β2-Microglobulin Regulates Extracellular Matrix Dynamics During Peripheral Nerve Injury
Abstract
1. Introduction
2. Materials and Methods
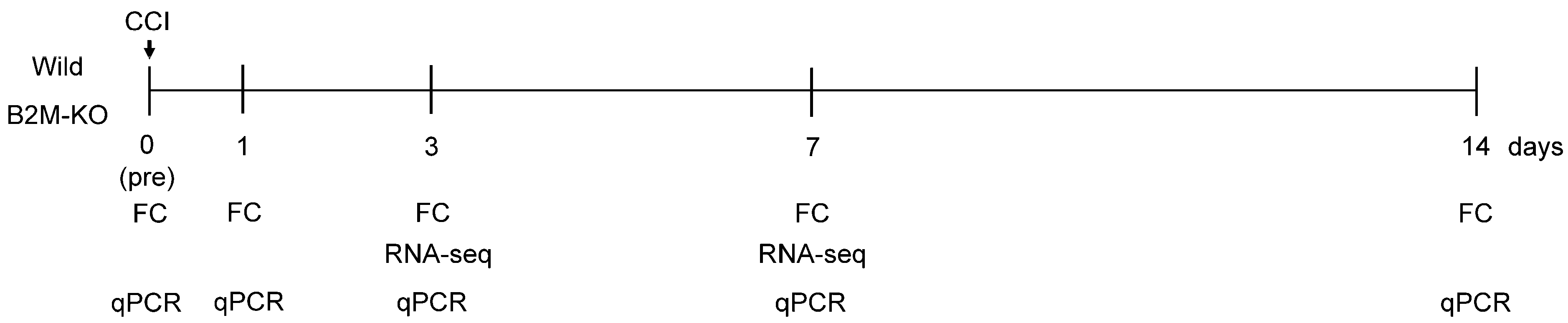
2.1. Sciatic Nerve Injury (SNI) Model
2.2. Flow Cytometric Analysis
2.3. Transcriptome Analysis
2.4. Statistical Analysis
3. Results
3.1. Differential Dynamics of CD4+ and CD8+ T-Cell Populations Between Wild-Type and B2M-KO Mice Following SNI
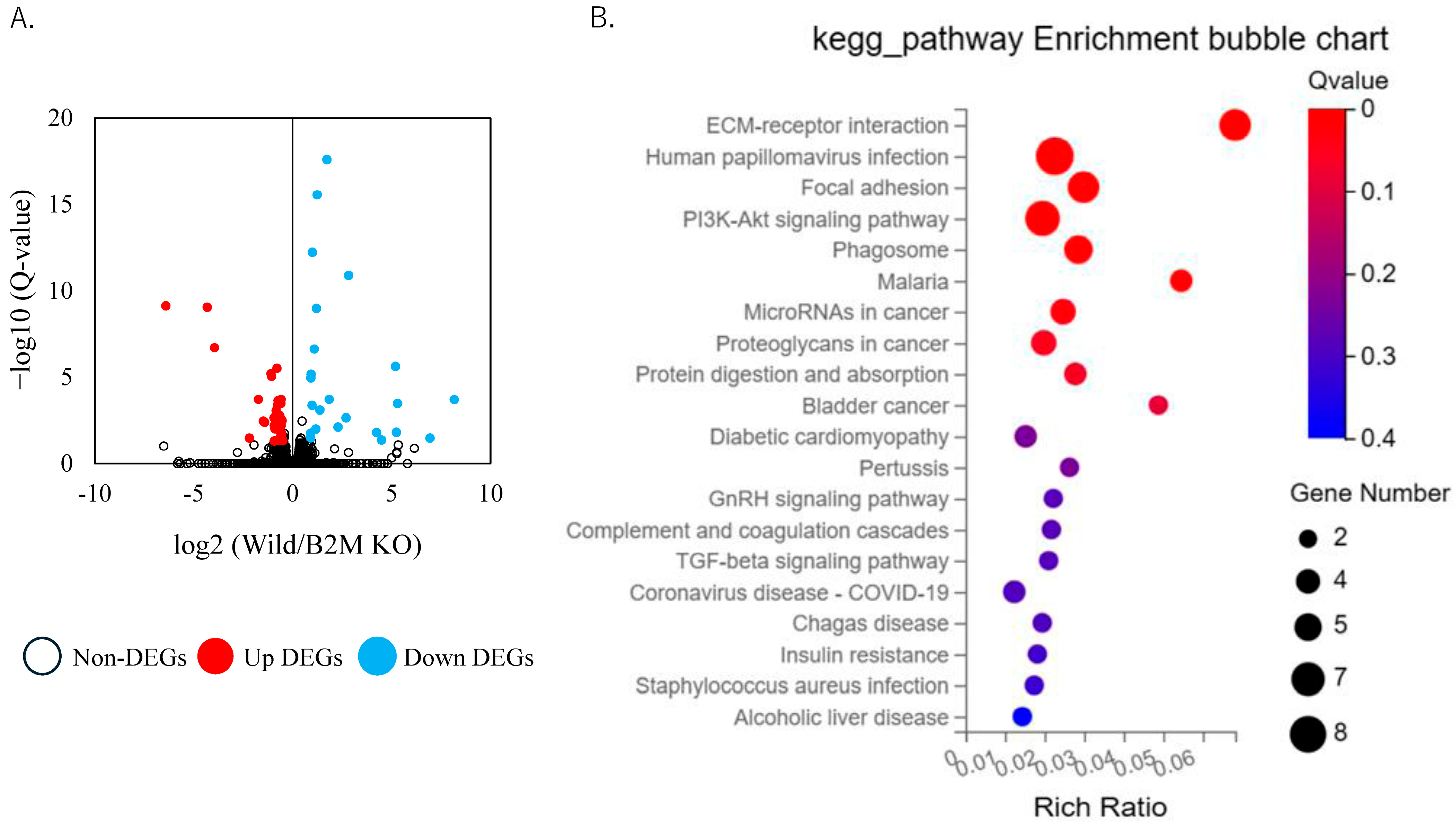
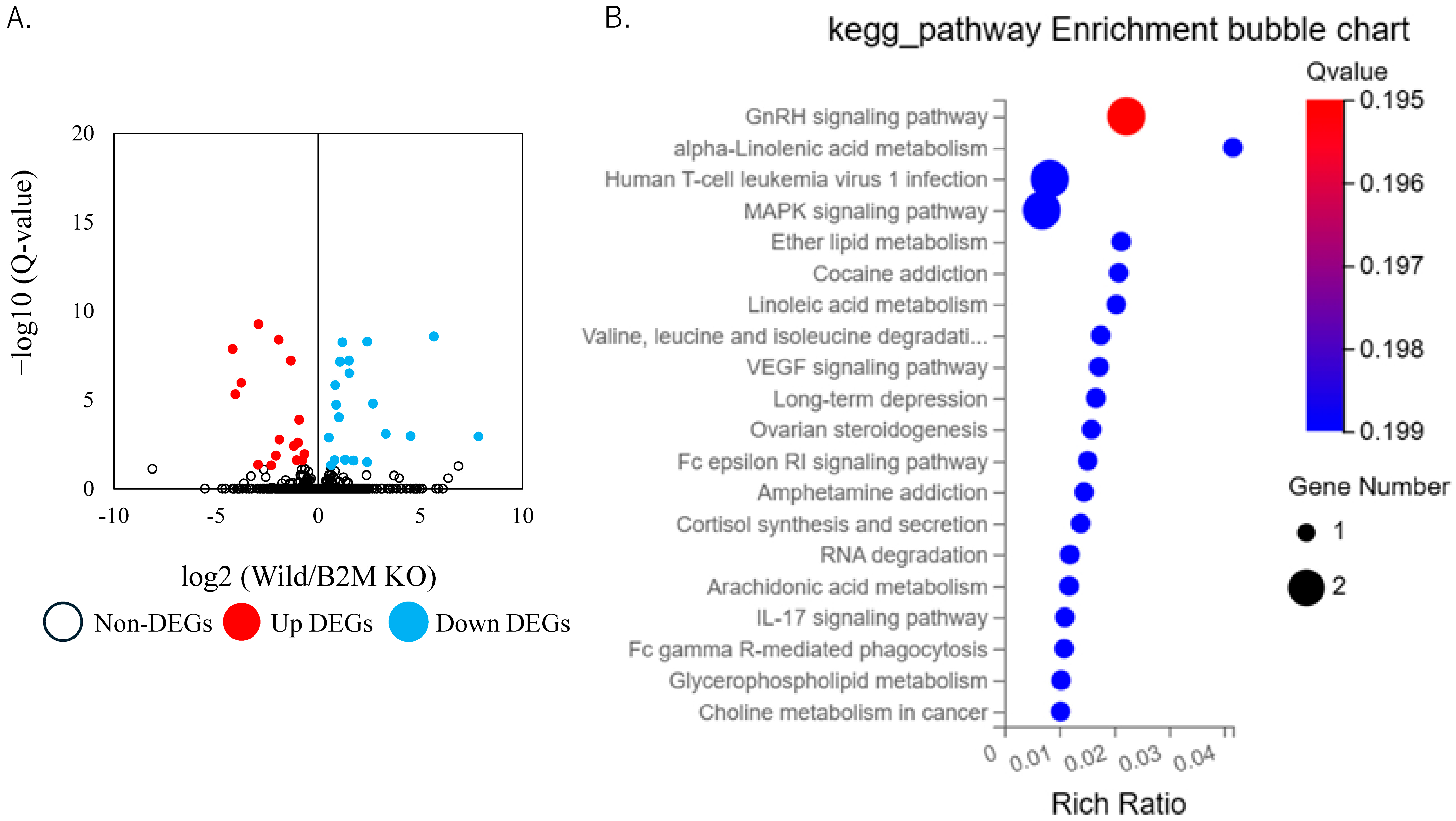
3.2. Altered Extracellular Matrix Dynamics and Remodeling Pathways in B2M-KO Mice Following SNI
3.3. Non-ECM Transcriptomic Changes Following Sciatic Nerve Injury
3.4. Validation of THBS1 Expression by qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | extracellular matrix |
| B2M | β2-microglobulin |
| TGF-β | transforming growth factor-beta |
| CCI | chronic constriction injury |
References
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle Nerve 2022, 66, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Braza, D.; Rice, J.B.; Dillingham, T. The incidence of peripheral nerve injury in extremity trauma. Am. J. Phys. Med. Rehabil. 2008, 87, 381–385. [Google Scholar] [CrossRef]
- Grosu-Bularda, A.; Vancea, C.V.; Hodea, F.V.; Cretu, A.; Bordeanu-Diaconescu, E.M.; Dumitru, C.S.; Ratoiu, V.A.; Teodoreanu, R.N.; Lascar, I.; Hariga, C.S. Optimizing Peripheral Nerve Regeneration: Surgical Techniques, Biomolecular and Regenerative Strategies-A Narrative Review. Int. J. Mol. Sci. 2025, 26, 3895. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cheng, N.; Deng, Y.; Xiang, P.; Liang, J.; Zhang, Z.; Hei, Z.; Li, X. Astrocyte senescence-like response related to peripheral nerve injury-induced neuropathic pain. Cell Mol. Biol. Lett. 2023, 28, 65. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J.; Yin, W.; Gao, P.; Fan, Y.; Wen, D.; Jiao, Y.; Yu, W. The peripheral Atf3 (+) neuronal population is responsible for nerve regeneration at the early stage of nerve injury revealed by single-cell RNA sequencing. Acta Biochim. Biophys. Sin. 2024, 57, 424–436. [Google Scholar] [CrossRef]
