Neurophysiology of Gaze Direction as Poly-Equilibrium
Abstract
1. Introduction
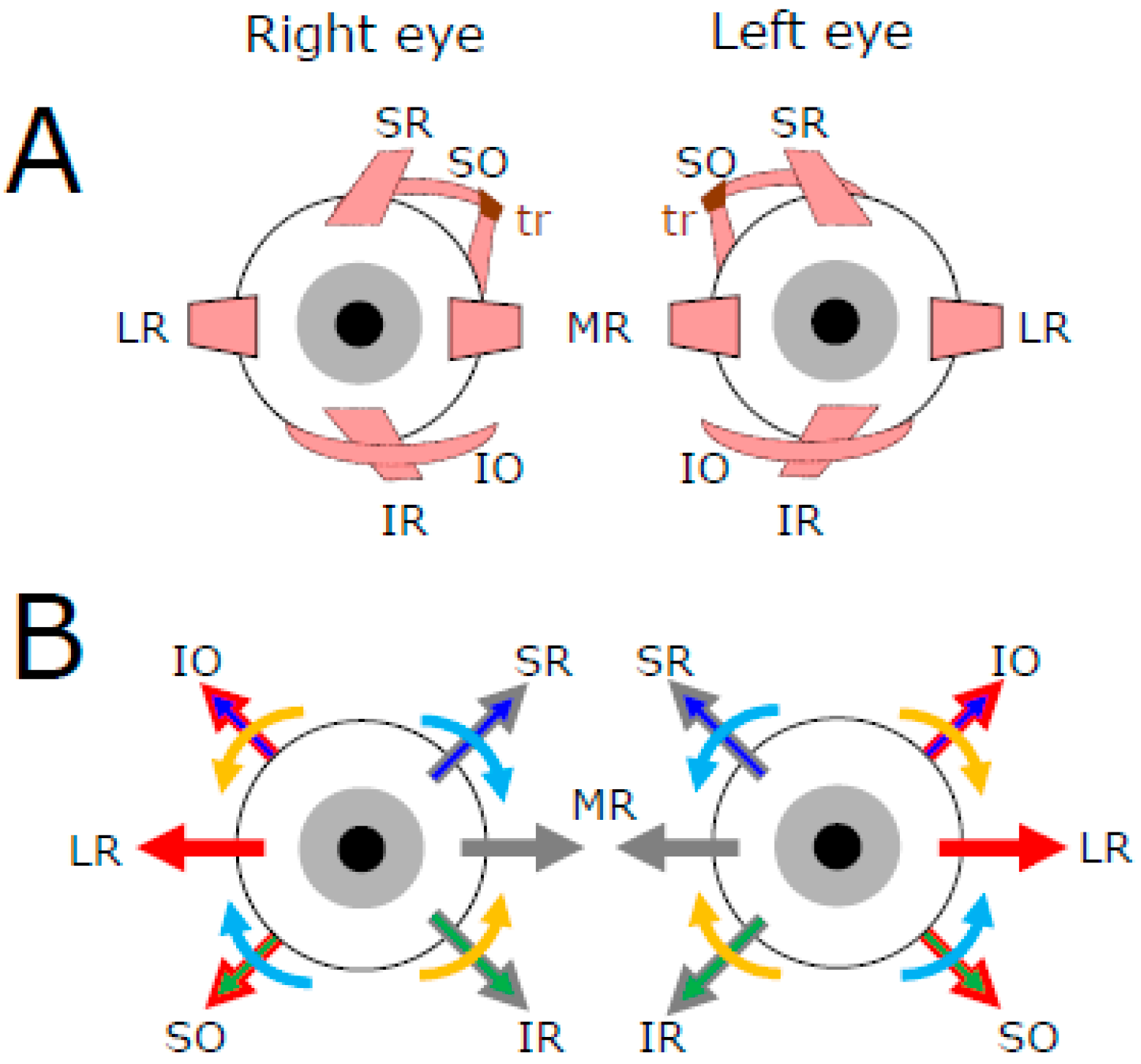
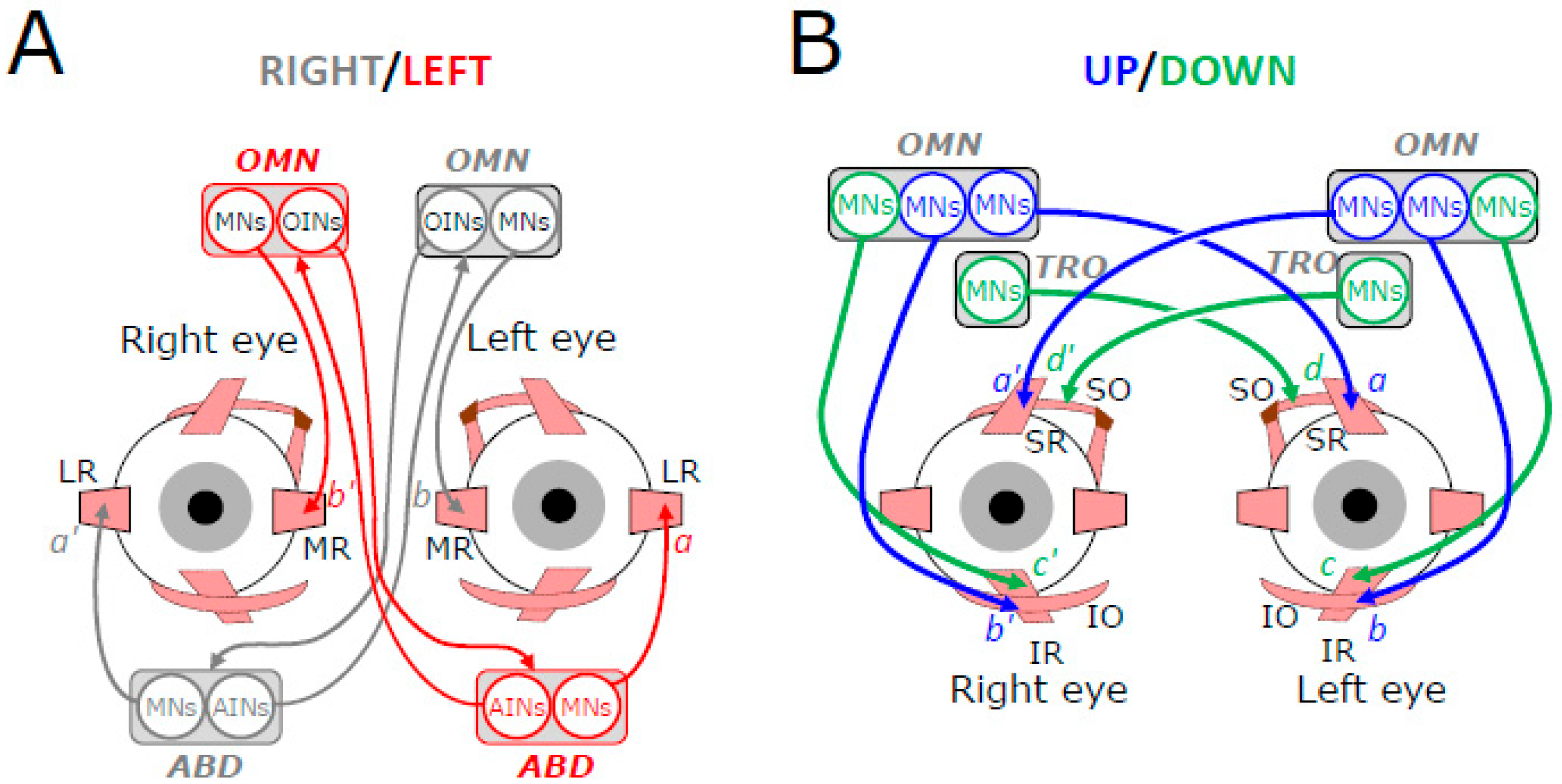
2. Motoneuronal Control of Extraocular Muscles Contraction
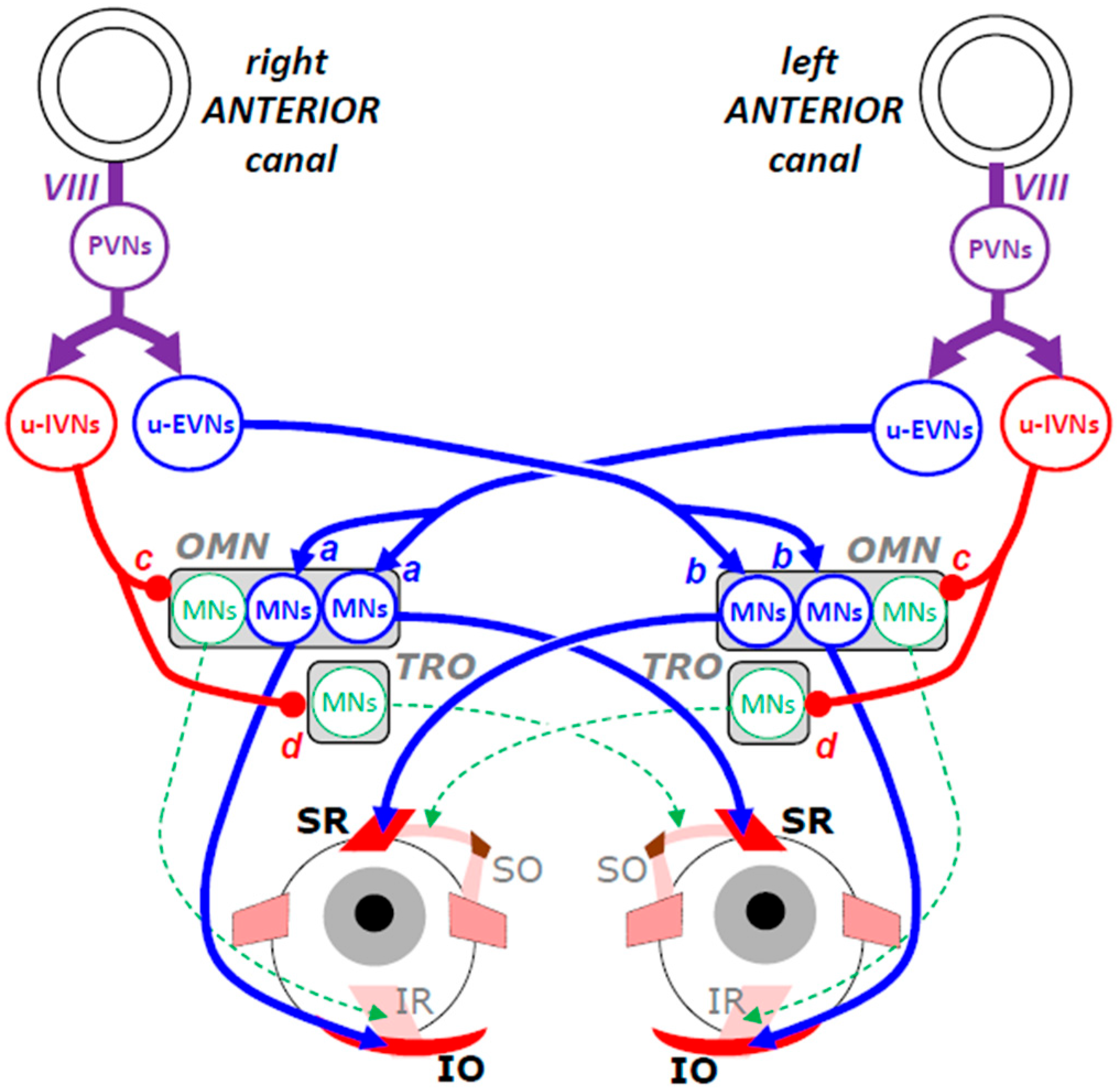
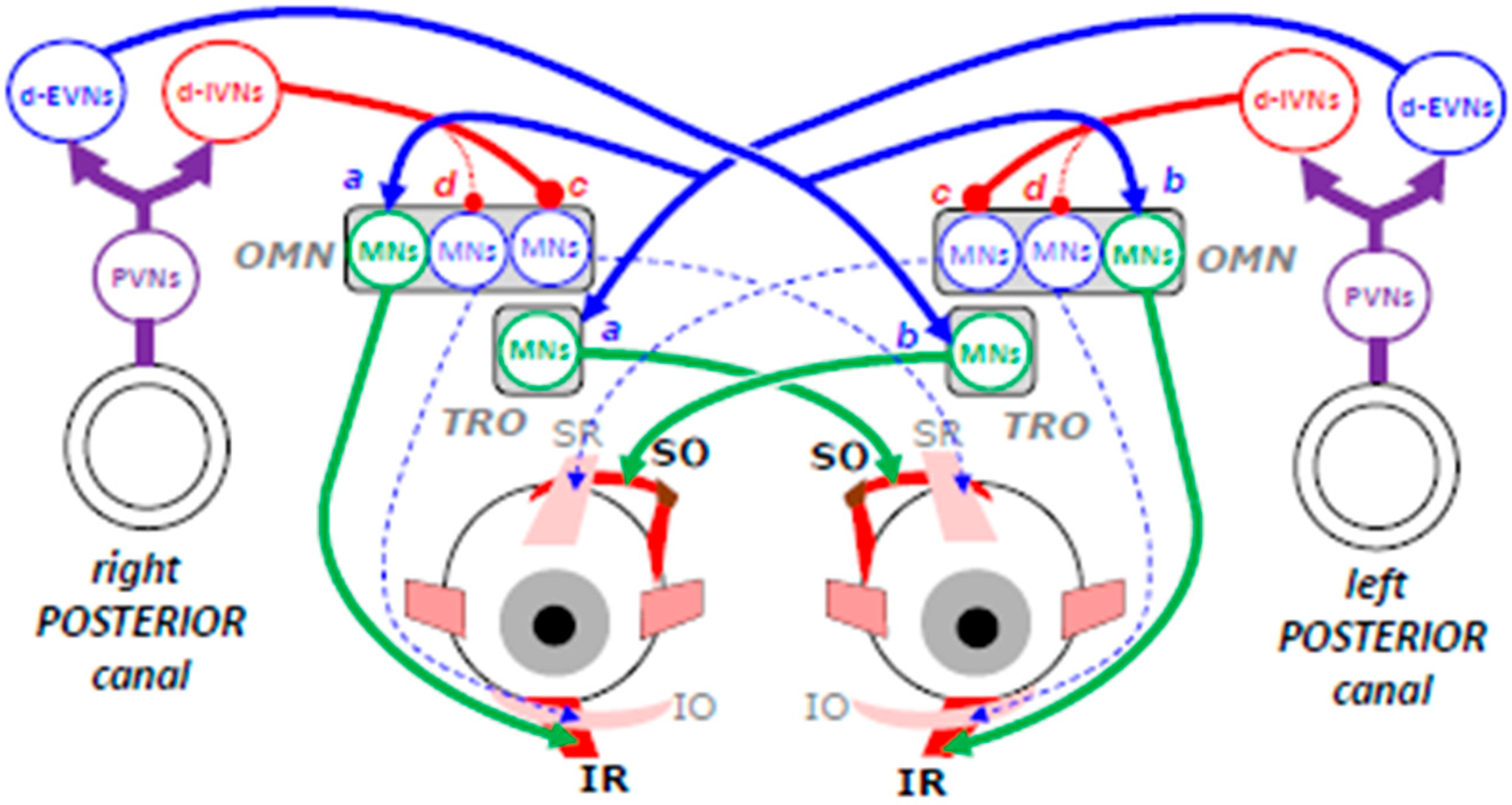
3. Vestibular Inputs to Extraocular Motoneurons
3.1. Inputs from Lateral Semicircular Canals
3.2. Inputs from Anterior Semicircular Canals
3.3. Inputs from Posterior Semicircular Canals
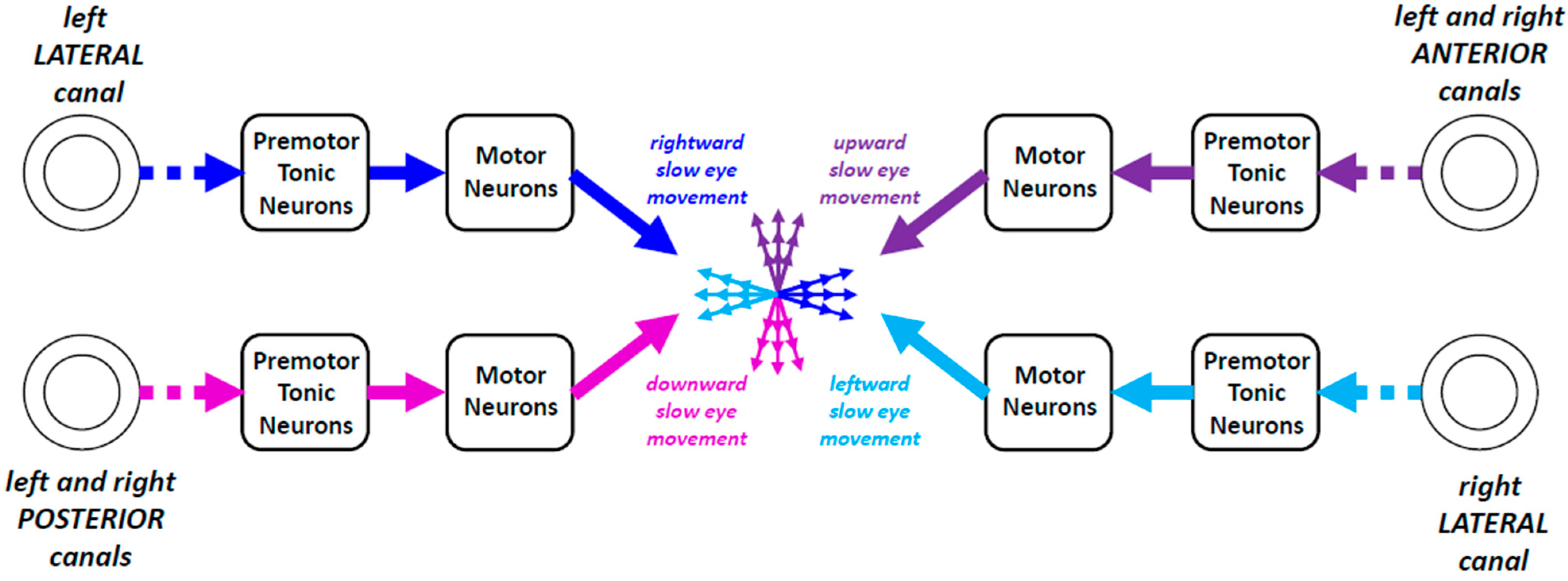
3.4. Gaze Direction as Equilibrium of Vestibulo-Oculomotor Commands
4. Visual Inputs to Extraocular Motoneurons
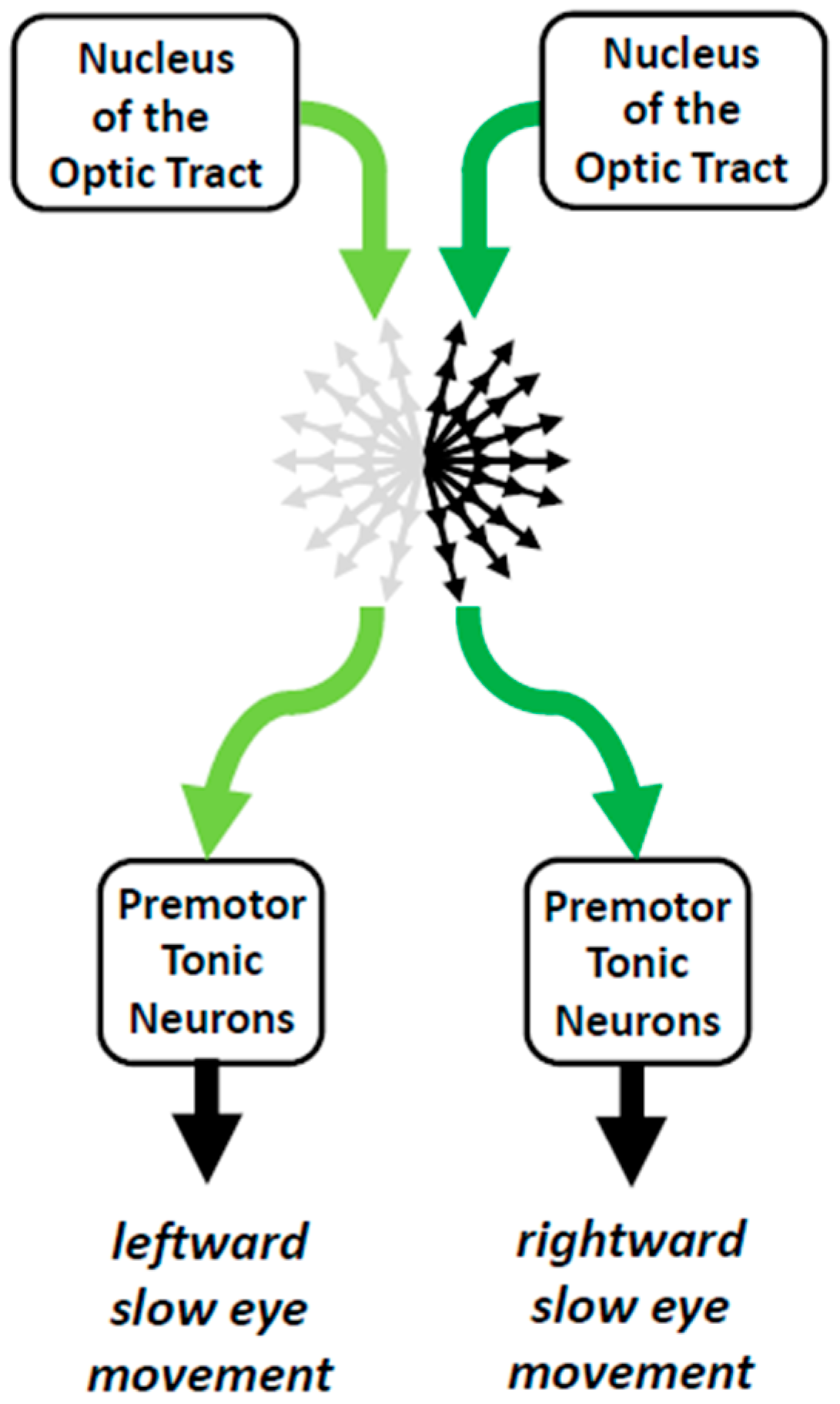
4.1. Nuclei of the Optic Tract and Visual Fixation
4.2. Caudal Fastigial Nuclei and Visual Fixation
4.3. Superior Colliculi and Visual Fixation
4.4. Additional Evidence from Clinical and Behavioral Studies
5. Conclusions: The Poly-Equilibrium Theory
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABD | abducens nucleus |
| AINs | abducens internuclear neurons |
| cFN | caudal fastigial nucleus |
| EVNs-1(2) | excitatory vestibular neurons of type 1 (type 2) |
| FEF | frontal eye field |
| IBTNs | inhibitory burst tonic neurons |
| IO | inferior oblique |
| IR | inferior rectus |
| IVNs-1(2) | inhibitory vestibular neurons of type 1 (type 2) |
| LR | lateral rectus |
| MNs | motoneurons |
| MR | medial rectus |
| MVN | medial vestibular nucleus |
| NOT | nucleus of the optic tract |
| NPH | nucleus prepositus hypoglossi |
| OMN | oculomotor nucleus |
| PVNs | primary vestibular neurons |
| PVP | position vestibular pause neurons |
| RIP | raphe interpositus |
| SC | superior colliculus |
| SO | superior oblique |
| SVN | superior vestibular nucleus |
| TRO | trochlear nucleus |
| VOR | vestibulo-ocular response |
References
- Sherrington, C.S. Further experimental note on the correlation of action of antagonistic muscles. BMJ 1893, 1, 1218. [Google Scholar] [CrossRef]
- Demer, J.L. Anatomy and physiology of the extraocular muscles and surrounding tissues. In Ophthalmology, 5th ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 1190–1194. [Google Scholar]
- Goffart, L.; Quinet, J.; Bourrelly, C. Neurophysiology of gaze orientation: Core neuronal networks. In Encyclopedia of the Human Brain, 2nd ed.; Grafman, J.H., Ed.; Elsevier: New York, NY, USA, 2005; Volume 1, pp. 681–699. [Google Scholar] [CrossRef]
- Wong, A.M. Listing’s law: Clinical significance and implications for neural control. Surv. Ophthalmol. 2004, 49, 563–575. [Google Scholar] [CrossRef]
- Thurtell, M.J.; Joshi, A.C.; Walker, M.F. Three-dimensional kinematics of saccadic and pursuit eye movements in humans: Relationship between Donders’ and Listing’s laws. Vis. Res. 2012, 60, 7–15. [Google Scholar] [CrossRef]
- Crawford, J.D.; Vilis, T. Symmetry of oculomotor burst neuron coordinates about Listing’s plane. J. Neurophysiol. 1992, 68, 432–448. [Google Scholar] [CrossRef]
- Helmchen, C.; Glasauer, S.; Buttner, U. Pathological torsional eye deviation during voluntary saccades: A violation of Listing’s law. J. Neurol. Neurosurg. Psychiatry 1997, 62, 253–260. [Google Scholar] [CrossRef]
