Natural Compounds That Target Glioma Stem Cells
Abstract
1. Introduction
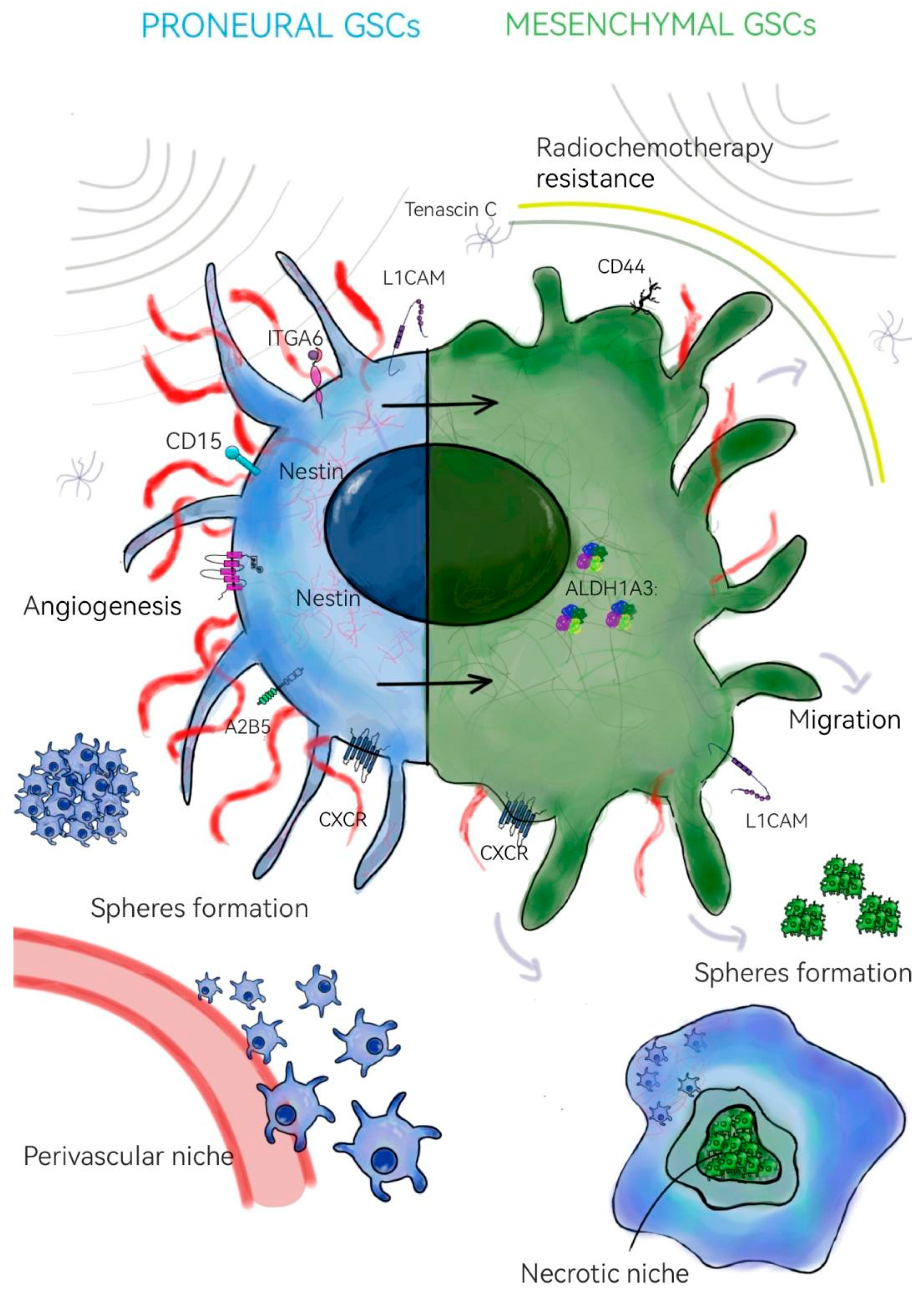
2. Glioma Stem Cells
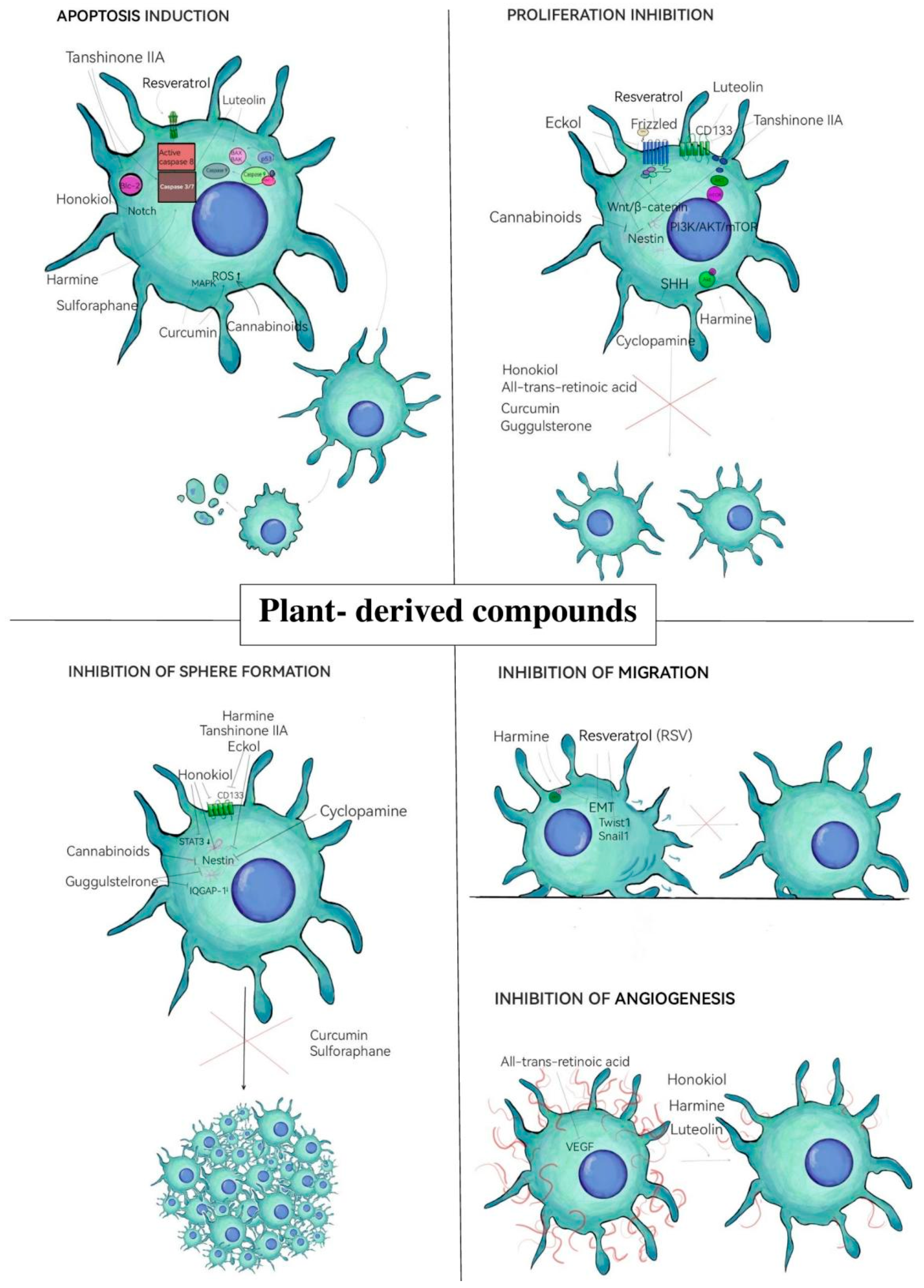
3. Natural Compounds Targeting GSCs
3.1. Phenolic Compounds and Flavonoids
3.1.1. Resveratrol
3.1.2. Luteolin
3.1.3. Curcumin
3.1.4. Apigenin
3.2. Alkaloids
3.2.1. Harmine
3.2.2. Cyclopamine
3.3. Terpenoids
3.3.1. Honokiol
3.3.2. Tanshinone IIA
3.3.3. Cannabinoids
3.4. Other Natural Compounds
3.4.1. Guggulsterone
3.4.2. Sulforaphane
3.4.3. Eckol
3.4.4. All-Trans-Retinoic Acid (Vit A Acid)
3.4.5. PBI-05204
3.4.6. RGWE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Protein Kinase B |
| ALDH1A3 | Aldehyde Dehydrogenase 1 Family Member A3 |
| ATP | Adenosine Triphosphate |
| ATRA | All-Trans-Retinoic Acid |
| Bax | BCL2-Associated X Protein |
| Bcl-2 | B-Cell Lymphoma 2 |
| BMPs | Bone Morphogenetic Proteins |
| CAPN1 | Calcium-Activated Neutral Protease 1 |
| CB1/2 | Cannabinoid Receptor Type 1/2 |
| CD133 | Cluster of Differentiation 133 |
| CD15 | Cluster of Differentiation 15 |
| CD44 | Cluster of Differentiation 44 |
| Chk1 and 2 | Checkpoint Kinase 1 and 2 |
| CK1 | Casein Kinase 1 |
| c-Myc | Cellular Myelocytomatosis Oncogene |
| CSCs | Cancer Stem Cells |
| CXCR4 | C-X-C Motif Chemokine Receptor 4 |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial–Mesenchymal Transition |
| ERK | Extracellular Signal-Regulated Kinase |
| FGF-4 | Fibroblast Growth Factor 4 |
| GBM | Glioblastoma |
| GFAP | Glial Fibrillary Acidic Protein |
| GLUT | Glucose Transporter |
| GPCRs | G-Protein-Coupled Receptors |
| GRP78 | 78 kDa Glucose-Regulated Protein |
| GSCs | Glioma Stem Cells |
| HIF-1/2-α | Hypoxia-Inducible Factor 1/2 Alpha |
| HOX | Homeobox Gene |
| IAP | Inhibitor of Apoptosis Protein |
| IL6 | Interleukin 6 |
| IQGAP1 | IQ Motif Containing GTPase-Activating Protein 1 |
| ITGA6 | Integrin Subunit Alpha 6 |
| JAK | Janus Kinase |
| JNK | c-Jun N-Terminal Kinase |
| KLF4 | Kruppel-Like Factor 4 |
| L1CAM | L1 Cell Adhesion Molecule |
| LEF | Lymphoid Enhancer-Binding Factor |
| MAPK | Mitogen-Activated Protein Kinase |
| MEK | Mitogen-Activated Protein Kinase |
| MES | Mesenchymal |
| MGMT | O6-Methylguanine-DNA Methyltransferase |
| MMP9 | Matrix Metalloproteinase 9 |
| MnSOD | Manganese Superoxide Dismutase |
| mTOR | Mammalian Target of Rapamycin |
| Musashi-1 | Musashi RNA-Binding Protein 1 |
| NANOG | Homeobox Transcription Factor NANOG |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cell |
| NRP | Neuropilin |
| O6MeG | O6-Methylguanine |
| OCT-4 | Octamer-Binding Transcription Factor 4 |
| PI3K/Akt | Phosphatidylinositol 3-Kinase/Protein Kinase B |
| PKM2 | Pyruvate Kinase M2 |
| PN | Proneuronal/Proneural |
| Ptch | Patched Receptor |
| PTK | Protein Tyrosine Kinase |
| Raf | Rapidly Accelerated Fibrosarcoma |
| RAREs | Retinoic Acid Response Elements |
| Ras | Rat Sarcoma |
| ROS | Reactive Oxygen Species |
| RSV | Resveratrol |
| RT | Radiotherapy |
| RTKs | Receptor Tyrosine Kinases |
| SALL4 | Sal-Like Protein 4 |
| SHH | Sonic Hedgehog Pathway |
| SMO | Smoothened Protein |
| Sox2 | SRY-Box Transcription Factor 2 |
| Src-FAK | Src Family Kinases–Focal Adhesion Kinase |
| STAT | Signal Transducer and Activator of Transcription |
| TCA | Tricarboxylic Acid Cycle (Krebs Cycle) |
| TCF | T-Cell Factor |
| TGF | Transforming Growth Factor |
| TMZ | Temozolomide |
| Ub | Ubiquitin |
| VEGF | Vascular Endothelial Growth Factor |
| WHO | World Health Organization |
References
- Molinaro, A.M.; Taylor, J.W.; Wiencke, J.K.; Wrensch, M.R. Genetic and molecular epidemiology of adult diffuse glioma. Nat. Rev. Neurol. 2019, 15, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, A.; Proietti, G.; Sica, G.; Scicchitano, B.M. Pathological and Molecular Features of Glioblastoma and Its Peritumoral Tissue. Cancers 2019, 11, 469. [Google Scholar] [CrossRef]
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in Glioblastoma Therapy: An Update on Current Approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef]