- Yong, N.; Guoping, C. The role and mechanism of the up-regulation of fibrinolytic activity in painful peripheral nerve injury. Neurochem. Res. 2009, 34, 587–592. [Google Scholar] [CrossRef]
- Wei, G.; Chen, C.; Li, X.; Wang, H.; Li, Z.; Gou, X.; Zhang, P. In situ piezoelectricity induces M2 polarization of macrophages to regulate Schwann cells for alleviating neuropathic pain of CCI rats. Biomater. Adv. 2025, 174, 214319. [Google Scholar] [CrossRef]
- Chen, P.; Cescon, M.; Megighian, A.; Bonaldo, P. Collagen VI regulates peripheral nerve myelination and function. FASEB J. 2014, 28, 1145–1156. [Google Scholar] [CrossRef]
- Chen, P.; Cescon, M.; Bonaldo, P. The Role of Collagens in Peripheral Nerve Myelination and Function. Mol. Neurobiol. 2015, 52, 216–225. [Google Scholar] [CrossRef]
- Bray, E.R.; Yungher, B.J.; Levay, K.; Ribeiro, M.; Dvoryanchikov, G.; Ayupe, A.C.; Thakor, K.; Marks, V.; Randolph, M.; Danzi, M.C.; et al. Thrombospondin-1 Mediates Axon Regeneration in Retinal Ganglion Cells. Neuron 2019, 103, 642–657.e7. [Google Scholar] [CrossRef]
- Sang, Q.; Sun, D.; Chen, Z.; Zhao, W. NGF and PI3K/Akt signaling participate in the ventral motor neuronal protection of curcumin in sciatic nerve injury rat models. Biomed. Pharmacother. 2018, 103, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Jalise, S.Z.; Habibi, S.; Fath-Bayati, L.; Habibi, M.A.; Ababzadeh, S.; Hosseinzadeh, F. Role and Interplay of Different Signaling Pathways Involved in Sciatic Nerve Regeneration. J. Mol. Neurosci. 2024, 74, 108. [Google Scholar] [CrossRef]
- Ding, Z.; Jiang, M.; Qian, J.; Gu, D.; Bai, H.; Cai, M.; Yao, D. Role of transforming growth factor-beta in peripheral nerve regeneration. Neural Regen. Res. 2024, 19, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Koller, B.H.; Marrack, P.; Kappler, J.W.; Smithies, O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 1990, 248, 1227–1230. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.; Paul, P.; Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Thams, S.; Lidman, O.; Piehl, F.; Hokfelt, T.; Karre, K.; Linda, H.; Cullheim, S. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc. Natl. Acad. Sci. USA 2004, 101, 17843–17848. [Google Scholar] [CrossRef]