- Helmchen, C.; Rambold, H.; Fuhry, L.; Büttner, U. Deficits in vertical and torsional eye movements after uni- and bilateral muscimol inactivation of the interstitial nucleus of Cajal of the alert monkey. Exp. Brain Res. 1998, 119, 436–452. [Google Scholar] [CrossRef]
- Tweed, D.; Glenn, B.; Vilis, T. Eye-head coordination during large gaze shifts. J. Neurophysiol. 1995, 73, 766–779. [Google Scholar] [CrossRef]
- Balliet, R.; Nakayama, K. Training of voluntary torsion. Invest. Ophthalm. Vis. Sci. 1978, 17, 303–314. [Google Scholar]
- Büttner-Ennever, J.A. The extraocular motor nuclei: Organization and functional neuroanatomy. In Progress in Brain Research; Büttner-Ennever, J.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 95–125. [Google Scholar]
- Clendaniel, R.A.; Mays, L.E. Characteristics of antidromically identified oculomotor internuclear neurons during vergence and versional eye movements. J. Neurophysiol. 1994, 71, 1111–1127. [Google Scholar] [CrossRef]
- Moschovakis, A.K. The neural integrators of the mammalian saccadic system. Front. Biosci. 1997, 2, 552–577. [Google Scholar] [CrossRef]
- Sylvestre, P.A.; Cullen, K.E. Quantitative analysis of abducens neuron discharge dynamics during saccadic and slow eye movements. J. Neurophysiol. 1999, 82, 2612–2632. [Google Scholar] [CrossRef]
- Walton, M.M.G. Reduced activity of vertically acting motoneurons during convergence. J. Neurophysiol. 2022, 128, 671–680. [Google Scholar] [CrossRef]
- Angelaki, D.E.; Hess, B.J.M. Self-motion-induced eye movements: Effects on visual acuity and navigation. Nat. Rev. Neurosci. 2005, 6, 966–976. [Google Scholar] [CrossRef]
- Cullen, K.E. The vestibular system: Multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012, 35, 185–196. [Google Scholar] [CrossRef]
- Graf, W.M. Visual-vestibular guided control of posture, movement and self-motion perception. In Encyclopedia of the Human Brain Grafman, 2nd ed.; Grafman, J.H., Ed.; Elsevier: Philadelphia, PA, USA, 2025; Volume 1, pp. 582–611. [Google Scholar] [CrossRef]
- Highstein, S.M.; Holstein, G.R. The anatomy of the vestibular nuclei. In Progress in Brain Research; Büttner-Ennever, J.A., Ed.; Elsevier: Philadelphia, PA, USA, 2006; Volume 151, pp. 157–203. [Google Scholar] [CrossRef]
- Büttner-Ennever, J.A. Patterns of connectivity in the vestibular nuclei. Ann. N. Y. Acad. Sci. 1992, 656, 363–378. [Google Scholar] [CrossRef]
- Ostriker, G.; Pellionisz, A.; Llinás, R. Tensorial computer model of gaze—I. Oculomotor activity is expressed in non-orthogonal natural coordinates. Neuroscience 1985, 14, 483–500. [Google Scholar] [CrossRef]
- Cox, P.G.; Jeffery, N. Morphology of the mammalian vestibulo-ocular reflex: The spatial arrangement of the human fetal semicircular canals and extraocular muscles. J. Morphol. 2007, 268, 878–890. [Google Scholar] [CrossRef]
- Cox, P.G.; Jeffery, N. Geometry of the semicircular canals and extraocular muscles in rodents, lagomorphs, felids and modern humans. Am. J. Anat. 2008, 213, 583–596. [Google Scholar] [CrossRef]
- McCrea, R.A.; Strassman, A.; May, E.; Highstein, S.M. Anatomical and physiological characteristics of vestibular neurons mediating the horizontal vestibulo-ocular reflex of the squirrel monkey. J. Comp. Neurol. 1987, 264, 547–570. [Google Scholar] [CrossRef]