- Zeng, J.; Zeng, X.X. Systems Medicine for Precise Targeting of Glioblastoma. Mol. Biotechnol. 2023, 65, 1565–1584. [Google Scholar] [CrossRef]
- Rodríguez-Camacho, A.; Flores-Vázquez, J.G.; Moscardini-Martelli, J.; Torres-Ríos, J.A.; Olmos-Guzmán, A.; Ortiz-Arce, C.S.; Cid-Sánchez, D.R.; Pérez, S.R.; Macías-González, M.D.S.; Hernández-Sánchez, L.C.; et al. Glioblastoma Treatment: State-of-the-Art and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 7207. [Google Scholar] [CrossRef]
- Alves, A.L.V.; Gomes, I.N.F.; Carloni, A.C.; Rosa, M.N.; da Silva, L.S.; Evangelista, A.F.; Reis, R.M.; Silva, V.A.O. Role of glioblastoma stem cells in cancer therapeutic resistance: A perspective on antineoplastic agents from natural sources and chemical derivatives. Stem Cell Res. Ther. 2021, 12, 206. [Google Scholar] [CrossRef]
- Liebelt, B.D.; Shingu, T.; Zhou, X.; Ren, J.; Shin, S.A.; Hu, J. Glioma Stem Cells: Signaling, Microenvironment, and Therapy. Stem Cells Int. 2016, 2016, 7849890. [Google Scholar] [CrossRef]
- Vira, D.; Basak, S.K.; Veena, M.S.; Wang, M.B.; Batra, R.K.; Srivatsan, E.S. Cancer stem cells, microRNAs, and therapeutic strategies including natural products. Cancer Metastasis Rev. 2012, 31, 733–751. [Google Scholar] [CrossRef]
- Schmidt-Kittler, O.; Ragg, T.; Daskalakis, A.; Granzow, M.; Ahr, A.; Blankenstein, T.J.F.; Kaufmann, M.; Diebold, J.; Arnholdt, H.; Muller, P.; et al. From latent disseminated cells to overt metastasis: Genetic analysis of systemic breast cancer progression. Proc. Natl. Acad. Sci. USA 2003, 100, 7737–7742. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Zhai, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer stem cells: Advances in knowledge and implications for cancer therapy. Sig. Transduct. Target Ther. 2024, 9, 170. [Google Scholar] [CrossRef]
- Safa, A.R. Resistance to drugs and cell death in cancer stem cells (CSCs). J. Transl. Sci. 2020, 6, 341. [Google Scholar] [CrossRef] [PubMed]
- Bayin, N.S.; Modrek, A.S.; Placantonakis, D.G. Glioblastoma stem cells: Molecular characteristics and therapeutic implications. World J. Stem Cells 2014, 6, 230–238. [Google Scholar] [CrossRef]
- Prager, B.C.; Bhargava, S.; Mahadev, V.; Hubert, C.G.; Rich, J.N. Glioblastoma Stem Cells: Driving Resiliency through Chaos. Trends Cancer 2020, 6, 223–235. [Google Scholar] [CrossRef]
- DeLay, M.; Jahangiri, A.; Carbonell, W.S.; Hu, Y.L.; Tsao, S.; Tom, M.W.; Paquette, J.; Tokuyasu, T.A.; Aghi, M.K. Microarray Analysis Verifies Two Distinct Phenotypes of Glioblastomas Resistant to Antiangiogenic Therapy. Clin. Cancer Res. 2012, 18, 2930–2942. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, Q.; Guryanova, O.A.; Huang, Z.; Huang, Q.; Rich, J.N.; Shideng, B. Elevated invasive potential of glioblastoma stem cells. Biochem. Biophys. Res. Commun. 2011, 406, 643–648. [Google Scholar] [CrossRef]
- Steponaitis, G.; Tamasauskas, A. Mesenchymal and Proneural Subtypes of Glioblastoma Disclose Branching Based on GSC Associated Signature. Int. J. Mol. Sci. 2021, 22, 4964. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Xu, S.; Liu, Z.; Cheng, Q. The adaptive transition of glioblastoma stem cells and its implications on treatments. Sig. Transduct. Target Ther. 2021, 6, 124. [Google Scholar] [CrossRef]
- Guardia, G.D.A.; Correa, B.R.; Araujo, P.R.; Qiao, M.; Burns, S.; Penalva, L.O.F.; Galante, P.A.F. Proneural and mesenchymal glioma stem cells display major differences in splicing and lncRNA profiles. npj Genom. Med. 2020, 5, 2. [Google Scholar] [CrossRef]
- Pleskač, P.; Fargeas, C.A.; Veselska, R.; Corbeil, D.; Skoda, J. Emerging roles of prominin-1 (CD133) in the dynamics of plasma membrane architecture and cell signaling pathways in health and disease. Cell Mol. Biol. Lett. 2024, 29, 41. [Google Scholar] [CrossRef]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.; Rich, J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, M.; Drakulic, D.; Lazic, A.; Ninkovic, D.S.; Schwirtlich, M.; Mojsin, M. SOX Transcription Factors as Important Regulators of Neuronal and Glial Differentiation During Nervous System Development and Adult Neurogenesis. Front. Mol. Neurosci. 2021, 14, 654031. [Google Scholar] [CrossRef] [PubMed]