- Muneshige, K.; Onuma, K.; Sukegawa, K.; Otake, Y.; Inoue, G.; Takaso, M.; Uchida, K. beta2-Microglobulin Elevates COL5A1 mRNA in the Subsynovial Connective Tissue of Patients Receiving Hemodialysis with Carpal Tunnel Syndrome. Cureus 2022, 14, e32423. [Google Scholar]
- Li, D.; Zhang, Q.; Li, L.; Chen, K.; Yang, J.; Dixit, D.; Gimple, R.C.; Ci, S.; Lu, C.; Hu, L.; et al. beta2-Microglobulin Maintains Glioblastoma Stem Cells and Induces M2-like Polarization of Tumor-Associated Macrophages. Cancer Res. 2022, 82, 3321–3334. [Google Scholar] [CrossRef]
- Sun, W.; Gui, L.; Zuo, X.; Zhang, L.; Zhou, D.; Duan, X.; Ren, W.; Xu, G. Human epithelial-type ovarian tumour marker beta-2-microglobulin is regulated by the TGF-beta signaling pathway. J. Transl. Med. 2016, 14, 75. [Google Scholar] [CrossRef]
- Ma, C.H.; Omura, T.; Cobos, E.J.; Latremoliere, A.; Ghasemlou, N.; Brenner, G.J.; van Veen, E.; Barrett, L.; Sawada, T.; Gao, F.; et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J. Clin. Investig. 2011, 121, 4332–4347. [Google Scholar] [CrossRef]
- Nishimoto, S.; Okada, K.; Tanaka, H.; Okamoto, M.; Fujisawa, H.; Okada, T.; Naiki, M.; Murase, T.; Yoshikawa, H. Neurotropin attenuates local inflammatory response and inhibits demyelination induced by chronic constriction injury of the mouse sciatic nerve. Biologicals 2016, 44, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Hofstetter, H.H.; Meuth, S.G.; Braeuninger, S.; Sommer, C.; Stoll, G. T cell infiltration after chronic constriction injury of mouse sciatic nerve is associated with interleukin-17 expression. Exp. Neurol. 2006, 200, 480–485. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Ruggiero, F. The collagen superfamily: From the extracellular matrix to the cell membrane. Pathol. Biol. 2005, 53, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Chiquet, M.; Birk, D.E.; Bonnemann, C.G.; Koch, M. Collagen XII: Protecting bone and muscle integrity by organizing collagen fibrils. Int. J. Biochem. Cell Biol. 2014, 53, 51–54. [Google Scholar] [CrossRef]