- Scudder, C.A.; Fuchs, A.F. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J. Neurophysiol. 1992, 68, 244–264. [Google Scholar] [CrossRef]
- Roy, J.E.; Cullen, K.E. Vestibuloocular reflex signal modulation during voluntary and passive head movements. J. Neurophysiol. 2002, 87, 2337–2357. [Google Scholar] [CrossRef]
- Roy, J.E.; Cullen, K.E. Brain stem pursuit pathways: Dissociating visual, vestibular, and proprioceptive inputs during combined eye-head gaze tracking. J. Neurophysiol. 2003, 90, 271–290. [Google Scholar] [CrossRef]
- Fuchs, A.F.; Ling, L.; Phillips, J.O. Behavior of the position vestibular pause (PVP) interneurons of the vestibuloocular reflex during head-free gaze shifts in the monkey. J. Neurophysiol. 2005, 94, 4481–4490. [Google Scholar] [CrossRef]
- Broussard, D.M.; DeCharms, R.C.; Lisberger, S.G. Inputs from the ipsilateral and contralateral vestibular apparatus to behaviorally characterized abducens neurons in rhesus monkeys. J. Neurophysiol. 1995, 74, 2445–2459. [Google Scholar] [CrossRef]
- Yokota, J.-I.; Reisine, H.; Cohen, B. Nystagmus induced by electrical stimulation of the vestibular and prepositus hypoglossi nuclei in the monkey: Evidence for site of induction of velocity storage. Exp. Brain Res. 1992, 92, 123–138. [Google Scholar] [CrossRef]
- Cannon, S.C.; Robinson, D.A. Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. J. Neurophysiol. 1987, 57, 1383–1409. [Google Scholar] [CrossRef]
- McFarland, J.L.; Fuchs, A.F. Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movement in behaving macaques. J. Neurophysiol. 1992, 68, 319–332. [Google Scholar] [CrossRef]
- Langer, T.; Kaneko, C.R.S.; Scudder, C.A.; Fuchs, A.F. Afferents to the abducens nucleus in the monkey and cat. J. Comp. Neurol. 1986, 245, 379–400. [Google Scholar] [CrossRef]
- Belknap, D.B.; McCrea, R.A. Anatomical connections of the prepositus and abducens nuclei in the squirrel monkey. J. Comp. Neurol. 1988, 268, 13–28. [Google Scholar] [CrossRef]
- Spencer, R.F.; Baker, R. GABA and glycine as inhibitory neurotransmitters in the vestibuloocular reflex. Ann. N. Y. Acad. Sci. 1992, 656, 602–611. [Google Scholar] [CrossRef]
- Ilg, U.; Hoffmann, K.-P. Responses of monkey nucleus of the optic tract neurons during pursuit and fixation. Neurosci. Res. 1991, 12, 101–110. [Google Scholar] [CrossRef]
- McCrea, R.A.; Strassman, A.; Highstein, S.M. Anatomical and physiological characteristics of vestibular neurons mediating the vertical vestibulo-ocular reflexes of the squirrel monkey. J. Comp. Neurol. 1987, 264, 571–594. [Google Scholar] [CrossRef]
- Schultheis, L.W.; Robinson, D.A. Directional plasticity of the vestibulo-ocular reflex in the cat. Ann. N. Y. Acad. Sci. 1981, 374, 504–512. [Google Scholar] [CrossRef]
- Schiller, P.H.; True, S.D.; Conway, J.L. Deficits in eye movements following frontal eye-field and superior colliculus ablations. J. Neurophysiol. 1980, 44, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Schiff, D.; Cohen, B.; Raphan, T. Nystagmus induced by stimulation of the nucleus of the optic tract in the monkey. Exp. Brain Res. 1988, 70, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mustari, M.J.; Fuchs, A.F. Discharge patterns of neurons in the pretectal nucleus of the optic tract (NOT) in the behaving primate. J. Neurophysiol. 1990, 64, 77–90. [Google Scholar] [CrossRef]
