Limited Evidence to Review—Is There an Association Between Cognition and Upper Extremity Motor Reaction Time in Older Adults?
Abstract
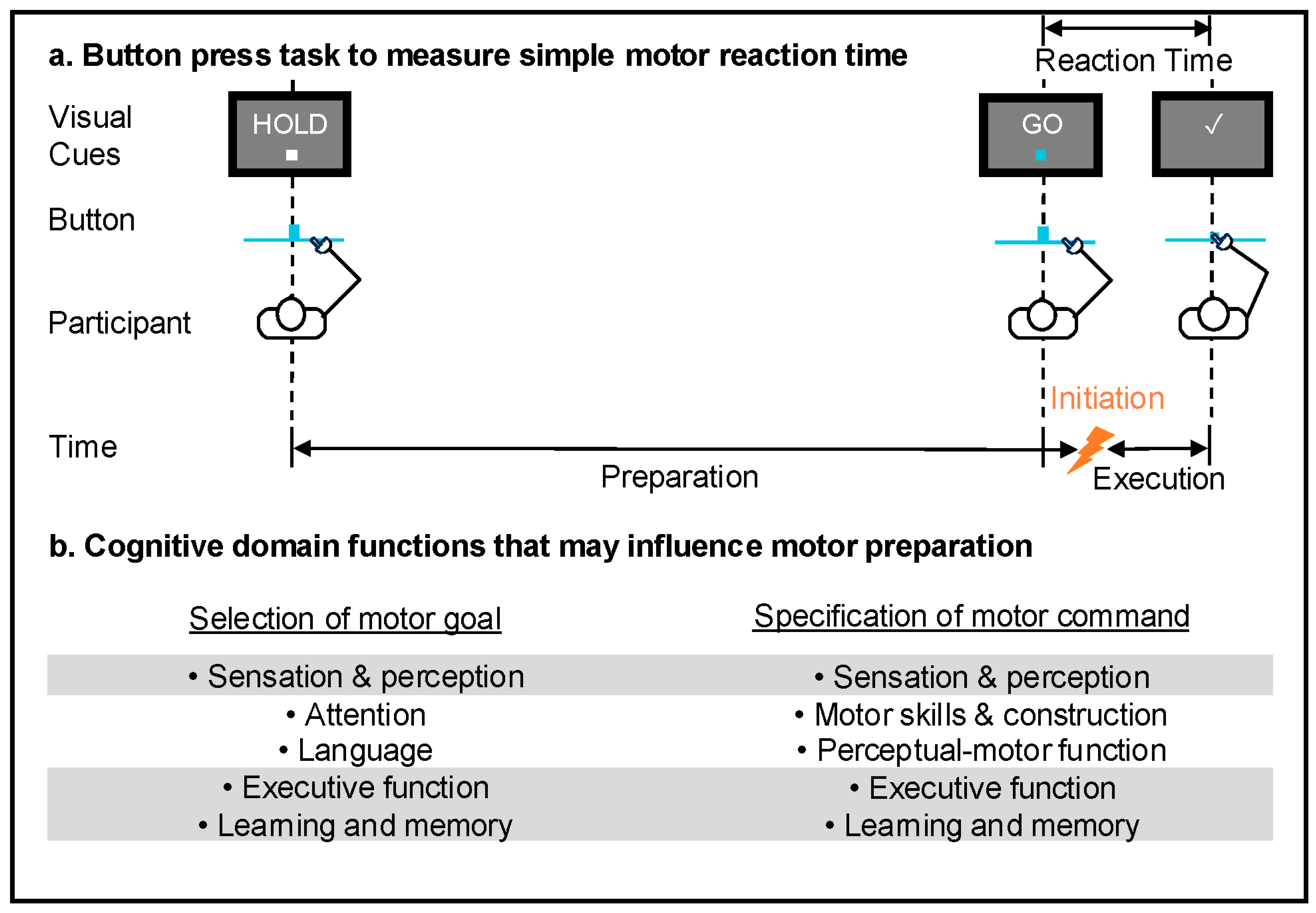
1. Introduction
2. Materials and Methods
- (1)
- The study population included healthy, community-dwelling older adults (age > 60 years)
- (2)
- A physical upper extremity movement task was performed (examples include reaching, finger tapping, and button press tasks)
- (3)
- At least one measure of cognitive assessment was performed
- (4)
- An RT measure was quantified from the upper extremity task

| PEDro Criteria | Study | ||||||||||||||||||||||||
| Bao et al. (2019) [44] | Chen et al. (2020) [45] | Ferreira et al. (2022) [46] | Hartle et al. (2022) [47] | Hennessy et al. (2025) [48] | Hong et al. (2020) [49] | Jardim et al. (2024) [50] | Juhasz et al. (2019) [51] | Jutten et al. (2023) [52] | Kimura et al. (2023) [53] | Kitchen and Miall (2019) [54] | Korthauer et al. (2019) [55] | Krumpolt et al. (2025) [56] | Mack et al. (2025) [57] | Qiu and Xiong (2017) [58] | Rattanavichit et al. (2022) [59] | Sleimen-Malkoun et al. (2013) [60] | Staub et al. (2014) [61] | Tait et al. (2024) [62] | Unger et al. (2025) [63] | Van Humbeeck et al. (2024) [64] | Vasquez et al. (2016) [65] | Welhaf et al. (2024) [66] | Worschech et al. (2024) [67] | Yao et al. (2016) [68] | |
| 1 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | yes | yes | yes | yes | yes | yes | yes |
| 2 | no | no | yes | no | no | no | no | no | no | no | no | no | no | yes | no | no | no | no | yes | no | no | no | no | no | no |
| 3 | no | no | no | no | no | no | no | no | no | no | no | no | no | yes | no | no | no | no | yes | no | no | no | no | no | no |
| 4 | no | yes | yes | yes | no | no | yes | no | yes | yes | yes | yes | no | yes | no | yes | no | no | yes | no | no | no | yes | yes | yes |
| 5 | no | no | no | no | no | no | no | no | no | no | no | no | no | yes | no | no | no | no | yes | no | no | no | no | no | no |
| 6 | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | yes | no | no | no | no | no | no |
| 7 | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no | no |
| 8 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | yes | yes | yes | yes | yes | yes |
| 9 | no | no | yes | no | no | no | no | no | no | no | no | no | yes | yes | no | no | no | no | yes | yes | no | no | no | no | no |
| 10 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | no |
| 11 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | no |
| Score | 4 | 5 | 7 | 5 | 4 | 4 | 5 | 4 | 5 | 5 | 5 | 4 | 4 | 9 | 4 | 5 | 4 | 3 | 9 | 5 | 4 | 4 | 5 | 4 | 3 |
3. Results
4. Discussion
4.1. Executive Function and Memory Are Associated with Choice and Complex RTs
4.2. The Interplay Between Cognitive and Motor Function Contributes to Interdependence of Select Measures
4.3. Advancing the Field of Sensorimotor Control for Older Adults
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RT | Reaction Time |
| PEDro | Physiotherapy Evidence Database. |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| MCI | Mild cognitive impairment |
| DRS | Dementia Rating Scale |
| ADL | Activities of Daily Living |
| IADL | Instrumental Activities of Daily Living |
| MoCA | Montreal Cognitive Assessment |
| TMT | Trail Making Test |
| MMSE | Mini-Mental State Examination |
| BDS | Blessed Dementia Scale |
| WAIS-R | Wechsler Adult Intelligence Scale—Revised |
| WCST | Wisconsin Card Sorting Test |
| CVLT | California Verbal Learning Test |
| WMS-R | Wechsler Memory Scale—Revised |
| BNT | Boston Naming Test |
| WMS | Weschler Memory Scale |
| WAIS-III | Wechsler Adult Intelligence Scale—3rd edition |
| DSCT | Digit Symbol Coding Test |
| CVFT | Category Verbal Fluency Test |
| TICS | Telephone Inventory of Cognitive Status |
| D-KEFS | Delis-Kaplan Executive Function System |
| WMS-III | Weschler Memory Scale—3rd edition |
| CBB | Cogstate Brief Battery |
| EEG | Electroencephalogram |
| ROCFT | Rey-Osterrieth Complex Figure Test |
| SCWT | Stroop Color and Word Test |
| SDMT | Symbol Digit Modalities Test |
| AVLT | Auditory Verbal Learning Test |
| COMP | CompCog computerized cognitive screening battery |
| Benton JoLO | Benton Judgement of Line Orientation |
| HVLT-R | Hopkins Verbal Learning Test—Revised |
| BVMT-R | Brief Visuospatial Memory Test—Revised |
| WMS-IV | Wechsler Memory Scale—4th edition |
| VTS | Vienna Test System |
| SPMSQ | Short Portable Mental Screening Questionnaire |
| WAIS-IV | Wechsler Adult Intelligence Scale—4th edition |
| PACC5 | Preclinical Alzheimer’s Cognitive Composite-5 |
| COWAT | Controlled Oral Word Association Test |
| INT | Internally driven uncertainty |
| EXT | Externally cued uncertainty |
| STM | CompCog Visual and Spatial Short Term Memory subtest |
References
- Egger, S.W.; Le, N.M.; Jazayeri, M. A neural circuit model for human sensorimotor timing. Nat. Commun. 2020, 11, 3933. [Google Scholar] [CrossRef] [PubMed]
