Emerging Therapies for Neurological Disorders: A Clinical Review of MANAGED (Music, Art, Nature-Based, Animal-Assisted, Game, Essential Oil, Dance) Care
Abstract
1. Introduction
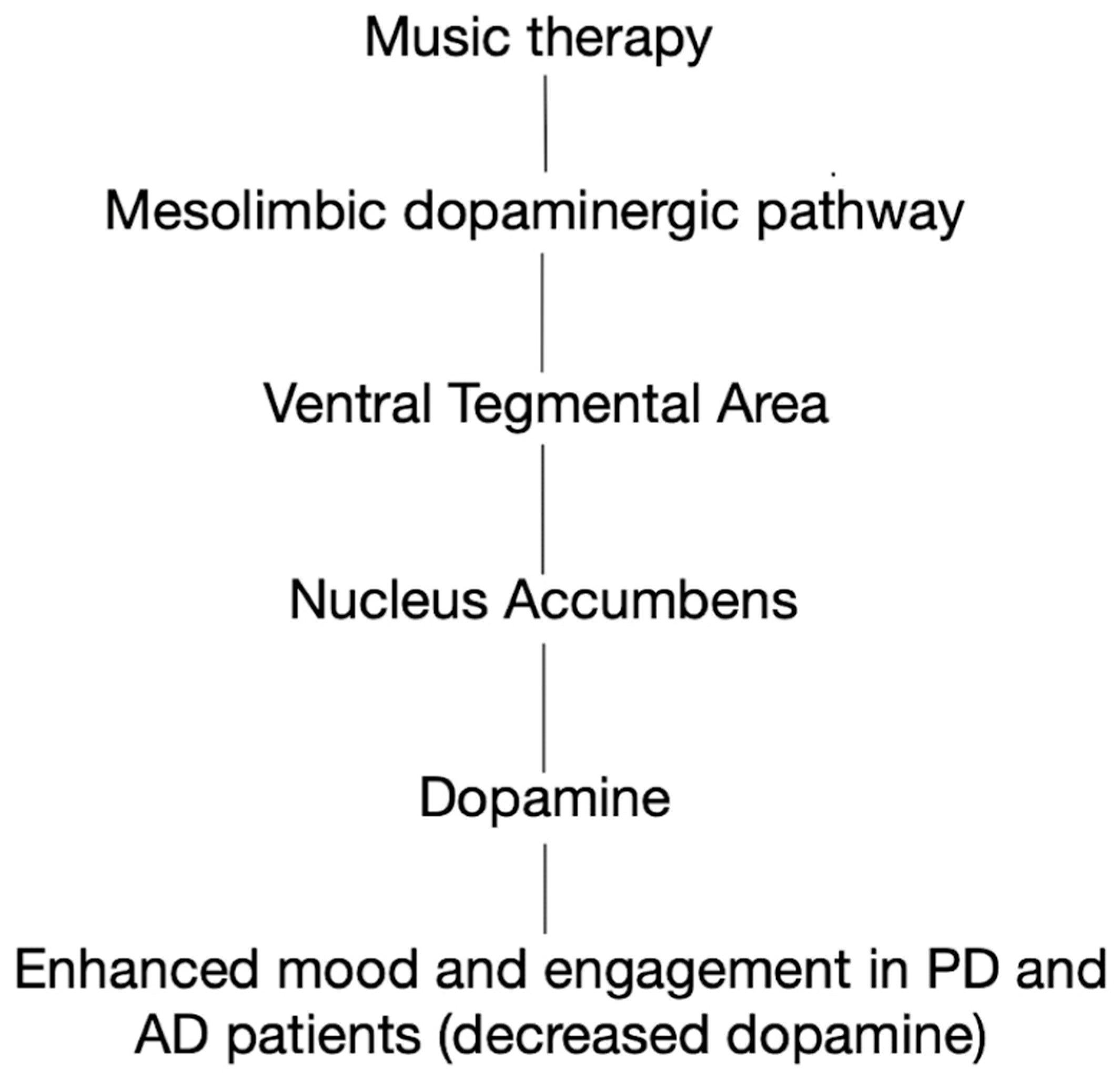
2. Music Therapy
3. Art Therapy
4. Shinrin-Yoku (“Forest Bathing”)
5. Animal-Assisted Therapy (AAT)
6. Game Therapy
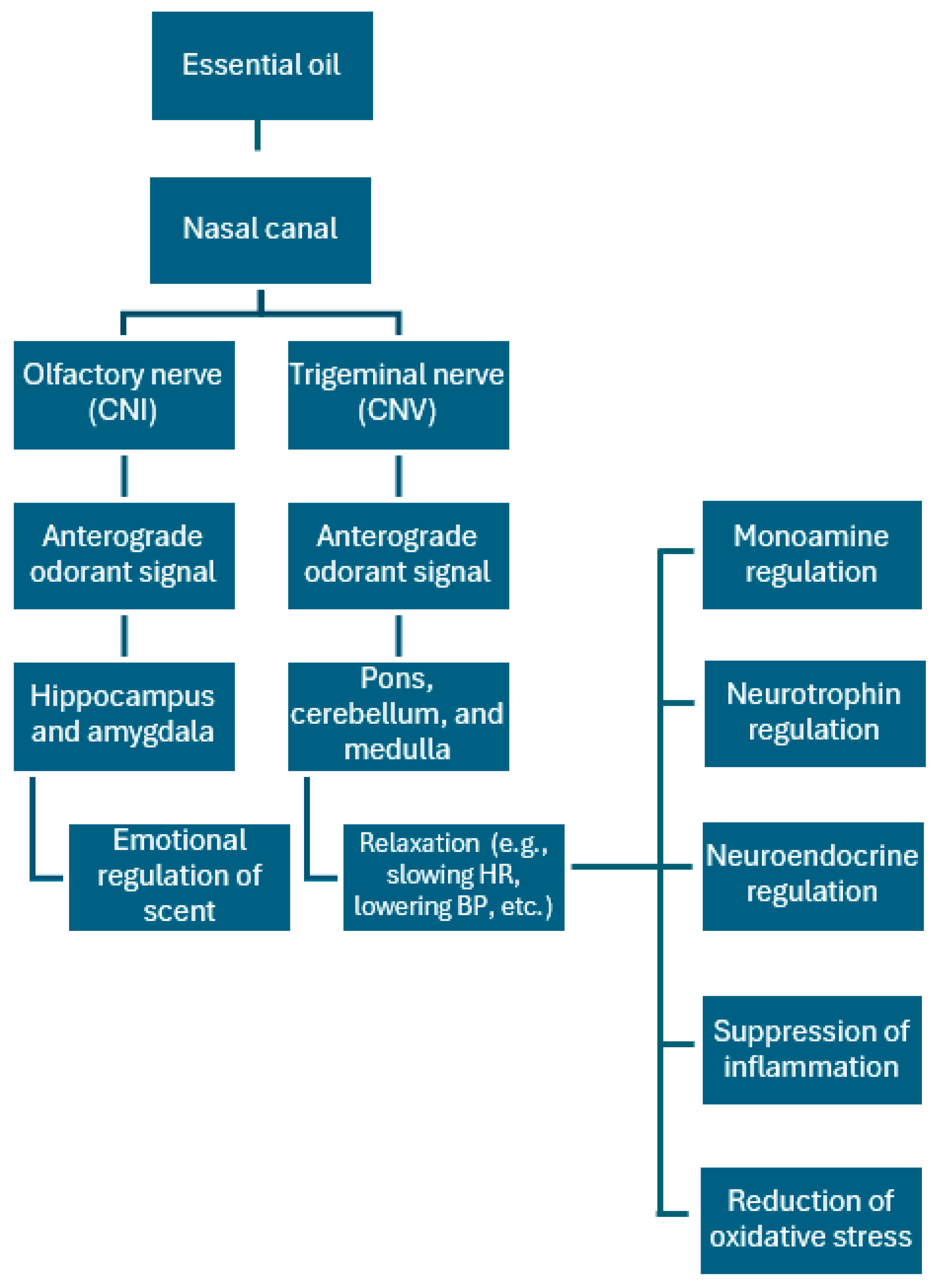
7. Essential Oil/Aromatherapy
8. Dance Movement Therapy (DMT)
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hong, G.G. Acupuncture: The Historical Basis and Its US Practitioners. Lab. Med. 1998, 29, 163–166. [Google Scholar] [CrossRef][Green Version]
- Guo, X.; Ma, T. Effects of Acupuncture on Neurological Disease in Clinical- and Animal-Based Research. Front. Integr. Neurosci. 2019, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S. Rehabilitative potential of Ayurveda for neurological deficits caused by traumatic spinal cord injury. J. Ayurveda Integr. Med. 2014, 5, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Nierhaus, T.; Pach, D.; Huang, W.; Long, X.; Napadow, V.; Roll, S.; Liang, F.; Pleger, B.; Villringer, A.; Witt, C.M. Differential cerebral response to somatosensory stimulation of an acupuncture point vs. two non-acupuncture points measured with EEG and fMRI. Front. Hum. Neurosci. 2015, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Bauchner, H.; Fontanarosa, P.B. Health Care Spending in the United States Compared With 10 Other High-Income Countries: What Uwe Reinhardt Might Have Said. JAMA 2018, 319, 990–992. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Sriramoju, B.; Kanwar, R.K. Neurological disorders and therapeutics targeted to surmount the blood-brain barrier. Int. J. Nanomed. 2012, 7, 3259–3278. [Google Scholar] [CrossRef]