- Hassn Mesrati, M.; Behrooz, A.B.; Y Abuhamad, A.; Syahir, A. Understanding Glioblastoma Biomarkers: Knocking a Mountain with a Hammer. Cells 2020, 9, 1236. [Google Scholar] [CrossRef]
- Bernal, A.; Arranz, L. Nestin-expressing progenitor cells: Function, identity and therapeutic implications. Cell Mol. Life Sci. 2018, 75, 2177–2195. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Yang, W.; Ouyang, Y.; Liu, Y.; Cai, Z. CXCR4 promotes tumor stemness maintenance and CDK4/6 inhibitors resistance in ER-positive breast cancer. Breast Cancer Res. 2025, 27, 15. [Google Scholar] [CrossRef]
- Fu, X.; Hong, L.; Gong, H.; Kan, G.; Zhang, P.; Cui, T.T.; Fan, G.; Si, X.; Zhu, J. Identification of a Nomogram with an Autophagy-Related Risk Signature for Survival Prediction in Patients with Glioma. IJGM 2022, 15, 1517–1535. [Google Scholar] [CrossRef]
- Cilibrasi, C.; Riva, G.; Romano, G.; Cadamuro, M.; Bazzoni, R.; Butta, V.; Paoletta, L.; Dalprà, L.; Strazzabosco, M.; Lavitrano, M.; et al. Resveratrol Impairs Glioma Stem Cells Proliferation and Motility by Modulating the Wnt Signaling Pathway. PLoS ONE 2017, 12, e0169854. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Navone, S.E.; Guarnaccia, L.; Rizzaro, M.D.; Begani, L.; Barilla, E.; Alotta, G.; Garzia, E.; Caroli, M.; Ampollini, A.; Violetti, A.; et al. Role of Luteolin as Potential New Therapeutic Option for Patients with Glioblastoma through Regulation of Sphingolipid Rheostat. Int. J. Mol. Sci. 2023, 25, 130. [Google Scholar] [CrossRef]
- Gersey, Z.C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Kobayashi, M.; Koyasu, S.; Chow, C.C.T.; Harada, H. HIF-1-Dependent Reprogramming of Glucose Metabolic Pathway of Cancer Cells and Its Therapeutic Significance. Int. J. Mol. Sci. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, H.; Jia, C.H.; Fan, K.; Xie, T.; Zhu, Z.Y.; Xie, M.L. Apigenin increases radiosensitivity of glioma stem cells by attenuating HIF-1α-medieted glycolisis. Med. Oncol. 2021, 38, 131. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.; Yu, S. Pharmacological effects of harmine and its derivatives: A review. Arch. Pharm. Res. 2020, 43, 1259–1275. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, X.; Yang, L.; Xue, G.; Wei, Q.; Han, Z.; Chen, H. Research progress on the antitumor effects of harmine. Front. Oncol. 2024, 14, 1382142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Lv, Y.X.; Guo, C.Y.; Xiao, Z.M.; Jiang, Q.G.; Kuang, H.; Zhang, W.H.; Hu, P. Harmine inhibits the proliferation and migration of glioblastoma cells via the FAK/AKT pathway. Life Sci. 2021, 270, 119112. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Han, D.; Liu, Y.; Hou, X.; Wu, L.; Li, H.; Yang, J.; Shen, C.; Yang, G.; Fu, C.; et al. Harmine hydrochloride inhibits Akt phosphorylation and depletes the pool of cancer stem-like cells of glioblastoma. J. Neurooncol. 2013, 112, 39–48. [Google Scholar] [CrossRef]
- Turner, M.; Cruz, R.; Mattos, J.; Baughman, N.; Elwell, J.; Fothergill, J.; Nielsen, A.; Brookhouse, J.; Bartlett, A.; Malek, P.; et al. Cyclopamine Bioactivity by Extraction Method from Veratrum californicum. Bioorganic Med. Chem. 2016, 24, 3752–3757. [Google Scholar] [CrossRef]
- Eimer, S.; Dugay, F.; Airiau, K.; Avril, T.; Quillien, V.; Belaud-Rotureau, M.A.; Belloc, F. Cyclopamine cooperates with EGFR inhibition to deplete stem-like cancer cells in glioblastoma-derived spheroid cultures. Neuro Oncol. 2012, 14, 1441–1451. [Google Scholar] [CrossRef]
- Bar, E.E.; Chaudhry, A.; Lin, A.; Fan, X.; Schreck, K.; Matsui, W.; Piccirillo, S.; Vescovi, A.L.; DiMeco, F.; Olivi, A.; et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells 2007, 25, 2524–2533. [Google Scholar] [CrossRef]