- Klemmer, E.T. Time uncertainty in simple reaction time. J. Exp. Psychol. 1956, 51, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, I.H.; Schurman, D.L.; Forester, G. Choice Reaction Time As a Function of Stimulus Uncertainty, Response Uncertainty, and Behavioral Hypotheses. J. Exp. Psychol. 1967, 74, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.A. Human movement initiation: Specification of arm, direction, and extent. J. Exp. Psychol. Gen. 1980, 109, 444–474. [Google Scholar] [CrossRef] [PubMed]
- Lara, A.H.; Elsayed, G.F.; Zimnik, A.J.; Cunningham, J.P.; Churchland, M.M. Conservation of preparatory neural events in monkey motor cortex regardless of how movement is initiated. eLife 2018, 22, e31826. [Google Scholar] [CrossRef] [PubMed]
- Orban de Xivry, J.; Legrain, V.; Lefèvre, P. Overlap of movement planning and movement execution reduces reaction time. J. Neurophysiol. 2017, 117, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Haith, A.M.; Huberdeau, D.M.; Krakauer, J.W. Hedging Your Bets: Intermediate Movements as Optimal Behavior in the Context of an Incomplete Decision. PLoS Comput. Biol. 2015, 11, e1004171. [Google Scholar] [CrossRef] [PubMed]
- Oostwoud Wijdenes, L.; Ivry, R.B.; Bays, P.M. Competition between movement plans increases motor variability: Evidence of a shared resource for movement planning. J. Neurophysiol. 2016, 116, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wilkinson, K.; Sainburg, R.L. Is Hand Selection Modulated by Cognitive–perceptual Load? Neuroscience 2018, 369, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.L.; Haith, A.M.; Krakauer, J.W. Motor Planning. Neuroscientist 2015, 21, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.D. Domains of cognition and their assessment. Dialogues Clin. Neurosci. 2019, 21, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying neurocognitive disorders: The DSM-5 approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, B. Screening for Cognitive Impairment in Geriatrics. Clin. Geriatr. Med. 2018, 34, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Johari, K.; den Ouden, D.; Behroozmand, R. Behavioral and neural correlates of normal aging effects on motor preparatory mechanisms of speech production and limb movement. Exp. Brain Res. 2019, 237, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Lavrencic, L.M.; Churches, O.F.; Keage, H.A.D. Cognitive reserve is not associated with improved performance in all cognitive domains. Appl. Neuropsychol. Adult 2018, 25, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. Trajectories of normal cognitive aging. Psychol. Aging 2019, 34, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Fozard, J.L.; Vercryssen, M.; Reynolds, S.L.; Hancock, P.A.; Quilter, R.E. Age Differences and changes in reaction time: The Baltimore longitudinal study of aging. J. Gerontol. 1994, 49, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.; Vantieghem, S.; Gorus, E.; Lauwers, E.; Fierens, Y.; Pool-Goudzwaard, A.; Bautmans, I. Age-related differences in muscle recruitment and reaction-time performance. Exp. Gerontol. 2015, 70, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.C.; Yap, P.; Tay, S.Y.; Koay, W.I.; Liew, T.M. Examining the validity and utility of Montreal Cognitive Assessment domain scores for early neurocognitive disorders. J. Am. Med. Dir. Assoc. 2023, 24, 314–320.e2. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Berroa, E.; Luo, X.; Schmeidler, J.; Rapp, M.A.; Dahlman, K.; Grossman, H.T.; Haroutunian, V.; Beeri, M.S. The MMSE orientation for time domain is a strong predictor of subsequent cognitive decline in the elderly. Int. J. Geriatr. Psychiatry 2009, 24, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Hedge, C.; Powell, G.; Sumner, P. The mapping between transformed reaction time costs and models of processing in aging and cognition. Psychol. Aging 2018, 33, 1093–1104. [Google Scholar] [CrossRef]
- Inzitari, M.; Baldereschi, M.; Carlo, A.D.; Bari, M.D.; Marchionni, N.; Scafato, E.; Farchi, G.; Inzitari, D. Impaired Attention Predicts Motor Performance Decline in Older Community-Dwellers With Normal Baseline Mobility: Results From the Italian Longitudinal Study on Aging (ILSA). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Lingo VanGilder, J.; Walter, C.S.; Hengge, C.R.; Schaefer, S.Y. Exploring the relationship between visuospatial function and age-related deficits in motor skill transfer. Aging Clin. Exp. Res. 2020, 32, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Robison, M.K.; Diede, N.T.; Nicosia, J.; Ball, B.H.; Bugg, J.M. A multimodal analysis of sustained attention in younger and older adults. Psychol. Aging 2022, 37, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.M.; Forrence, A.D.; Costello, M.G.; Zackowski, K.; Haith, A.M. Age-related increases in reaction time result from slower preparation, not delayed initiation. J. Neurophysiol. 2022, 128, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.R.; Poyser, C.; Schiessl, I. Age-Related Deficits in Visuospatial Memory Are due to Changes in Preparatory Set and Eye–Hand Coordination. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2015, 70, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Raw, R.K.; Wilkie, R.M.; Allen, R.J.; Warburton, M.; Leonetti, M.; Williams, J.H.G.; Mon-Williams, M. Skill acquisition as a function of age, hand and task difficulty: Interactions between cognition and action. PLoS ONE 2019, 14, e0211706. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Cheng, L.; Yang, C. An investigation of the influence of age on eye fatigue and hand operation performance in a virtual environment. Vis. Comput. 2021, 37, 2301–2313. [Google Scholar] [CrossRef]