- Christoph Diener, H.; Kastrup, O. Chapter 106—Neurological and General Side Effects of Drug Therapy. In Neurological Disorders, 2nd ed.; Brandt, T., Caplan, L.R., Dichgans, J., Diener, H.C., Kennard, C., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 1507–1524. Available online: https://www.sciencedirect.com/science/article/pii/B9780121258313503014 (accessed on 27 November 2024).
- Lee, D.J.; Lozano, A.M. The Future of Surgical Treatments for Parkinson’s Disease. J. Park. Dis. 2018, 8, S79–S83. [Google Scholar] [CrossRef]
- Pathan, A. Limitations of Alzheimer’s Disease Medications. NeuroPharmac. J. 2023, 11–17. [Google Scholar] [CrossRef]
- Rajahthurai, S.D.; Farrukh, M.J.; Makmor-Bakry, M.; Tan, H.J.; Fatokun, O.; Mohd Saffian, S.; Ramatillah, D.L. Use of Complementary and Alternative Medicine and Adherence to Medication Therapy Among Stroke Patients: A Meta-analysis and Systematic Review. Front. Pharmacol. 2022, 13, 870641. [Google Scholar] [CrossRef]
- Wells, R.E.; Baute, V.; Wahbeh, H. Complementary and Integrative Medicine for Neurologic Conditions. Med. Clin. N. Am. 2017, 101, 881–893. [Google Scholar] [CrossRef]
- Thaut, M.H. Music as therapy in early history. Prog. Brain Res. 2015, 217, 143–158. [Google Scholar] [CrossRef] [PubMed]
- T Zaatar, M.; Alhakim, K.; Enayeh, M.; Tamer, R. The transformative power of music: Insights into neuroplasticity, health and disease. Brain Behav. Immun. Health 2024, 35, 100716. [Google Scholar] [CrossRef]
- Sun, J.; Chen, W. Music therapy for coma patients: Preliminary results. Eur. Rev. 2015, 19, 7. [Google Scholar]
- Chang, Y.-S.; Chu, H.; Yang, C.-Y.; Tsai, J.-C.; Chung, M.-H.; Liao, Y.-M.; Chi, M.; Liu, M.F.; Chou, K.-R. The efficacy of music therapy for people with dementia: A meta-analysis of randomised controlled trials. J. Clin. Nurs. 2015, 24, 3425–3440. [Google Scholar] [CrossRef]