- Ron, S.; Robinson, D.A. Eye movements evoked by cerebellar stimulation in the alert monkey. J. Neurophysiol. 1973, 36, 1004–1022. [Google Scholar] [CrossRef]
- Belknap, D.B.; Noda, H. Eye movements evoked by microstimulation in the flocculus of the alert macaque. Exp. Brain Res. 1987, 67, 352–362. [Google Scholar] [CrossRef]
- Heinen, S.; Oh, D.; Keller, E. Characteristics of nystagmus evoked by electrical stimulation of the uvular/nodular lobules of the cerebellum in monkey. J. Vestib. Res. 1992, 2, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, B.; Weber, J.T. The pretectal complex of the monkey: A reinvestigation of the morphology and retinal terminations. J. Comp. Neurol. 1985, 232, 425–442. [Google Scholar] [CrossRef]
- Gamlin, P.D. The pretectum: Connections and oculomotor-related roles. Prog. Brain Res. 2006, 151, 379–405. [Google Scholar] [CrossRef]
- Distler, C.; Mustari, M.J.; Hoffmann, K. Cortical projections to the nucleus of the optic tract and dorsal terminal nucleus and to the dorsolateral pontine nucleus in macaques: A dual retrograde tracing study. J. Comp. Neurol. 2002, 444, 144–158. [Google Scholar] [CrossRef]
- Mustari, M.J.; Fuchs, A.F.; Kaneko, C.R.S.; Robinson, F.R. Anatomical connections of the primate pretectal nucleus of the optic tract. J. Comp. Neurol. 1994, 349, 111–128. [Google Scholar] [CrossRef]
- Ilg, U.J.; Hoffmann, K. Responses of neurons of the nucleus of the optic tract and the dorsal terminal nucleus of the accessory optic tract in the awake monkey. Eur. J. Neurosci. 1996, 8, 92–105. [Google Scholar] [CrossRef]
- Ilg, U.J.; Bremmer, F.; Hoffmann, K.-P. Optokinetic and pursuit system: A case report. Behav. Brain Res. 1993, 57, 21–29. [Google Scholar] [CrossRef]
- Yakushin, S.B.; Gizzi, M.; Reisine, H.; Raphan, T.; Büttner-Ennever, J.; Cohen, B. Functions of the nucleus of the optic tract (NOT). Exp. Brain Res. 2000, 131, 433–447. [Google Scholar] [CrossRef]
- Inoue, Y.; Takemura, A.; Mustari, M.; Kawano, K. Role of the pretectal nucleus of the optic tract in short-latency ocular following responses in monkeys. Exp. Brain Res. 2000, 131, 269–281. [Google Scholar] [CrossRef]
- Goffart, L.; Bourrelly, C.; Quinton, J.-C. Neurophysiology of visually guided eye movements: Critical review and alternative viewpoint. J. Neurophysiol. 2018, 120, 3234–3245. [Google Scholar] [CrossRef]
- Takemura, A.; Murata, Y.; Kawano, K.; Miles, F.A. Deficits in short-latency tracking eye movements after chemical lesions in monkey cortical areas MT and MST. J. Neurosci. 2007, 27, 529–541. [Google Scholar] [CrossRef]
- Ventre, J.; Faugier-Grimaud, S. Effects of posterior parietal lesions (area 7) on VOR in monkeys. Exp. Brain Res. 1986, 62, 654–658. [Google Scholar] [CrossRef]
- Sparks, D.L. Functional properties of neurons in the monkey superior colliculus: Coupling of neuronal activity and saccade onset. Brain Res. 1978, 156, 1–16. [Google Scholar] [CrossRef]