- Hsieh, S.; Lin, Y. The boundary condition for observing compensatory responses by the elderly in a flanker-task paradigm. Biol. Psychol. 2014, 103, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Dully, J.; McGovern, D.P.; O’Connell, R.G. The impact of natural aging on computational and neural indices of perceptual decision making: A review. Behav. Brain Res. 2018, 355, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hanes, D.P.; Schall, J.D. Neural control of voluntary movement initiation. Science 1996, 274, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Bennet, R.; Reiner, M. How Long Is Too Long: An Individual Time-Window for Motor Planning. Front. Hum. Neurosci. 2019, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Langsdorf, L.; Maresch, J.; Hegele, M.; McDougle, S.D.; Schween, R. Prolonged response time helps eliminate residual errors in visuomotor adaptation. Psychon. Bull. Rev. 2021, 28, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Johari, K.; den Ouden, D.; Behroozmand, R. Effects of aging on temporal predictive mechanisms of speech and hand motor reaction time. Aging Clin. Exp. Res. 2018, 30, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Ghez, C.; Favilla, M.; Ghilardi, M.F.; Gordon, J.; Bermejo, R.; Pullman, S. Discrete and continuous planning of hand movements and isometric force trajectories. Exp. Brain Res. 1997, 115, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Hening, W.; Favilla, M.; Ghez, C. Trajectory control in targeted force impulses. V: Gradual specification of response amplitude. Exp. Brain Res. 1988, 71, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Haith, A.M.; Pakpoor, J.; Krakauer, J.W. Independence of Movement Preparation and Movement Initiation. J. Neurosci. 2016, 36, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Mutter, S.A.; Holder, J.M.; Mashburn, C.A.; Luna, C.M. Aging and the Role of Attention in Associative Learning. Psychol. Aging 2019, 34, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, R.; Haegens, S.; Jazayeri, M.; Merchant, H.; Sternad, D.; Song, J. Neural encoding and representation of time for sensorimotor control and learning. J. Neurosci. 2021, 41, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.E.; Avraham, G.; Ivry, R.B. The psychology of reaching: Action selection, movement implementation, and sensorimotor learning. Annu. Rev. Psychol. 2021, 72, 61–95. [Google Scholar] [CrossRef] [PubMed]
- Reinkensmeyer, D.J.; Burdet, E.; Casadio, M.; Krakauer, J.W.; Kwakkel, G.; Lang, C.E.; Swinnen, S.P.; Ward, N.S.; Schweighofer, N. Computational neurorehabilitation: Modeling plasticity and learning to predict recovery. J. Neuroeng. Rehabil. 2016, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, A.P.; de Vet, H.C.W.; de Bie, R.A.; Kessels, A.G.H.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi List: A Criteria List for Quality Assessment of Randomized Clinical Trials for Conducting Systematic Reviews Developed by Delphi Consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Su, L.; Kinnaird, C.; Kabeto, M.; Shull, P.B.; Sienko, K.H. Vibrotactile display design: Quantifying the importance of age and various factors on reaction times. PLoS ONE 2019, 14, e0219737. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, H.; Xu, P.; Wang, J.; Qiu, Y.; Feng, W.; Luo, Y.; Hu, L.; Guan, Q. The weakened relationship between prestimulus alpha oscillations and response time in older adults with mild cognitive impairment. Front. Hum. Neurosci. 2020, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Marmeleira, J.; Del Pozo-Cruz, J.; Bernardino, A.; Leite, N.; Brandão, M.; Raimundo, A. Acute Effects of Augmented Reality Exergames versus Cycle Ergometer on Reaction Time, Visual Attention, and Verbal Fluency in Community Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 14667. [Google Scholar] [CrossRef] [PubMed]
- Hartle, L.; Martorelli, M.; Balboni, G.; Souza, R.; Charchat-Fichman, H. Diagnostic accuracy of CompCog: Reaction time as a screening measure for mild cognitive impairment. Arq. Neuropsiquiatr. 2022, 80, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, C.; Pace, T.; Blatch-Williams, R.; Van Timmeren, T.; De Wit, S.; Andrews, S.C. Switching gears: Age-related differences in goal-directed and habitual behavior. Neuropsychology 2025, 39, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Alvarado, R.L.; Jog, A.; Greve, D.N.; Salat, D.H. Serial reaction time task performance in older adults with neuropsychologically Defined mild cognitive impairment. J. Alzheimers Dis. 2020, 74, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Jardim, N.Y.V.; Bento-Torres, N.V.O.; Tomás, A.M.; Da Costa, V.O.; Bento-Torres, J.; Picanço-Diniz, C.W. Unexpected cognitive similarities between older adults and young people: Scores variability and cognitive performances. Arch. Gerontol. Geriatr. 2023, 117, 105206. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, D.; Nemeth, D.; Janacsek, K. Is there more room to improve? The lifespan trajectory of procedural learning and its relationship to the between- and within-group differences in average response times. PLoS ONE 2019, 14, e0215116. [Google Scholar] [CrossRef] [PubMed]
