effect on Gastrointestinal and Neurobehavioral Symptoms in Autism Spectrum Disorder. Gut Microbes 2025, 17, 2499580. [Google Scholar] [CrossRef]
- Amadi, C.N.; Orish, C.N.; Frazzoli, C.; Orisakwe, O.E. Dietary Interventions for Autism Spectrum Disorder: An Updated Systematic Review of Human Studies. Psychiatriki 2022, 33, 228–242. [Google Scholar] [CrossRef]
- Yang, J.; Fu, X.; Liao, X.; Li, Y. Effects of Gut Microbial-Based Treatments on Gut Microbiota, Behavioral Symptoms, and Gastrointestinal Symptoms in Children with Autism Spectrum Disorder: A Systematic Review. Psychiatry Res. 2020, 293, 113471. [Google Scholar] [CrossRef]
| Brain Area | General Role | Relevance in ASD |
|---|---|---|
| Cerebral Cortex | High order cognitive processes | Abnormal folding and altered connectivity |
| Basal Ganglia | Motor control, higher-order cognition, speech | Volumetric changes and reduced connectivity |
| Amygdala | Emotion regulation, fear processing, social salience | Enlargement and hypoactivation |
| Hippocampus | Memory formation, reasoning, social interaction | Structural differences and altered connectivity |
| Thalamus | Relay center of connectivity between cortical and subcortical regions | Atypical neural connectivity |
| Hypothalamus | Neuroendocrine regulation of social behavior | Reduced neuronal density and alterations in the oxytocinergic system |
| Brainstem | Sensory processing and autonomic regulation | Reduced volume, abnormal auditory processing, and autonomic dysregulation |
| Cerebellum | Motor coordination, cognitive and social modulation | Reduced and abnormal Purkinje neurons, with structural and functional anomalies |
| Model | Core Assumption | Relevance in ASD |
|---|---|---|
| Broken Mirror Hypothesis | Dysfunction of the mirror neuron system (MNS) leads to social and communicative alterations | Initially influential in linking neural activity to autism-related social deficits |
| EP-M Model (Emulation–Prediction Model) | The MNS is intact in ASD; but deficits occur in prediction and mimicry-based imitation | Provides a more dynamic account of action understanding, linking MNS activity to broader predictive processing systems |
| STORM Model (Social Top-Down Response Modulation) | The MNS is intact in ASD; impairments arise from atypical top-down modulation of MNS by prefrontal control systems | Suggests that social difficulties result from impaired connectivity rather than a broken MNS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzo, J.; Hernández-Aguilar, M.E.; Toledo-Cárdenas, M.R.; Herrera-Covarrubias, D.; Coria-Avila, G.A.; Libreros-Jiménez, H.M.; Fernández-Cañedo, L.; Ortega-Pineda, L.A. The Long and Winding Road to Understanding Autism. NeuroSci 2025, 6, 84. https://doi.org/10.3390/neurosci6030084
Manzo J, Hernández-Aguilar ME, Toledo-Cárdenas MR, Herrera-Covarrubias D, Coria-Avila GA, Libreros-Jiménez HM, Fernández-Cañedo L, Ortega-Pineda LA. The Long and Winding Road to Understanding Autism. NeuroSci. 2025; 6(3):84. https://doi.org/10.3390/neurosci6030084
Chicago/Turabian StyleManzo, Jorge, María Elena Hernández-Aguilar, María Rebeca Toledo-Cárdenas, Deissy Herrera-Covarrubias, Genaro A. Coria-Avila, Hugo M. Libreros-Jiménez, Lauro Fernández-Cañedo, and Lizbeth A. Ortega-Pineda. 2025. "The Long and Winding Road to Understanding Autism" NeuroSci 6, no. 3: 84. https://doi.org/10.3390/neurosci6030084
APA StyleManzo, J., Hernández-Aguilar, M. E., Toledo-Cárdenas, M. R., Herrera-Covarrubias, D., Coria-Avila, G. A., Libreros-Jiménez, H. M., Fernández-Cañedo, L., & Ortega-Pineda, L. A. (2025). The Long and Winding Road to Understanding Autism. NeuroSci, 6(3), 84. https://doi.org/10.3390/neurosci6030084