- Arora, S.; Singh, S.; Piazza, G.A.; Contreras, C.M.; Panyam, J.; Singh, A.P. Honokiol: A novel natural agent for cancer prevention and therapy. Curr. Mol. Med. 2013, 12, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xue, W.; Schachner, M.; Zhao, W. Honokiol Eliminates Glioma/Glioblastoma Stem Cell-Like Cells Via JAK-STAT3 Signaling and Inhibits Tumor Progression by Targeting Epidermal Growth Factor Receptor. Cancers 2018, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Lai, I.C.; Shih, P.H.; Yao, C.J.; Yeh, C.T.; Wang-Peng, J.; Lui, T.N.; Chuang, S.E.; Hu, T.S.; Lai, T.Y.; Lai, G.M. Elimination of cancer stem-like cells and potentiation of temozolomide sensitivity by Honokiol in glioblastoma multiforme cells. PLoS ONE 2015, 10, e0114830. [Google Scholar] [CrossRef]
- You, S.; He, X.; Wang, M.; Mao, L.; Zhang, L. Tanshinone IIA Suppresses Glioma Cell Proliferation, Migration and Invasion Both in vitro and in vivo Partially Through miR-16-5p/Talin-1 (TLN1) Axis. Cancer Manag. Res. 2020, 12, 11309–11320. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, M.; Liu, J.; Zhao, X.; Zhang, Y.; Fang, L. Tanshinone IIA: A Review of its Anticancer Effects. Front. Pharmacol. 2021, 11, 611087. [Google Scholar] [CrossRef]
- Yang, L.; Guo, H.; Dong, L.; Wang, L.; Liu, C.; Wang, X. Tanshinone IIA inhibits the growth, attenuates the stemness and induces the apoptosis of human glioma stem cells. Oncol. Rep. 2014, 32, 1303–1311. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Sandalcioglu, I.E.; Karsak, M. Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Front. Mol. Neurosci. 2018, 11, 159. [Google Scholar] [CrossRef]
- Aguado, T.; Carracedo, A.; Julien, B.; Velasco, G.; Milman, G.; Mechoulam, R.; Alvarez, L.; Guzmán, M.; Galve-Roperh, I. Cannabinoids Induce Glioma Stem-like Cell Differentiation and Inhibit Gliomagenesis. J. Biol. Chem. 2007, 282, 6854–6862. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.; Judkins, J.; Salomonis, N.; Matlaf, L.; Soteropoulos, P.; McAllister, S.; Soroceanu, L. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015, 6, e1601. [Google Scholar] [CrossRef]
- Dixit, D.; Ghildiyal, R.; Anto, N.P.; Ghosh, S.; Sharma, V.; Sen, E. Guggulsterone sensitizes glioblastoma cells to Sonic hedgehog inhibitor SANT-1 induced apoptosis in a Ras/NFκB dependent manner. Cancer Lett. 2013, 336, 347–358. [Google Scholar] [CrossRef]
- Balenci, L.; Clarke, I.D.; Dirks, P.B.; Assard, N.; Ducray, F.; Jouvet, A.; Belin, M.; Honnorat, J.; Baudier, J. IQGAP1 Protein Specifies Amplifying Cancer Cells in Glioblastoma Multiforme. Cancer Res. 2006, 66, 9074–9082. [Google Scholar] [CrossRef] [PubMed]
- Otoo, R.A.; Allen, A.R. Sulforaphane’s Multifaceted Potential: From Neuroprotection to Anticancer Action. Molecules 2023, 28, 6902. [Google Scholar] [CrossRef] [PubMed]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Bijangi-Vishehsaraei, K.; Reza Saadatzadeh, M.; Wang, H.; Nguyen, A.; Kamocka, M.M.; Cai, W.; Cohen-Gadol, A.A.; Halum, S.L.; Sarkaria, J.N.; Pollok, K.E.; et al. Sulforaphane suppresses the growth of glioblastoma cells, glioblastoma stem cell-like spheroids, and tumor xenografts through multiple cell signaling pathways. J. Neurosurg. 2017, 127, 1219–1230. [Google Scholar] [CrossRef]
- Hyun, K.; Yoon, C.; Kim, R.; Lim, E.; An, S.; Park, M.; Hyun, J.; Suh, Y.; Kim, M.; Lee, S. Eckol suppresses maintenance of stemness and malignancies in glioma stem-like cells. Toxicol. Appl. Pharmacol. 2011, 254, 32–40. [Google Scholar] [CrossRef]
- Manandhar, B.; Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing Eckol as a Therapeutic Aid: A Systematic Review. Mar. Drugs 2019, 17, 361. [Google Scholar] [CrossRef]
- Ling, G.Q.; Liu, Y.J.; Ke, Y.Q.; Chen, L.; Jiang, X.D.; Jiang, C.L.; Ye, W. All-trans retinoic acid impairs the vasculogenic mimicry formation ability of U87 stem-like cells through promoting differentiation. Mol. Med. Rep. 2015, 12, 165–172. [Google Scholar] [CrossRef]
- Broussy, S. Vascular Endothelial Growth Factor (VEGF) and VEGF Receptor Inhibitors in Health and Disease. Pharmaceuticals 2024, 17, 959. [Google Scholar] [CrossRef]