- Jutten, R.J.; Amariglio, R.E.; Maruff, P.; Properzi, M.J.; Rentz, D.M.; Johnson, K.A.; Sperling, R.A.; Papp, K.V. Increased Intraindividual Variability in Reaction Time Performance Is Associated With Emerging Cognitive Decline in Cognitively Unimpaired Adults. Neuropsychology 2023, 38, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Hirano, D.; Yano, H.; Taniguchi, K.; Taniguchi, T. Relationship between reaction time variability on go/no go tasks and neuropsychological functioning in younger and older adults. J. Clin. Exp. Neuropsychol. 2023, 45, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, N.M.; Miall, R.C. Proprioceptive deficits in inactive older adults are not reflected in fast targeted reaching movements. Exp. Brain Res. 2019, 237, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Korthauer, L.E.; Salmon, D.P.; Festa, E.K.; Galasko, D.; Heindel, W.C. Alzheimer’s disease and the processing of uncertainty during choice task performance: Executive dysfunction within the Hick–Hyman law. J. Clin. Exp. Neuropsychol. 2019, 41, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Krumpolt, M.; Rahil, D.; Schumacher, A.; Sannemann, L.; Witte, K. Gender-specific improvements in cognitive resources. Z. Gerontol. Geriat. 2025, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.; Marie, D.; Worschech, F.; Krüger, T.H.C.; Sinke, C.; Altenmüller, E.; James, C.E.; Kliegel, M. Effects of a 1-Year Piano Intervention on Cognitive Flexibility in Older Adults. Psychol. Aging 2025, 40, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Xiong, S. New Hick’s law based reaction test App reveals “information processing speed” better identifies high falls risk older people than “simple reaction time”. Int. J. Ind. Ergon. 2017, 58, 25–32. [Google Scholar] [CrossRef]
- Rattanavichit, Y.; Chaikeeree, N.; Boonsinsukh, R.; Kitiyanant, K. The age differences and effect of mild cognitive impairment on perceptual-motor and executive functions. Front. Psychol. 2022, 13, 906898. [Google Scholar] [CrossRef] [PubMed]
- Sleimen-Malkoun, R.; Temprado, J.; Berton, E. Age-related dedifferentiation of cognitive and motor slowing: Insight from the comparison of Hick–Hyman and Fitts’ laws. Front. Aging Neurosci. 2013, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Staub, B.; Doignon-Camus, N.; Bacon, E.; Bonnefond, A. Investigating sustained attention ability in the elderly by using two different approaches: Inhibiting ongoing behavior versus responding on rare occasions. Acta Psychol. 2014, 146, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tait, J.L.; Duckham, R.L.; Rantalainen, T.; Milte, C.M.; Main, L.C.; Nowson, C.A.; Sanders, K.M.; Taaffe, D.R.; Hill, K.D.; Abbott, G.; et al. Effects of a 6-month dual-task, power-based exercise program on cognitive function, neurological and inflammatory markers in older adults: Secondary analysis of a cluster randomised controlled trial. GeroScience 2024, 47, 1251–1268. [Google Scholar] [CrossRef] [PubMed]
- Unger, K.; Wylie, J.; Karbach, J. Age-related changes in the effects of induced positive affect on executive control in younger and older adults—Evidence from a task-switching paradigm. Aging Neuropsychol. Cogn. 2024, 32, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Van Humbeeck, N.; Van Wilderode, M.; Kliegl, R.; Van Wieringen, A.; Krampe, R.T. Multitasking across the lifespan in different task contexts. Sci. Rep. 2024, 14, 11817. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, B.P.; Binns, M.A.; Anderson, N.D. Staying on Task: Age-Related Changes in the Relationship Between Executive Functioning and Response Time Consistency. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2016, 71, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Welhaf, M.S.; Wilks, H.; Aschenbrenner, A.J.; Balota, D.A.; Schindler, S.E.; Benzinger, T.L.S.; Gordon, B.A.; Cruchaga, C.; Xiong, C.; Morris, J.C.; et al. Naturalistic assessment of reaction time variability in older adults at risk for Alzheimer’s disease. J. Int. Neuropsychol. Soc. 2024, 30, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Worschech, F.; Passarotto, E.; Losch, H.; Oku, T.; Lee, A.; Altenmüller, E. What Does It Take to Play the Piano? Cognito-Motor Functions Underlying Motor Learning in Older Adults. Brain Sci. 2024, 14, 405. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Stawski, R.S.; Hultsch, D.F.; MacDonald, S.W.S. Selective attrition and intraindividual variability in response time moderate cognitive change. J. Clin. Exp. Neuropsychol. 