- Sihvonen, A.J.; Särkämö, T.; Leo, V.; Tervaniemi, M.; Altenmüller, E.; Soinila, S. Music-based interventions in neurological rehabilitation. Lancet Neurol. 2017, 16, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Large infarcts in the middle cerebral artery territory. Neurology 1998, 50, 1940–1943. [CrossRef]
- Liao, H.; Jiang, G.; Wang, X. Music therapy as a non-pharmacological treatment for epilepsy. Expert Rev. Neurother. 2015, 15, 993–1003. [Google Scholar] [CrossRef]
- Thaut, M.H.; Rice, R.R.; Braun Janzen, T.; Hurt-Thaut, C.P.; McIntosh, G.C. Rhythmic auditory stimulation for reduction of falls in Parkinson’s disease: A randomized controlled study. Clin. Rehabil. 2019, 33, 34–43. [Google Scholar] [CrossRef]
- Cubo, E.; Leurgans, S.; Goetz, C.G. Short-term and practice effects of metronome pacing in Parkinson’s disease patients with gait freezing while in the ‘on’ state: Randomized single blind evaluation. Parkinsonism Relat. Disord. 2004, 10, 507–510. [Google Scholar] [CrossRef]
- Duncan, R.P.; Earhart, G.M. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil. Neural Repair 2012, 26, 132–143. [Google Scholar] [CrossRef]
- Butala, A.; Li, K.; Swaminathan, A.; Dunlop, S.; Salnikova, Y.; Ficek, B.; Portnoff, B.; Harper, M.; Vernon, B.; Turk, B.; et al. Parkinsonics: A Randomized, Blinded, Cross-Over Trial of Group Singing for Motor and Nonmotor Symptoms in Idiopathic Parkinson Disease. Park. Dis. 2022, 2022, 4233203. [Google Scholar] [CrossRef] [PubMed]
- Machado Sotomayor, M.J.; Arufe-Giráldez, V.; Ruíz-Rico, G.; Navarro-Patón, R. Music Therapy and Parkinson’s Disease: A Systematic Review from 2015–2020. Int. J. Environ. Res. Public Health 2021, 18, 11618. [Google Scholar] [CrossRef] [PubMed]
- O’Kelly, J.; Bodak, R. Development of the Music Therapy Assessment Tool for Advanced Huntington’s Disease: A Pilot Validation Study. J. Music Ther. 2016, 53, 232–256. [Google Scholar] [CrossRef] [PubMed]