- Wang, R.; Liu, C. All-trans retinoic acid therapy induces asymmetric division of glioma stem cells from the U87MG cell line. Oncol. Lett. 2019, 18, 3646–3654. [Google Scholar] [CrossRef]
- Chakraborty, S.; Wei, D.; Tran, M.; Lang, F.F.; Newman, R.A.; Yang, P. PBI-05204, a supercritical CO2 extract of Nerium oleander, supresses glioblastoma stem cells by inhibiting GRP78 and inducing programmed necroptotic cell death. Neoplasia 2024, 54, 101008. [Google Scholar] [CrossRef]
- Camerino, I.; Franco, P.; Bajetto, A.; Thellung, S.; Florio, T.; Stoppelli, M.P.; Colucci-D’Amato, L. Ruta graveolens, but Not Rutin, Inhibits Survival, Migration, Invasion, and Vasculogenic Mimicry of Glioblastoma Cells. Int. J. Mol. Sci. 2024, 25, 11789. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Liu, W.; Geng, Y.; Shi, Y.; Tian, Y.; Zhang, B.; Luo, Y.; Sun, X. New drug discovery and development from natural products: Advances and strategies. Pharmacol. Ther. 2024, 264, 108752. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Gupta, J.K.; Chanchal, D.K.; Shinde, M.G.; Kumar, S.; Jain, D.; Almarhoon, Z.M.; Alshahrani, A.M.; Calina, D.; Sharifi-Rad, J.; et al. Natural products as drug leads: Exploring their potential in drug discovery and development. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 4673–4687. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.D.; Geetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef]
- Brown, K.; Theofanous, D.; Britton, R.G.; Aburido, G.; Pepper, C.; Sri Undru, S.; Howells, L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. Int. J. Mol. Sci. 2024, 25, 747. [Google Scholar] [CrossRef]
- Lv, J.; Song, X.; Luo, Z.; Huang, D.; Xiao, L.; Zou, K. Luteolin: Exploring its therapeutic potential and molecular mechanisms in pulmonary diseases. Front. Pharmacol. 2025, 16, 1535555. [Google Scholar] [CrossRef]
- Jayawickreme, D.K.; Ekwosi, C.; Anand, A.; Andres-Mach, M.; Wlaź, P.; Socała, K. Luteolin for neurodegenerative diseases: A review. Pharmacol. Rep. 2024, 76, 644–664. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Barreto, G.E.; Henney, N.C.; Majeed, M.; Sahebkar, A. Neuroprotection by curcumin: A review on brain delivery strategies. Int. J. Pharm. 2020, 30, 119476. [Google Scholar] [CrossRef]
- Panknin, T.M.; Howe, C.L.; Hauer, M.; Bucchireddigari, B.; Rossi, A.M.; Funk, J.L. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2023, 24, 4476. [Google Scholar] [CrossRef] [PubMed]
- DeRango-Adem, E.F.; Blay, J. Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers? Front. Pharmacol. 2021, 12, 681477. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Ables, J.L.; Israel, L.; Wood, O.; Govindarajulu, U.; Fremont, R.T.; Banerjee, R.; Liu, H.; Cohen, J.; Wang, P.; Kumar, K.; et al. A Phase 1 single ascending dose study of pure oral harmine in healthy volunteers. J. Psychopharmacol. 2024, 38, 911–923. [Google Scholar] [CrossRef]
- Li, Y.; Sattler, R.; Yang, E.J.; Nunes, A.; Ayukawa, Y.; Akhtar, S.; Ji, G.; Zhang, P.W.; Rothstein, J.D. Harmine, a natural beta-carboline alkaloid, upregulates astroglial glutamate transporter expression. Neuropharmacology 2011, 60, 1168–1175. [Google Scholar] [CrossRef]
- Tremblay, M.R.; Nevalainen, M.; Nair, S.J.; Porter, J.R.; Castro, A.C.; Behnke, M.L.; Yu, L.C.; Hagel, M.; White, K.; Faia, K.; et al. Semisynthetic cyclopamine analogues as potent and orally bioavailable hedgehog pathway antagonists. Med. Chem. 2008, 51, 6646–6649. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, T.K.; Carpenter, R.L.; Qasem, S.; Chan, M.; Lo, H.W. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers 2016, 8, 22. [Google Scholar] [CrossRef]
- Yang, J.; Shang, J.; Yang, L.; Wei, D.; Wang, X.; Deng, Q.; Zhong, Z.; Ye, Y.; Zhou, M. Nanotechnology-Based Drug Delivery Systems for Honokiol: Enhancing Therapeutic Potential and Overcoming Limitations. Int. J. Nanomed. 2023, 18, 6639–6665. [Google Scholar] [CrossRef]