- Sances, S.; Ho, R.; Vatine, G.; West, D.; Laperle, A.; Meyer, A.; Godoy, M.; Kay, P.S.; Mandefro, B.; Hatata, S.; et al. Human iPSC-Derived Endothelial Cells and Microengineered Organ-Chip Enhance Neuronal Development. Stem Cell Rep. 2018, 10, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Chernousov, M.A.; Baylor, K.; Stahl, R.C.; Stecker, M.M.; Sakai, L.Y.; Lee-Arteaga, S.; Ramirez, F.; Carey, D.J. Fibrillin-2 is dispensable for peripheral nerve development, myelination and regeneration. Matrix Biol. 2010, 29, 357–368. [Google Scholar] [CrossRef]
- Zhu, S.; Ye, L.; Bennett, S.; Xu, H.; He, D.; Xu, J. Molecular structure and function of microfibrillar-associated proteins in skeletal and metabolic disorders and cancers. J. Cell Physiol. 2021, 236, 41–48. [Google Scholar] [CrossRef]
- Ching, S.; Zhang, H.; Chen, Q.; Quan, N. Differential expression of extracellular matrix and adhesion molecule genes in the brain of juvenile versus adult mice in responses to intracerebroventricular administration of IL-1. Neuroimmunomodulation 2007, 14, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Dong, J.; Zhang, F.; Zhao, D.; Yang, Q.; Wen, J.; Sun, Y.; Wei, J.; Liu, Z. Entrectinib can induce nerve cell damage by inhibiting PI3K-AKT and TGF-beta signaling pathways. Front. Pharmacol. 2025, 16, 1489210. [Google Scholar] [CrossRef]
- Zhou, L.; Kong, G.; Palmisano, I.; Cencioni, M.T.; Danzi, M.; De Virgiliis, F.; Chadwick, J.S.; Crawford, G.; Yu, Z.; De Winter, F.; et al. Reversible CD8 T cell-neuron cross-talk causes aging-dependent neuronal regenerative decline. Science 2022, 376, eabd5926. [Google Scholar] [CrossRef]
- Bali, K.K.; Kuner, R. Therapeutic potential for leukocyte elastase in chronic pain states harboring a neuropathic component. Pain 2017, 158, 2243–2258. [Google Scholar] [CrossRef]