2016, 38, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Williams, J.; Wilmut, K. Constraints on motor planning across the life span: Physical, cognitive, and motor factors. Psychol. Aging 2020, 35, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Liu-Ambrose, T.Y.; Ashe, M.C.; Graf, P.; Beattie, B.L.; Khan, K.M. Increased risk of falling in older community-dwelling women with mild cognitive impairment. Phys. Ther. 2008, 88, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Buracchio, T.J.; Mattek, N.C.; Dodge, H.H.; Hayes, T.L.; Pavel, M.; Howieson, D.B.; Kaye, J.A. Executive function predicts risk of falls in older adults without balance impairment. BMC Geriatr. 2011, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Butler, A.A.; Lord, S.R.; Delbaere, K.; Kurrle, S.E.; Mikolaizak, A.S.; Close, J. Inaccurate judgement of reach is associated with slow reaction time, poor balance, impaired executive function and predicts prospective falls in older people with cognitive impairment. Exp. Gerontol. 2018, 114, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bouazzaoui, B.; Isingrini, M.; Fay, S.; Angel, L.; Vanneste, S.; Clarys, D.; Taconnat, L. Aging and self-reported internal and external memory strategy uses: The role of executive functioning. Acta Psychol. 2010, 135, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Pizzonia, K.L.; Suhr, J.A. Systematic Review of Correlates of Internal and External Memory Strategy Use in Older Adults. J. Appl. Gerontol. 2022, 41, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Schweiger, A.; Herman, T.; Yogev-Seligmann, G.; Giladi, N. Dual-task decrements in gait: Contributing factors among healthy older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, C.J.; van Deudekom, F.J.; van Campen, J.P.; Appels, B.A.; de Vries, O.J.; Pijnappels, M. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. J. Neuroeng. Rehabil. 2011, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.W.; Chan, V.W.; Wong, H.H.; Yip, T.W.; Lu, X. The effect of performing a dual-task on postural control and selective attention of older adults when stepping backward. J. Phys. Ther. Sci. 2016, 28, 2806–2811. [Google Scholar] [CrossRef] [PubMed]
- Van Iersel, M.B.; Kessels, R.P.C.; Bloem, B.R.; Verbeek, A.L.; Olde Rikkert, M.G.M. Executive functions are associated with gait and balance in community-living elderly people. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Lindenberger, U.; Marsiske, M.; Baltes, P.B. Memorizing while walking: Increase in dual-task costs from young adulthood to old age. Psychol. Aging 2000, 15, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Allali, G.; Assal, F.; Kressig, R.W.; Dubost, V.; Herrmann, F.R.; Beauchet, O. Impact of Impaired Executive Function on Gait Stability. Dement. Geriatr. Cogn. Disord. 2008, 26, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Springer, S.; Giladi, N.; Peretz, C.; Yogev, G.; Simon, E.S.; Hausdorff, J.M. Dual-tasking effects on gait variability: The role of aging, falls, and executive function. Mov. Disord. 2006, 21, 950–957. [Google Scholar] [CrossRef] [PubMed]
- van het Reve, E.; de Bruin, E.D. Strength-balance supplemented with computerized cognitive training to improve dual task gait and divided attention in older adults: A multicenter randomized-controlled trial. BMC Geriatr. 2014, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Giraudeau, C.; Musielak, C.; Hervé, C.; Seren, D.; Chasseigne, G.; Mullet, E. Aging, Functional Learning, and Inhibition. Exp. Aging Res. 2016, 42, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Verneau, M.; van der Kamp, J.; Savelsbergh, G.J.P.; de Looze, M.P. Optimising assembly learning in older adults through the manipulation of instruction. Ergonomics 2014, 57, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Berghuis, K.M.M.; Veldman, M.P.; Solnik, S.; Koch, G.; Zijdewind, I.; Hortobágyi, T. Neuronal mechanisms of motor learning and motor memory consolidation in healthy old adults. Age 2015, 37, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Voelcker-Rehage, C. Motor-skill learning in older adults—A review of studies on age-related differences. Eur. Rev. Aging Phys. Act. 2008, 5, 5–16. [Google Scholar] [CrossRef]
- Kobayashi-Cuya, K.E.; Sakurai, R.; Suzuki, H.; Ogawa, S.; Takebayashi, T.; Fujiwara, Y. Observational Evidence of the Association Between Handgrip Strength, Hand Dexterity, and Cognitive Performance in Community-Dwelling Older Adults: A Systematic Review. J. Epidemiol. 2018, 28, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Konczak, J.; Sciutti, A.; Avanzino, L.; Squeri, V.; Gori, M.; Masia, L.; Abbruzzese, G.; Sandini, G. Parkinson’s disease accelerates age-related decline in haptic perception by altering somatosensory integration. Brain 2012, 135, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Parthasharathy, M.; Mantini, D.; Orban De Xivry, J. Increased upper-limb sensory attenuation with age. J. Neurophysiol. 2022, 127, 474–492. [Google Scholar] [CrossRef] [PubMed]
- Juravle, G.; Binsted, G.; Spence, C. Tactile suppression in goal-directed movement. Psychon. Bull. Rev. 2017, 24, 1060–1076. [Google Scholar] [CrossRef] [PubMed]
- Kilteni, K.; Ehrsson, H.H. Predictive attenuation of touch and tactile gating are distinct perceptual phenomena. iScience 2022, 25, 104077. [Google Scholar] [CrossRef] [PubMed]