- Freedman, E.G.; Sparks, D.L. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: Evidence for a gaze displacement command. J. Neurophysiol. 1997, 78, 1669–1690. [Google Scholar] [CrossRef]
- Goffart, L.; Pélisson, D. Orienting gaze shifts during muscimol inactivation of caudal fastigial nucleus in the cat. I. Gaze Dysmetria. J. Neurophysiol. 1998, 79, 1942–1958. [Google Scholar] [CrossRef][Green Version]
- Robinson, F.R.; Straube, A.; Fuchs, A.F. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J. Neurophysiol. 1993, 70, 1741–1758. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Sato, H.; Noda, H. Saccadic burst neurons in the fastigial nucleus are not involved in compensating for orbital nonlinearities. J. Neurophysiol. 1994, 71, 1976–1980. [Google Scholar] [CrossRef]
- Goffart, L.; Chen, L.L.; Sparks, D.L. Deficits in saccades and fixation during muscimol inactivation of the caudal fastigial nucleus in the rhesus monkey. J. Neurophysiol. 2004, 92, 3351–3367. [Google Scholar] [CrossRef]
- Guerrasio, L.; Quinet, J.; Büttner, U.; Goffart, L. Fastigial oculomotor region and the control of foveation during fixation. J. Neurophysiol. 2010, 103, 1988–2001. [Google Scholar] [CrossRef]
- Sato, H.; Noda, H. Saccadic dysmetria induced by transient functional decortication of the cerebellar vermis. Exp. Brain Res. 1992, 88, 455–458. [Google Scholar] [CrossRef]
- Quinet, J.; Goffart, L. Saccade dysmetria in head-unrestrained gaze shifts after muscimol inactivation of the caudal fastigial nucleus in the monkey. J. Neurophysiol. 2005, 93, 2343–2349. [Google Scholar] [CrossRef]
- Kurkin, S.; Akao, T.; Fukushima, J.; Shichinohe, N.; Kaneko, C.R.S.; Belton, T.; Fukushima, K. No-go neurons in the cerebellar oculomotor vermis and caudal fastigial nuclei: Planning tracking eye movements. Exp. Brain Res. 2013, 232, 191–210. [Google Scholar] [CrossRef]
- Noda, H.; Sugita, S.; Ikeda, Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J. Comp. Neurol. 1990, 302, 330–348. [Google Scholar] [CrossRef]
- Goffart, L.; Hafed, Z.M.; Krauzlis, R.J. Visual fixation as equilibrium: Evidence from superior colliculus inactivation. J. Neurosci. 2012, 32, 10627–10636. [Google Scholar] [CrossRef]
- Hafed, Z.M.; Goffart, L.; Krauzlis, R.J. Superior colliculus inactivation causes stable offsets in eye position during tracking. J. Neurosci. 2008, 28, 8124–8137. [Google Scholar] [CrossRef]
- Hafed, Z.M.; Goffart, L.; Krauzlis, R.J. A neural mechanism for microsaccade generation in the primate superior colliculus. Science 2009, 323, 940–943. [Google Scholar] [CrossRef]
- Hafed, Z.M.; Krauzlis, R.J. Similarity of superior colliculus involvement in microsaccade and saccade generation. J. Neurophysiol. 2012, 107, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Shadmehr, R. Distinct neural circuits for control of movement vs. holding still. J. Neurophysiol. 2017, 117, 1431–1460. [Google Scholar] [CrossRef] [PubMed]
- Parr, T.; Friston, K.J. The active construction of the visual world. Neuropsychologia 2017, 104, 92–101. [Google Scholar] [CrossRef]