- Hu, P.; Bembrick, A.L.; Keay, K.A.; McLachlan, E.M. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav. Immun. 2007, 21, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Bombeiro, A.L.; Thome, R.; Oliveira Nunes, S.L.; Monteiro Moreira, B.; Verinaud, L.; Oliveira, A.L. MHC-I and PirB Upregulation in the Central and Peripheral Nervous System following Sciatic Nerve Injury. PLoS ONE 2016, 11, e0161463. [Google Scholar]
- Kawaguchi, T.; Qin, L.; Shimomura, T.; Kondo, J.; Matsumoto, K.; Denda, K.; Kitamura, N. Purification and cloning of hepatocyte growth factor activator inhibitor type 2, a Kunitz-type serine protease inhibitor. J. Biol. Chem. 1997, 272, 27558–27564. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.R.; Lee, J.; Lee, D.; Nho, B.; Kim, S. Hepatocyte Growth Factor (HGF) Promotes Peripheral Nerve Regeneration by Activating Repair Schwann Cells. Sci. Rep. 2018, 8, 8316. [Google Scholar] [CrossRef] [PubMed]
- Near, S.L.; Whalen, L.R.; Miller, J.A.; Ishii, D.N. Insulin-like growth factor II stimulates motor nerve regeneration. Proc. Natl. Acad. Sci. USA 1992, 89, 11716–11720. [Google Scholar] [CrossRef]
- Recio-Pinto, E.; Rechler, M.M.; Ishii, D.N. Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J. Neurosci. 1986, 6, 1211–1219. [Google Scholar] [CrossRef]


| Primer | Sequence (5′–3′) | Product Size (bp) |
|---|---|---|
| Thbs1-F | TAGCTGGAAATGTGGTGCGT | 123 |
| Thbs1-R | TTGCACCGATGTTCTCCGTT | |
| Gapdh-F | AACTTTGGCATTGTGGAAGG | 223 |
| Gapdh-R | ACACATTGGGGGTAGGAACA |
| Gene ID | Gene Symbol | log2 (FC) | Q-Value |
|---|---|---|---|
| 12816 | Col12a1 | −0.584 | 6.27 × 10−3 |
| 12818 | Col14a1 | −0.593 | 3.37 × 10−4 |
| 14118 | Fbn1 | −0.530 | 3.18 × 10−3 |
| 108075 | Ltbp4 | −0.500 | 4.58 × 10−2 |
| 67532 | Mfap1a | −1.094 | 6.25 × 10−6 |
| 17390 | Mmp2 | −0.610 | 2.89 × 10−3 |
| 21825 | Thbs1 | −0.568 | 2.89 × 10−3 |
| 21827 | Thbs3 | −0.581 | 2.60 × 10−3 |
| 21923 | Tnc | −0.583 | 1.93 × 10−4 |
| 81877 | Tnxb | −0.795 | 3.12 × 10−6 |
| Day 3 | Day 7 | ||||||
|---|---|---|---|---|---|---|---|