- Klever, L.; Voudouris, D.; Fiehler, K.; Billino, J. Age effects on sensorimotor predictions: What drives increased tactile suppression during reaching? J. Vis. 2019, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Qualls, C.E.; Bliwise, N.G.; Stringer, A.Y. Short Forms of The Benton Judgment of Line Orientation Test: Development and Psychometric Properties. Arch. Clin. Neuropsychol. 2000, 15, 159–163. [Google Scholar] [PubMed]
- Treccani, B.; Cubelli, R. The need for a revised version of the Benton judgment of line orientation test. J. Clin. Exp. Neuropsychol. 2011, 33, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Aman, J.E.; Elangovan, N.; Yeh, I.; Konczak, J. The effectiveness of proprioceptive training for improving motor function: A systematic review. Front. Hum. Neurosci. 2015, 8, 1075. [Google Scholar] [CrossRef] [PubMed]
- Sarlegna, F.R.; Sainburg, R.L. The roles of vision and proprioception in the planning of reaching movements. Adv. Exp. Med. Biol. 2009, 629, 317–335. [Google Scholar] [PubMed]
- Dion, C.; Arias, F.; Amini, S.; Davis, R.; Penney, D.; Libon, D.J.; Price, C.C. Cognitive Correlates of Digital Clock Drawing Metrics in Older Adults with and without Mild Cognitive Impairment. J. Alzheimers Dis. 2020, 75, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Chun, C.T.; Seward, K.; Patterson, A.; Melton, A.; Macdonald-Wicks, L. Evaluation of Available Cognitive Tools Used to Measure Mild Cognitive Decline: A Scoping Review. Nutrients 2021, 13, 3974. [Google Scholar] [CrossRef] [PubMed]
- Hazan, E.; Frankenburg, F.; Brenkel, M.; Shulman, K. The test of time: A history of clock drawing. Int. J. Geriat. Psychiatry 2017, 33, e22–e30. [Google Scholar] [CrossRef] [PubMed]
- Talwar, N.A.; Churchill, N.W.; Hird, M.A.; Pshonyak, I.; Tam, F.; Fischer, C.E.; Graham, S.J.; Schweizer, T.A. The neural correlates of the clock-drawing test in healthy aging. Front. Hum. Neurosci. 2019, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Eknoyan, D.; Hurley, R.A.; Taber, K.H. The clock drawing task: Common errors and functional neuroanatomy. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Gainotti, G.; Trojano, L. Constructional apraxia. Handb. Clin. Neurol. 2018, 151, 331–348. [Google Scholar] [PubMed]
- Hagger-Johnson, G.; Deary, I.J.; Davies, C.A.; Weiss, A.; Batty, G.D. Reaction Time and Mortality from the Major Causes of Death: The NHANES-III Study. PLoS ONE 2014, 9, e82959. [Google Scholar] [CrossRef] [PubMed]
- Kochan, N.A.; Bunce, D.; Pont, S.; Crawford, J.D.; Brodaty, H.; Sachdev, P.S. Is intraindividual reaction time variability an independent cognitive predictor of mortality in old age? Findings from the Sydney Memory and Ageing Study. PLoS ONE 2017, 12, e0181719. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Cao, S.; Liu, W.; Li, L.; Jiang, J.; Wu, L. Is simple reaction time or choice reaction time an indicator of all-cause mortality or CVD mortality? Public Health 2021, 199, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Clark, B.C. Dynapenia and aging: An update. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67A, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Lee, S.; Song, S. A review of sarcopenia pathophysiology, diagnosis, treatment and future direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef] [PubMed]
- Hedman, L.D.; Rogers, M.W.; Hanke, T.A. Neurologic Professional Education: Linking the Foundation Science of Motor Control With Physical Therapy Interventions for Movement Dysfunction. Neurol. Rep. 1996, 20, 9–13. [Google Scholar] [CrossRef]
- Schenkman, M.; Deutsch, J.E.; Gill-Body, K.M. An Integrated Framework for Decision Making in Neurologic Physical Therapist Practice. Phys. Ther. 2006, 86, 1681–1702. [Google Scholar] [CrossRef] [PubMed]
- Weinrich, M.; Wise, S.P. The premotor cortex of the monkey. J. Neurosci. 1982, 2, 1329–1345. [Google Scholar] [CrossRef] [PubMed]
- Riehle, A.; Requin, J. Monkey primary motor and premotor cortex: Single-cell activity related to prior information about direction and extent of an intended movement. J. Neurophysiol. 1989, 61, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Riehle, A.; Requin, J. Neuronal correlates of the specification of movement direction and force in four cortical areas of the monkey. Behav. Brain Res. 1995, 70, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, K.; Li, N. Neural mechanisms of movement planning: Motor cortex and beyond. Curr. Opin. Neurobiol. 2018, 49, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.K.; Chen, S.; Daie, K.; Finkelstein, A.; Fontolan, L.; Romani, S.; Svoboda, K. Neural algorithms and circuits for motor planning. Annu. Rev. Neurosci. 2022, 45, 249–271. [Google Scholar] [CrossRef] [PubMed]
| A. Studies that Directly Assessed an Association | ||||
| Study Population | Inclusion and Exclusion Criteria | Cognitive Domain or Subdomain [test] | Movement and RT Measures | Results and Associations |
| Kimura et al. (2023) [53] | ||||