- Bodeck, S.; Lappe, C.; Evers, S. Tic-reducing effects of music in patients with Tourette’s syndrome: Self-reported and objective analysis. J. Neurol. Sci. 2015, 352, 41–47. [Google Scholar] [CrossRef]
- Wittwer, J.E.; Winbolt, M.; Morris, M.E. A Home-Based, Music-Cued Movement Program Is Feasible and May Improve Gait in Progressive Supranuclear Palsy. Front. Neurol. 2019, 10, 116. [Google Scholar] [CrossRef]
- Chatterjee, D.; Hegde, S.; Thaut, M. Neural plasticity: The substratum of music-based interventions in neurorehabilitation. NeuroRehabilitation 2021, 48, 155–166. [Google Scholar] [CrossRef]
- Li, K.; Cui, C.; Zhang, H.; Jia, L.; Li, R.; Hu, H.-Y. Exploration of combined physical activity and music for patients with Alzheimer’s disease: A systematic review. Front. Aging Neurosci. 2022, 14, 962475. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, J.-K.; Yeo, M.S. Dual-Task-Based Music Therapy to Improve Executive Functioning of Elderly Patients with Early Stage Alzheimer’s Disease: A Multiple Case Study. Int. J. Environ. Res. Public Health 2022, 19, 11940. [Google Scholar] [CrossRef]
- Matziorinis, A.M.; Koelsch, S. The promise of music therapy for Alzheimer’s disease: A review. Ann. N. Y. Acad. Sci. 2022, 1516, 11–17. [Google Scholar] [CrossRef]
- Ettinger, T.; Berberian, M.; Acosta, I.; Cucca, A.; Feigin, A.; Genovese, D.; Pollen, T.; Rieders, J.; Kilachand, R.; Gomez, C.; et al. Art therapy as a comprehensive complementary treatment for Parkinson’s disease. Front. Hum. Neurosci. 2023, 17, 1110531. [Google Scholar] [CrossRef]
- Deshmukh, S.R.; Holmes, J.; Cardno, A. Art Therapy for People with Dementia; Deshmukh, S.R., Ed.; Cochrane Library: London, UK, 2018; Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011073.pub2/full (accessed on 27 November 2024).