- Faysal, M.; Khan, J.; Zehravi, M.; Nath, N.; Singh, L.P.; Kakkar, S.; Perusomula, R.; Khan, P.A.; Nainu, F.; Asiri, M.; et al. Neuropharmacological potential of honokiol and its derivatives from Chinese herb Magnolia species: Understandings from therapeutic viewpoint. Chin. Med. 2023, 18, 154. [Google Scholar] [CrossRef]
- Ashour, A.A.; Ramadan, A.A.; Abdelmonsif, D.A.; El-Kamel, A.H. Enhanced oral bioavailability of Tanshinone IIA using lipid nanocapsules: Formulation, in-vitro appraisal and pharmacokinetics. Int. J. Pharm. 2020, 586, 119598. [Google Scholar] [CrossRef]
- Sherawat, K.; Mehan, S. Tanshinone-IIA mediated neuroprotection by modulating neuronal pathways. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 1647–1667. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zou, J.; Cao, D.; Ma, X. Pharmacological basis of tanshinone and new insights into tanshinone as a multitarget natural product for multifaceted diseases. Biomed. Pharmacother. 2020, 130, 110599. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Jensen, S.S.; Kolli, A.R.; Nikolajsen, G.N.; Bruun, H.Z.; Hoeng, J. Strategies to Improve Cannabidiol Bioavailability and Drug Delivery. Pharmaceuticals 2024, 17, 244. [Google Scholar] [CrossRef] [PubMed]
- Calapai, F.; Cardia, L.; Sorbara, E.E.; Navarra, M.; Gangemi, S.; Calapai, G.; Mannucci, C. Cannabinoids, Blood-Brain Barrier, and Brain Disposition. Pharmaceutics 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Volkow, N.D.; Friemel, C.M.; Lorenzetti, V.; Freeman, T.P.; Hall, W. Cannabis, cannabinoids and health: A review of evidence on risks and medical benefits. Eur. Arch. Psychiatry Clin. Neurosci. 2025, 275, 281–292. [Google Scholar] [CrossRef]
- Hourfane, S.; Mechqoq, H.; Bekkali, A.Y.; Rocha, J.M.; El Aouad, N. A Comprehensive Review on Cannabis sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities. Plants 2023, 12, 1245. [Google Scholar] [CrossRef]
- Girisa, S.; Parama, D.; Harsha, C.; Banik, K.; Kunnumakkara, A.B. Potential of guggulsterone, a farnesoid X receptor antagonist, in the prevention and treatment of cancer. Explor. Target Antitumor Ther. 2020, 1, 313–342. [Google Scholar] [CrossRef]
- Xu, H.B.; Tang, Z.Q.; Wang, J.; Kong, P.S. Z-guggulsterone regulates MDR1 expression mainly through the pregnane X receptor-dependent manner in human brain microvessel endothelial cells. Eur. J. Pharmacol. 2020, 874, 173023. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Banik, K.; Bordoloi, D.; Harsha, C.; Sailo, B.L.; Padmavathi, G.; Roy, N.K.; Gupta, S.C.; Aggarwal, B.B. Googling the Guggul (Commiphora and Boswellia) for Prevention of Chronic Diseases. Front. Pharmacol. 2018, 9, 686. [Google Scholar] [CrossRef]
- Hanlon, N.; Coldham, N.; Gielbert, A.; Kuhnert, N.; Sauer, M.J.; King, L.J.; Ioannides, C. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br. J. Nutr. 2008, 99, 559–564. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, T.; Mao, L.; Zhang, F. Sulforaphane Protects against Brain Diseases: Roles of Cytoprotective Enzymes. Austin. J. Cerebrovasc. Dis. Stroke 2017, 4, 1054. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 2017, 9, eaah4477. [Google Scholar] [CrossRef]
- Shin, H.C.; Rosenfeld, C.; Guttendorf, R.J.; Wade, S.B.; Park, Y.J.; Kim, J.H.; Kim, S.H.; Lee, B.H.; Hwang, H.J. A Pharmacokinetic and Bioavailability Study of Ecklonia cava Phlorotannins Following Intravenous and Oral Administration in Sprague-Dawley Rats. Mar. Drugs 2024, 22, 500. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, M.J.; Lee, H.L.; Moon, J.H.; Jeong, H.R.; Kim, H.J.; Heo, H.J. Water Extract of Ecklonia cava Protects against Fine Dust (PM2.5)-Induced Health Damage by Regulating Gut Health. J. Microbiol. Biotechnol. 2022, 32, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wei, S.; Hu, X.; Liu, J.; Wang, J. Protective Effect and Mechanisms of Eckol on Chronic Ulcerative Colitis Induced by Dextran Sulfate Sodium in Mice. Mar. Drugs 2023, 21, 376. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tretinoin (accessed on 7 May 2025).