| 72 ± 3 years n = 31 ∧ 77% male | Inclusion No diagnosis of MCI or dementia Clinical DRS = 0 Able to perform ADLs and IADLs MoCA ≥ 26 (Japanese version) Exclusion Diagnosis of dementia, MCI, mental disorder, cerebrovascular disease | Global [MoCA] Processing Speed [TMT-A, TMT-B] Executive Function a [TMT-B] Attention [TMT-A] Language b [Verbal Fluency Test] | Right thumb button press Complex RT Go/No-go task Feature: stimulus letter Frequency: 75% Go, 25% No-go | Intraindividual variability in RT in older adults was associated with poorer performance in global cognition and cognitive domains of processing speed, attention, and language. Intraindividual variability in RT was not associated with a working memory measure of executive function. There were no age-related differences in adjusted intraindividual variability in RT. |
| Korthauer et al. (2019) [55] | ||||
| Externally cued task 77 ± 6 years n = 12 ∧ 25% male Internally driven task 76 ± 5 years n = 14 ∧ 28% male | Inclusion Cognitively healthy Exclusion History of alcohol use History of substance use Learning disabilities Serious psychiatric illness | Global [MMSE, BDS, DRS] Processing Speed [WAIS-R (Digit Symbol Modalities), TMT-A] Visuospatial Ability [WAIS-R (Block Design)] Executive function [TMT-B, Letter Fluency, modified WCST, Clock Drawing Test] Attention c [WAIS-R (Digit Span)] Learning and Memory [CVLT, WMS-R (Visual Reproduction and Logical Memory)] Language [BNT, WAIS-R (Vocabulary and Category Fluency)] | Card sorting, externally cued uncertainty Choice RT 2–5 choices Feature: shape, number of sorting piles cued the stimulus-response uncertainty Card sorting, internally driven uncertainty Choice RT 5 choices Feature: shape, relative frequency of shapes varied but was not cued by the number of sorting piles | For the externally cued uncertainty task, longer RTs were associated with poorer memory in the highest uncertainty condition. For the internally driven uncertainty task, longer RTs were associated with poorer executive function. The associations of global cognition, processing speed, attention, and language with RT were not assessed. |
| Staub et al. (2014) [61] | ||||
| 65 years (60–74) n = 30 ∧ 47% male | Inclusion No neurological or psychiatric disease Normal or corrected-to-normal vision | Attention d [Sustained Attention to Response Task] | Key press Complex RT Digits 1–9 presented on screen Target: “3” Response Task: press key only for target Response Inhibition Task: press key for all digits except target | Attention deficits in the response task for older adults were suggested as error rates and RTs increased over time. In the response inhibition task, no deficits in attention were found for older adults as error rates decreased over time while RTs increased. |
| Welhaf et al. (2024) [66] | ||||
| 76 ± 6 years (62–76) n = 345 ∧ 41% male | Inclusion Clinical Dementia Rating = 0 | Processing Speed [Digit Span Forward, Number Symbol Test] Attention [Stroop Incongruent, CVOE Switch] Learning and Memory e [WMS Paired Associative Recall, Free and Cued Selective Reminding Test, Craft Story 21 (Immediate and Delayed Recall)] Language f [CVFT, Multilingual Naming Test] | Touch screen tap Ambulatory Research in Cognition Symbols task Choice RT 2 choices Feature: shape pairs | Greater RT variability was correlated with poorer performance in processing speed, attention, episodic memory, and semantic memory. |
| Yao et al. (2016) [68] | ||||
| 74 ± 6 years (64–92) n = 304 32% male | Inclusion Older adults concerned about cognitive function Exclusion Diagnosis of dementia or MMSE < 24 History of significant head injury Neurological or major medical illnesses Severe sensory impairment Substance or alcohol use Psychiatric diagnosis Psychotropic drug use Not fluent in English | Perceptual Speed [TMT-A, TMT-B] Executive Function g [WAIS-III] Memory e [Immediate free recall] Language [Vocabulary] | Key press Choice RT 4 choices Feature: location Choice/Complex RT 4 choices Feature: location of previous cue Complex RT Stimuli choices varied in shape (square, circle) and color (red, green) Stimulus feature cued at beginning of trial | Greater RT variability in choice and complex motor tasks was associated with poorer cognitive function in perceptual speed, executive function, and memory. RT variability was not associated with language ability. Over a five-year period, memory function was associated with decreased RT in choice tasks, while both perceptual speed and memory function were associated with decreased RT in complex tasks. |
| B. Studies that indirectly assessed an association | ||||
| Study Population | Inclusion and Exclusion Criteria | Cognitive Domain or Subdomain [test] | Movement and RT Measures | Results and Associations |
| Bao et al. (2019) [44] | ||||
| 71 ± 6 years n = 9 44% male | Inclusion No neurological conditions No joint replacements Stand independently > 1 min Exclusion Impaired sensation on the dorsal aspect of the dominant foot | Attention [Backwards counting by 3s] | Thumb trigger press Complex RT Respond to vibrotactile stimuli while performing cognitive task | Attention was divided with a dual task paradigm and affected both RT and cognitive performance.