- Takahashi, M.; Sugiuchi, Y.; Na, J.; Shinoda, Y. Brainstem circuits triggering saccades and fixation. J. Neurosci. 2021, 42, 789–803. [Google Scholar] [CrossRef]
- Takahashi, M.; Veale, R. Pathways for naturalistic looking behavior in primate I: Behavioral characteristics and brainstem circuits. Neuroscience 2023, 532, 133–163. [Google Scholar] [CrossRef]
- Munoz, D.P.; Guitton, D. Fixation and orientation control by the tecto-reticulo-spinal system in the cat whose head is unrestrained. Rev. Neurol. 1989, 145, 567–579. [Google Scholar]
- Munoz, D.P.; Wurtz, R.H. Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J. Neurophysiol. 1993, 70, 576–589. [Google Scholar] [CrossRef]
- Munoz, D.P.; Wurtz, R.H. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J. Neurophysiol. 1993, 70, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Dorris, M.C.; Paré, M.; Munoz, D.P. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J. Neurosci. 1997, 17, 8566–8579. [Google Scholar] [CrossRef] [PubMed]
- Everling, S.; Paré, M.; Dorris, M.C.; Munoz, D.P. Comparison of the discharge characteristics of brain stem omnipause neurons and superior colliculus fixation neurons in monkey: Implications for control of fixation and saccade behavior. J. Neurophysiol. 1998, 79, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.W.; Keller, E.L.; Gandhi, N.J.; Das, S. Two-dimensional saccade-related population activity in superior colliculus in monkey. J. Neurophysiol. 1998, 80, 798–817. [Google Scholar] [CrossRef]
- Martinez-Conde, S.; Macknik, S.L. Fixational eye movements across vertebrates: Comparative dynamics, physiology, and perception. J. Vis. 2008, 8, 28. [Google Scholar] [CrossRef]
- Sparks, D.; Rohrer, W.; Zhang, Y. The role of the superior colliculus in saccade initiation: A study of express saccades and the gap effect. Vis. Res. 2000, 40, 2763–2777. [Google Scholar] [CrossRef]
- Kaneko, C.R. Effect of ibotenic acid lesions of the omnipause neurons on saccadic eye movements in rhesus macaques. J. Neurophysiol. 1996, 75, 2229–2242. [Google Scholar] [CrossRef]
- Soetedjo, R.; Kaneko, C.R.S.; Fuchs, A.F. Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. J. Neurophysiol. 2002, 87, 679–695. [Google Scholar] [CrossRef][Green Version]
- Moschovakis, A.; Scudder, C.; Highstein, S. The microscopic anatomy and physiology of the mammalian saccadic system. Prog. Neurobiol. 1996, 50, 133–254. [Google Scholar] [CrossRef]
- Scudder, C.A.; Kaneko, C.R.S.; Fuchs, A.F. The brainstem burst generator for saccadic eye movements: A modern synthesis. Exp. Brain Res. 2002, 142, 439–462. [Google Scholar] [CrossRef]
- Munoz, D.P.; Wurtz, R.H. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J. Neurophysiol. 1995, 73, 2313–2333. [Google Scholar] [CrossRef]
- Goffart, L. Orienting gaze toward a visual target: Neurophysiological synthesis with epistemological considerations. Vision 2025, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.A.; Levy, R.; Munoz, D.P. Distinct sensory-and goal-related signals underlie the gap effect in the superior colliculus. Eur. J. Neurosci. 2022, 55, 205–226. [Google Scholar] [CrossRef]