- Lippmann, E.S.; Al-Ahmad, A.; Azarin, S.M.; Palecek, S.P.; Shusta, E.V. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014, 4, 4160. [Google Scholar] [CrossRef] [PubMed]
- Ferla, V.; Sciumé, M.; Gianelli, U.; Baldini, L.; Fracchiolla, N.S. Multiple adverse drug reactions during all-trans retinoic acid treatment for acute promyelocytic leukemia: Differentiation syndrome, bradycardia, intestinal necrosis. Explor. Target Antitumor Ther. 2020, 1, 109–116. [Google Scholar] [CrossRef]
- Abdennour, S.; Derouiche, M.; Romeuf, L.; Gaillard, Y.; Dalia, F.; Azzouz, M. Pharmacokinetics of Oleandrin Following Administration of a Nerium oleander Extract in Mice. BJMS 2024, 11, 326–330. [Google Scholar] [CrossRef]
- Roth, M.T.; Cardin, D.B.; Borazanci, E.H.; Steinbach, M.; Picozzi, V.J.; Rosemury, A.; Wadlow, R.C.; Newman, R.A.; Berlin, J. A Phase II, Single-Arm, Open-Label, Bayesian Adaptive Efficacy and Safety Study of PBI-05204 in Patients with Stage IV Metastatic Pancreatic Adenocarcinoma. Oncologist 2020, 25, e1446–e1450. [Google Scholar] [CrossRef]
- Campanile, M.; Cuomo, O.; Brancaccio, P.; Vinciguerra, A.; Casamassa, A.; Pastorino, O.; Volpicelli, F.; Gentile, M.T.; Amoroso, S.; Annunziato, L.; et al. Ruta graveolens water extract (RGWE) ameliorates ischemic damage and improves neurological deficits in a rat model of transient focal brain ischemia. Biomed. Pharmacother. 2022, 154, 113587. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Phenolic Compounds from New Natural Sources-Plant Genotype and Ontogenetic Variation. Molecules 2023, 28, 1731. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Seidel, V.; Izabela, M.; Monserrat-Mequida, M.; Sureda, A.; Ormazabal, V.; Zuniga, F.A.; Mangalpady, S.S.; Pezzani, R.; Ydyrys, A.; et al. Phenolic compounds as Nrf2 inhibitors: Potential applications in cancer therapy. Cell Commun. Signal. 2023, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Sig. Transduct. Target Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef]
- Jin, S.; White, E. Tumor suppression by autophagy through the management of metabolic stress. Autophagy 2008, 4, 563–566. [Google Scholar] [CrossRef]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; De Lillo, A.; Laino, L.; Lo Muzio, L. Biological and therapeutic activities, and anticancer properties of curcumin (Review). Exp. Ther. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 3, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Silke, J.; Meier, P. Inhibitor of apoptosis (IAP) proteins-modulators of cell death and inflammation. Cold Spring Harb. Perspect Biol. 2013, 5, a008730. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Sig. Transduct. Target Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Recent Adv. Nat. Prod. Anal. 2020, 505–567. [Google Scholar] [CrossRef]
- Carballo, G.B.; Honorato, J.R.; de Lopes, G.P.F.; Spohr, T.C.L.S.E. A highlight on Sonic hedgehog pathway. Cell Commun. Signal 2018, 16, 11. [Google Scholar] [CrossRef]
- Lipinski, R.J.; Hutson, P.R.; Hannam, P.W.; Nydza, R.J.; Washington, I.M.; Moore, R.W.; Girdaukas, G.G.; Peterson, R.E.; Bushman, W. Dose- and Route-Dependent Teratogenicity, Toxicity, and Pharmacokinetic Profiles of the Hedgehog Signaling Antagonist Cyclopamine in the Mouse. Toxicol. Sci. 2008, 104, 189–197. [Google Scholar] [CrossRef]
- Li, R.; Cai, L.; Ding, J.; Hu, C.M.; Wu, T.N.; Hu, X.Y. Inhibition of hedgehog signal pathway by cyclopamine attenuates inflammation and articular cartilage damage in rats with adjuvant-induced arthritis. J. Pharm. Pharmacol. 2015, 67, 963–971. [Google Scholar] [CrossRef]
- Meth, M.J.; Weinberg, J.M. Cyclopamine: Inhibiting hedgehog in the treatment of psoriasis. Cutis 2006, 78, 185–188. [Google Scholar]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech. 2015, 5, 129–151. [Google Scholar] [CrossRef]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal 2009, 11, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Faiz, M.B.; Naeem, F.; Irfan, M.; Aslam, M.A.; Estevinho, L.M.; Ateşşahin, D.A.; Alshahrani, A.M.; Calina, D.; Khan, K.; Sharifi-Rad, J. Exploring the therapeutic potential of cannabinoids in cancer by modulating signaling pathways and addressing clinical challenges. Discov. Oncol. 2024, 15, 490. [Google Scholar] [CrossRef]
- Adarsh Krishna, T.P.; Ajeesh Krishna, T.P.; Edachery, B.; Antony Ceasar, S. Guggulsterone—A potent bioactive phytosteroid: Synthesis, structural modification, and its improved bioactivities. RSC Med. Chem. 2023, 15, 55–69. [Google Scholar] [CrossRef]
- Adams, M.K.; Belyaeva, O.V.; Kedishvili, N.Y. Generation and isolation of recombinant retinoid oxidoreductase complex. Methods Enzymol. 2020, 637, 77–93. [Google Scholar] [CrossRef]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Bi, G.; Liang, J.; Bian, Y.; Shan, G.; Besskaya, V.; Wang, Q.; Zhan, C. The immunomodulatory role of all-trans retinoic acid in tumor microenvironment. Clin. Exp. Med. 2023, 23, 591–606. [Google Scholar] [CrossRef]
- Colapietro, A.; Yang, P.; Rossetti, A.; Mancini, A.; Vitale, F.; Martellucci, S.; Conway, T.C.; Chakraborty, S.; Marampon, F.; Mattei, V.; et al. The Botanical Drug PBI-05204, a Supercritical CO2 Extract of Nerium Oleander, Inhibits Growth of Human Glioblastoma, Reduces Akt/mTOR Activities, and Modulates GSC Cell-Renewal Properties. Front. Pharmacol. 2020, 11, 552428. [Google Scholar] [CrossRef]