|
| Juhasz et al. (2019) [51] | ||||
| 66 ± 6 years (61–85) n = 26 ∧ 19% male | Inclusion None Exclusion Participants with response time or accuracy < 3 standard deviations of the group Developmental, psychiatric, or neurological disorders | Learning [General skill learning, triplet learning] | Key press Complex RT Alternating serial RT 4 choices Feature: location, frequency of repeated key responses | General skill learning measured by the change in RT with task performance was correlated with average RT in older adults. This correlation was decreased when general skill learning was normalized by the average RT. Triplet learning, measured by the change in RT for low- and high-frequency key press triplets, was not correlated with average RT in older adults. |
| Jutten et al. (2023) [52] | ||||
| 77 ± 5 years (68–89) n = 109 39% male | Inclusion Age > 65 years Clinical DRS = 0 MMSE > 25 Delayed Recall of Logical Memory Story A > cutoff adjusted by age and education Geriatric Depression Scale < 11 Exclusion History of alcohol use History of drug use History of head trauma Current serious medical or psychiatric illness | Global [Preclinical Alzheimer’s Cognitive Composite-5, including MMSE, WMS-R (Logical), DSCT, free and cued selective reminding test, and CVFT] Processing Speed [TMT-A, DSCT] Executive Function [Controlled Oral Word Association Test, TMT-B/A] Memory [WMS-R (Logical), selective reminding test, free and cued selective reminding test] | Touch screen tapCBB Simple RT (detection) 1-choice task Choice RT (identification) 2 choices Feature: color Complex RT Stimulus: playing cards Determine if you have seen the stimulus before (one-card learning). Determine if the stimulus is the same as the previous (one back). | Intraindividual variability in RT was measured each month for one year. An association between cognitive tests and RT was not assessed for a single session. Greater variability in combined simple and choice RT was associated with poorer baseline performance in global cognition, processing speed, and memory without adjustment for mean RT. Greater variability in complex RT was associated with poorer baseline performance in executive function with and without adjustment for mean RT. |
| Vasquez et al. (2016) [65] | ||||
| 74 ± 6 years (65–85) n = 48 ∧ 27% male | Inclusion Normal cognitive function assessed by the modified TICS Exclusion History of significant head injury Neurological or major medical illness Radiation to the head Drug abuse Current use of psychiatric medications Not fluent in English | Global [MMSE, WAIS-III] Processing Speed [WAIS-III (Digit Symbol Coding)] Executive Function [WCST, D-KEFS (Trail Making and Color-Word Interference)] Learning and Memory [WMS-III (Logical Memory and Digit Span h forwards and backwards)] Language [BNT, D-KEFS (Fluency h)] | Screen tap with a stylus Complex RT Playing cards moved horizontally across the screen Target: 8 of spades Features: color, suit, and number; distractor cards shared 0, 1, or 2 features with the target | When combined across young and old adults, age-related differences in RT distribution were associated with executive function.
The associations of executive function, learning and memory, and language with RT were not assessed directly. |
| RT Measure | ||||
| Simple | Choice | Complex | ||
| Cognitive Domain—Subdomain | Global Cognition | None | Weak | None |
| Sensation and Perception | None | None | None | |
| Motor Skills and Construction | None | None | None | |
| Perceptual Motor Function —Processing/ Perceptual Speed | None | Weak | Weak | |
| —Visuospatial Ability | None | None | None | |
| Executive Function | None | Weak | Weak | |
| Attention | None | Weak | Weak | |
| Learning and Memory —Learning | None | None | Weak | |
| —Memory | None | Moderate | Weak | |
| Language | None | None | Weak | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, A.; Weaver, N.; So, M.E.; Jaffri, A.; Heckman, R.L. Limited Evidence to Review—Is There an Association Between Cognition and Upper Extremity Motor Reaction Time in Older Adults? NeuroSci 2025, 6, 71. https://doi.org/10.3390/neurosci6030071
Jones A, Weaver N, So ME, Jaffri A, Heckman RL. Limited Evidence to Review—Is There an Association Between Cognition and Upper Extremity Motor Reaction Time in Older Adults? NeuroSci. 2025; 6(3):71. https://doi.org/10.3390/neurosci6030071
Chicago/Turabian StyleJones, Alexandria, Natalie Weaver, Mardon E. So, Abbis Jaffri, and Rosalind L. Heckman. 2025. "Limited Evidence to Review—Is There an Association Between Cognition and Upper Extremity Motor Reaction Time in Older Adults?" NeuroSci 6, no. 3: 71. https://doi.org/10.3390/neurosci6030071
APA StyleJones, A., Weaver, N., So, M. E., Jaffri, A., & Heckman, R. L. (2025). Limited Evidence to Review—Is There an Association Between Cognition and Upper Extremity Motor Reaction Time in Older Adults? NeuroSci, 6(3), 71. https://doi.org/10.3390/neurosci6030071