- Sparks, D.; Lee, C.; Rohrer, W. Population coding of the direction, amplitude, and velocity of saccadic eye movements by neurons in the superior colliculus. Cold Spring Harb. Symp. Quant. Biol. 1990, 55, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Crosby, E.C. Relations of brain centers to normal and abnormal eye movements in the horizontal plane. J. Comp. Neurol. 1953, 99, 437–479. [Google Scholar] [CrossRef]
- Latto, R.; Cowey, A. Fixation changes after frontal eye-field lesions in monkeys. Brain Res. 1971, 30, 25–36. [Google Scholar] [CrossRef]
- Dias, E.C.; Segraves, M.A. Muscimol-induced inactivation of monkey frontal eye field: Effects on visually and memory-guided saccades. J. Neurophysiol. 1999, 81, 2191–2214. [Google Scholar] [CrossRef]
- Lynch, J.C.; Graybiel, A.M.; Lobeck, L.J. The differential projection of two cytoarchitectonic subregions of the inferior parietal lobule of macaque upon the deep layers of the superior colliculus. J. Comp. Neurol. 1985, 235, 241–254. [Google Scholar] [CrossRef]
- Paré, M.; Wurtz, R.H. Progression in neuronal processing for saccadic eye movements from parietal cortex area LIP to superior colliculus. J. Neurophysiol. 2001, 85, 2545–2562. [Google Scholar] [CrossRef]
- Barbas, H.; Mesulam, M. Organization of afferent input to subdivisions of area 8 in the rhesus monkey. J. Comp. Neurol. 1981, 200, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A.; Asanuma, C.; Essick, G.; Siegel, R.M. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J. Comp. Neurol. 1990, 296, 65–113. [Google Scholar] [CrossRef]
- Tian, J.R.; Lynch, J.C. Corticocortical input to the smooth and saccadic eye movement subregions of the frontal eye field in Cebus monkeys. J. Neurophysiol. 1996, 76, 2754–2771. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.C.; McLaren, J.W. Deficits of visual attention and saccadic eye movements after lesions of parietooccipital cortex in monkeys. J. Neurophysiol. 1989, 61, 74–90. [Google Scholar] [CrossRef]
- Li, C.-S.R.; Mazzoni, P.; Andersen, R.A. Effect of reversible inactivation of macaque lateral intraparietal area on visual and memory saccades. J. Neurophysiol. 1999, 81, 1827–1838. [Google Scholar] [CrossRef]
- Wardak, C.; Olivier, E.; Duhamel, J.-R. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J. Neurosci. 2002, 22, 9877–9884. [Google Scholar] [CrossRef]
- Simon, J.; Morgan, S.; Pexman, J.; Hill, M.D.; Buchan, A. CT assessment of conjugate eye deviation in acute stroke. Neurology 2003, 60, 135–137. [Google Scholar] [CrossRef]
- Becker, E.; Karnath, H.-O. Neuroimaging of eye position reveals spatial neglect. Brain 2010, 133, 909–914. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berger, M.F.; Johannsen, L.; Karnath, H.-O. Time course of eye and head deviation in spatial neglect. Neuropsychology 2008, 22, 697–702. [Google Scholar] [CrossRef]
- Reinhard, J.I.; Damm, I.; Ivanov, I.V.; Trauzettel-Klosinski, S. Eye movements during saccadic and fixation tasks in patients with homonymous hemianopia. J. Neuro-Ophthalmol. 2014, 34, 354–361. [Google Scholar] [CrossRef]
- Snodderly, D.M. Effects of light and dark environments on macaque and human fixational eye movements. Vis. Res. 1987, 27, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Barash, S.; Melikyan, A.; Sivakov, A.; Tauber, M. Shift of visual fixation dependent on background illumination. J. Neurophysiol. 1998, 79, 2766–2781. [Google Scholar] [CrossRef]