- Coimbra, A.T.; Ferreira, S.; Duarte, A.P. Genus Ruta: A natural source of high value products with biological and pharmacological properties. J. Ethnopharmacol. 2020, 260, 113076. [Google Scholar] [CrossRef]
- Parray, S.A.; Bhat, J.U.; Ahmad, G.; Jahan, N.; Sofi, G.; Ifs, M. Ruta graveolens: From traditional system of medicine to modern pharmacology: An overview. Am. J. Pharm. Tech. Res. 2012, 2, 239–252. [Google Scholar]
| Compound | Chemical Structure | Type of GSCs | Mechanisms of Action | Literature | |
|---|---|---|---|---|---|
| Phenolic compounds and flavonoids | Resveratrol | 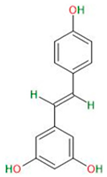 |
|
| [28] |
| Luteolin | 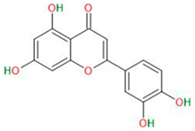 |
|
| [29,30] | |
| Curcumin | 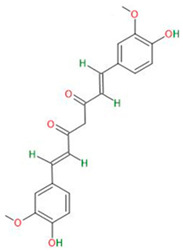 |
|
| [31] | |
| Apigenin |  |
|
| [32,33] | |
| Alkaloids | Harmine |  |
|
| [34,35,36,37] |
| Cyclopamine |  |
|
| [38,39,40] | |
| Terpenoids | Honokiol | 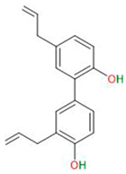 |
|
| [41,42,43] |
| Tanshinone IIa |  |
|
| [44,45,46] | |
| Cannabinoids |  |
|
| [47,48,49] | |
| Other natural compounds | Guggulsterone |  |
|
| [50,51] |
| Sulforaphane | 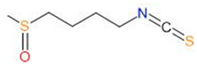 |
|
| [52,53,54] | |
| Eckol | 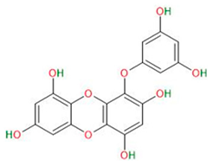 |
|
| [55,56] | |
| All-trans retinoic acid (ATRA) |  |
|
| [57,58,59] | |
| PBI-05204 | mixture of compoundsisolated from Nerium oleander |
|
| [60] | |
| RGWE | mixture of compoundsisolated from Ruta graveolens |
|
| [61] | |
| Compound | Oral Bioavailability | BBB Permeability | Side Effects in Clinical Trials | |
|---|---|---|---|---|
| Phenolic compounds and flavonoids | Resveratrol | 77–80% [64] | + [65] | Well tolerated; relatively few gastrointestinal side effects have been reported (diarrhea, constipation, nausea, abdominal cramps, vomiting, fatty diarrhea, heartburn, reflux, and bloating) [66]. |
| Luteolin | 17.5–53.9% [67] | + [68] | No significant side effects have been reported [67]. | |
| Curcumin | <1% [69] | - [70] | Mild gastrointestinal symptoms (diarrhea, abdominal pain, flatulence, indigestion, nausea, vomiting, and constipation), headache and dizziness, hair loss, and fever. Individual cases of muscle atrophy and kidney damage [71]. | |
| Apigenin | 30% [72] | + [73] | Well tolerated; no side effects have been reported [73]. | |
| Alkaloids | Harmine | 5–10% [74] | + [75] | Well tolerated; can cause drowsiness, impaired concentration, and dizziness, but not very often. At higher doses, mild nausea and vomiting occurred [74]. |
| Cyclopamine | 80% [76] | + [77] | Due to strong side effects in mouse models (weight loss, dehydration, and death), the compound has not been used in clinical trials [77]. | |
| Terpenoids | Honokiol | 5% [78] | + [78] | Well tolerated; relatively few side effects have been reported (heartburn, trembling hands, sexual dysfunction, thyroid failure, fatigue, and headaches) [79]. |
| Tanshinone IIa | <4% [80] | + [81] | No significant side effects have been reported [82]. | |
| Cannabinoids | 6–19% [83] | + [84] | Compounds used in medicine. Can be addictive and intoxicating (depending on the type—THC or CBD) and can cause psychiatric, gastrointestinal, and cardiovascular problems [85,86]. | |
| Other natural compounds | Guggulsterone | 43% [87] | + [88] | Well tolerated; mild side effects causing dermatologic hypersensitivity have been reported [89]. |
| Sulforaphane | 82% [90] | + [91] | Well tolerated; mild side effects (constipation and diarrhea) have been reported [92]. | |
| Eckol | <1% [93] | - [94] | Clinical trials have not yet been conducted, and no data on side effects in mice are available [95]. | |
| All-trans retinoic acid (ATRA) | <50% [96] | + [97] | Well tolerated; mild side effects (fatigue, headache, fever, dermatitis, weakness, and gastrointestinal symptoms) have been reported [98]. | |
| PBI-05204 | Different for each component of the extract; about 63% [99] | + [60] | Well tolerated; relatively few side effects have been reported (vomiting, nausea, decreased appetite, and diarrhea) [100]. | |
| RGWE | Not specified; different for each component of the extract | Not specified, different for each component of the extract | Clinical trials have not yet been conducted, and no data on side effects in rats are available [101]. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaroshenko, M.; Christoff, M.; Ścibiorski, M.; Surowiec, K.; Jakubowicz-Gil, J.; Sumorek-Wiadro, J. Natural Compounds That Target Glioma Stem Cells. NeuroSci 2025, 6, 52. https://doi.org/10.3390/neurosci6020052
Yaroshenko M, Christoff M, Ścibiorski M, Surowiec K, Jakubowicz-Gil J, Sumorek-Wiadro J. Natural Compounds That Target Glioma Stem Cells. NeuroSci. 2025; 6(2):52. https://doi.org/10.3390/neurosci6020052
Chicago/Turabian StyleYaroshenko, Mariia, Monika Christoff, Mateusz Ścibiorski, Karolina Surowiec, Joanna Jakubowicz-Gil, and Joanna Sumorek-Wiadro. 2025. "Natural Compounds That Target Glioma Stem Cells" NeuroSci 6, no. 2: 52. https://doi.org/10.3390/neurosci6020052
APA StyleYaroshenko, M., Christoff, M., Ścibiorski, M., Surowiec, K., Jakubowicz-Gil, J., & Sumorek-Wiadro, J. (2025). Natural Compounds That Target Glioma Stem Cells. NeuroSci, 6(2), 52. https://doi.org/10.3390/neurosci6020052