- Lo, T.L.T.; Lee, J.L.C.; Ho, R.T.H. Creative Arts-Based Therapies for Stroke Survivors: A Qualitative Systematic Review. Front. Psychol. 2019, 9, 1538. [Google Scholar] [CrossRef]
- Brown, S.E.; Shella, T.; Pestana-Knight, E. Development and use of the art therapy seizure assessment sculpture on an inpatient epilepsy monitoring unit. Epilepsy Behav. Case Rep. 2018, 9, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Windle, G.; Gregory, S.; Howson-Griffiths, T.; Newman, A.; O’Brien, D.; Goulding, A. Exploring the theoretical foundations of visual art programmes for people living with dementia. Dementia 2018, 17, 702–727. [Google Scholar] [CrossRef] [PubMed]
- Oepen, R.; Gruber, H. Art-based interventions and art therapy to promote health of migrant populations—A systematic literature review of current research. Arts Health 2024, 16, 266–284. [Google Scholar] [CrossRef]
- Smirnoff, L.; Pham, K. A Role for Visual Art Therapy in the Management of Migraine. Curr. Pain Headache Rep. 2024, 28, 189–194. [Google Scholar] [CrossRef]
- Raudenská, J.; Šteinerová, V.; Vodičková, Š.; Raudenský, M.; Fulková, M.; Urits, I.; Viswanath, O.; Varrassi, G.; Javůrková, A. Arts Therapy and Its Implications in Chronic Pain Management: A Narrative Review. Pain Ther. 2023, 12, 1309–1337. [Google Scholar] [CrossRef]
- Aguilar, B.A. The Efficacy of Art Therapy in Pediatric Oncology Patients: An Integrative Literature Review. J. Pediatr. Nurs. 2017, 36, 173–178. [Google Scholar] [CrossRef]
- Campbell, M.; Decker, K.P.; Kruk, K.; Deaver, S.P. Art Therapy and Cognitive Processing Therapy for Combat-Related PTSD: A Randomized Controlled Trial. Art Ther. J. Am. Art Ther. Assoc. 2016, 33, 169–177. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Havlena, J.; Krezinski, A.J. Art therapy focus groups for children and adolescents with epilepsy. Epilepsy Behav. EB 2012, 24, 227–233. [Google Scholar] [CrossRef]
- Akhan, L.U.; Kurtuncu, M.; Celik, S. The Effect of Art Therapy with Clay on Hopelessness Levels Among Neurology Patients. Rehabil. Nurs. Off. J. Assoc. Rehabil. Nurses 2017, 42, 39–45. [Google Scholar] [CrossRef]
- Kanesaki, H.; Watanabe, K.; Osugi, K.; Ohara, H.; Takada, K.; Kinoshita, M. Utility of scratch art therapy in adult epilepsy patients with difficulties in social adaptation. Epileptic. Disord. 2023, 25, 702–711. [Google Scholar] [CrossRef]
- Oliva, A.; Iosa, M.; Antonucci, G.; De Bartolo, D. Are neuroaesthetic principles applied in art therapy protocols for neurorehabilitation? A systematic mini-review. Front. Psychol. 2023, 14, 1158304. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Furuyashiki, A.; Tabuchi, K.; Norikoshi, K.; Kobayashi, T.; Oriyama, S. A comparative study of the physiological and psychological effects of forest bathing (Shinrin-yoku) on working age people with and without depressive tendencies. Environ. Health Prev. Med. 2019, 24, 46. [Google Scholar] [CrossRef]