- Goffart, L.; Quinet, J.; Chavane, F.; Masson, G.S. Influence of background illumination on fixation and visually guided saccades in the rhesus monkey. Vis. Res. 2006, 46, 149–162. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Newsome, W.T.; Maunsell, J.H. The visual field representation in striate cortex of the macaque monkey: Asymmetries, anisotropies, and individual variability. Vis. Res. 1984, 24, 429–448. [Google Scholar] [CrossRef]
- Hafed, Z.M.; Chen, C.-Y. Sharper, stronger, faster upper visual field representation in primate superior colliculus. Curr. Biol. 2016, 26, 1647–1658. [Google Scholar] [CrossRef]
- Böhmer, A.; Straumann, D. Pathomechanism of mammalian downbeat nystagmus due to cerebellar lesion: A simple hypothesis. Neurosci. Lett. 1998, 250, 127–130. [Google Scholar] [CrossRef]
- Marti, S.; Straumann, D.; Büttner, U.; Glasauer, S. A model-based theory on the origin of downbeat nystagmus. Exp. Brain Res. 2008, 188, 613–631. [Google Scholar] [CrossRef]
- Goffart, L. Kinematics and the neurophysiological study of visually-guided eye movements. In Progress in Brain Research; Ramat, S., Shaikh, A.G., Eds.; Elsevier: Philadelphia, PA, USA, 2019; Volume 249, pp. 375–384. [Google Scholar] [CrossRef]
- Schiller, P.H.; Stryker, M. Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J. Neurophysiol. 1972, 35, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D. Eye movements evoked by collicular stimulation in the alert monkey. Vis. Res. 1972, 12, 1795–1808. [Google Scholar] [CrossRef]
- Stanford, T.R.; Freedman, E.G.; Sparks, D.L. Site and parameters of microstimulation: Evidence for independent effects on the properties of saccades evoked from the primate superior colliculus. J. Neurophysiol. 1996, 76, 3360–3381. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, R.H.; E Goldberg, M. Activity of superior colliculus in behaving monkey. 3. Cells discharging before eye movements. J. Neurophysiol. 1972, 35, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L.; Holland, R.; Guthrie, B.L. Size and distribution of movement fields in the monkey superior colliculus. Brain Res. 1976, 113, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Hafed, Z.M.; Goffart, L. Gaze direction as equilibrium: More evidence from spatial and temporal aspects of small-saccade triggering in the rhesus macaque monkey. J. Neurophysiol. 2020, 123, 308–322. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goffart, L. Neurophysiology of Gaze Direction as Poly-Equilibrium. NeuroSci 2025, 6, 85. https://doi.org/10.3390/neurosci6030085
Goffart L. Neurophysiology of Gaze Direction as Poly-Equilibrium. NeuroSci. 2025; 6(3):85. https://doi.org/10.3390/neurosci6030085
Chicago/Turabian StyleGoffart, Laurent. 2025. "Neurophysiology of Gaze Direction as Poly-Equilibrium" NeuroSci 6, no. 3: 85. https://doi.org/10.3390/neurosci6030085
APA StyleGoffart, L. (2025). Neurophysiology of Gaze Direction as Poly-Equilibrium. NeuroSci, 6(3), 85. https://doi.org/10.3390/neurosci6030085