| Gene ID | Gene Symbol | log2 (FC) | Q-Value | Gene ID | Gene Symbol | log2 (FC) | Q-Value |
| 12010 | B2m | −6.752 | 1.5 × 10−301 | 12010 | B2m | −7.459 | 8.4 × 10−140 |
| 78655 | Eif3j1 | −6.601 | 5.9 × 10−82 | 78655 | Eif3j1 | −6.853 | 1.2 × 10−60 |
| 15018 | H2-Q7 | −6.407 | 7.5 × 10−10 | 433466 | Jmjd7 | −4.193 | 1.4 × 10−8 |
| 20732 | Spint1 | −4.317 | 8.9 × 10−10 | 225594 | Gm4841 | −4.057 | 4.8 × 10−6 |
| 433466 | Jmjd7 | −3.954 | 1.9 × 10−7 | 20732 | Spint1 | −3.762 | 1.1 × 10−6 |
| 211429 | Pla2g4b | −2.181 | 3.2 × 10−2 | 626578 | Gbp10 | −2.944 | 4.4 × 10−2 |
| 433470 | AA467197 | −1.734 | 2.0 × 10−4 | 433470 | AA467197 | −2.922 | 5.6 × 10−10 |
| 13386 | Dlk1 | −1.480 | 3.5 × 10−3 | 14562 | Gdf3 | −2.298 | 4.7 × 10−2 |
| 16002 | Igf’2 | −1.411 | 4.0 × 10−3 | 211429 | Pla2g4b | −2.070 | 1.4 × 10−2 |
| 56357 | Ivd | −1.062 | 8.8 × 10−6 | 30939 | Pttg1 | −1.935 | 4.1 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirasawa, E.; Uchida, K.; Onuma, K.; Inoue, G.; Eshima, K.; Satoh, M.; Miyagi, M.; Toyomura, Y.; Norisugi, A.; Takaso, M. β2-Microglobulin Regulates Extracellular Matrix Dynamics During Peripheral Nerve Injury. NeuroSci 2025, 6, 59. https://doi.org/10.3390/neurosci6030059
Shirasawa E, Uchida K, Onuma K, Inoue G, Eshima K, Satoh M, Miyagi M, Toyomura Y, Norisugi A, Takaso M. β2-Microglobulin Regulates Extracellular Matrix Dynamics During Peripheral Nerve Injury. NeuroSci. 2025; 6(3):59. https://doi.org/10.3390/neurosci6030059
Chicago/Turabian StyleShirasawa, Eiki, Kentaro Uchida, Kenji Onuma, Gen Inoue, Koji Eshima, Masashi Satoh, Masayuki Miyagi, Yoji Toyomura, Akira Norisugi, and Masashi Takaso. 2025. "β2-Microglobulin Regulates Extracellular Matrix Dynamics During Peripheral Nerve Injury" NeuroSci 6, no. 3: 59. https://doi.org/10.3390/neurosci6030059
APA StyleShirasawa, E., Uchida, K., Onuma, K., Inoue, G., Eshima, K., Satoh, M., Miyagi, M., Toyomura, Y., Norisugi, A., & Takaso, M. (2025). β2-Microglobulin Regulates Extracellular Matrix Dynamics During Peripheral Nerve Injury. NeuroSci, 6(3), 59. https://doi.org/10.3390/neurosci6030059