- James, P.; Hart, J.E.; Banay, R.F.; Laden, F. Exposure to Greenness and Mortality in a Nationwide Prospective Cohort Study of Women. Environ. Health Perspect. 2016, 124, 1344–1352. [Google Scholar] [CrossRef]
- Li, Q. Effects of forest environment (Shinrin-yoku/Forest bathing) on health promotion and disease prevention—the Establishment of “Forest Medicine”. Environ. Health Prev. Med. 2022, 27, 43. [Google Scholar] [CrossRef]
- Lee, S.-H.; Sohn, J.-H.; Sung, J.H.; Han, S.-W.; Lee, M.; Kim, Y.; Kim, J.H.; Jeon, J.P.; Lee, J.J.; Kim, C. The impact of forest therapy on functional recovery after acute ischemic stroke. Urban For. Urban Green. 2024, 101, 128537. [Google Scholar] [CrossRef]
- Lorraine Ernst, R.N. Animal-Assisted Therapy: An Exploration of Its History, Healing Benefits, and How Skilled Nursing Facilities Can Set Up Programs. Ann. Long-Term Care 2014, 22, 27–32. [Google Scholar]
- Doan, T.; Pennewitt, D.; Patel, R. Animal assisted therapy in pediatric mental health conditions: A review. Curr. Probl. Pediatr. Adolesc. Health Care 2023, 53, 101506. [Google Scholar] [CrossRef]
- Charry-Sánchez, J.D.; Pradilla, I.; Talero-Gutiérrez, C. Animal-assisted therapy in adults: A systematic review. Complement. Ther. Clin. Pract. 2018, 32, 169–180. [Google Scholar] [CrossRef]
- O’Haire, M.E.; Rodriguez, K.E. Preliminary efficacy of service dogs as a complementary treatment for posttraumatic stress disorder in military members and veterans. J. Consult. Clin. Psychol. 2018, 86, 179–188. [Google Scholar] [CrossRef]
- Lai, N.M.; Chang, S.M.W.; Ng, S.S.; Tan, S.L.; Chaiyakunapruk, N.; Stanaway, F. Animal-assisted therapy for dementia. Cochrane Database Syst. Rev. 2019, 11, CD013243. [Google Scholar] [CrossRef]
- Muñoz-Lasa, S.; López de Silanes, C.; Atín-Arratibel, M.Á.; Bravo-Llatas, C.; Pastor-Jimeno, S.; Máximo-Bocanegra, N. Effects of hippotherapy in multiple sclerosis: Pilot study on quality of life, spasticity, gait, pelvic floor, depression and fatigue. Med. Clin. 2019, 152, 55–58. [Google Scholar] [CrossRef]
- Machová, K.; Procházková, R.; Říha, M.; Svobodová, I. The Effect of Animal-Assisted Therapy on the State of Patients’ Health After a Stroke: A Pilot Study. Int. J. Environ. Res. Public Health 2019, 16, 3272. [Google Scholar] [CrossRef]
- Marcus, D.A. The Science Behind Animal-Assisted Therapy. Curr. Pain Headache Rep. 2013, 17, 322. [Google Scholar] [CrossRef] [PubMed]
- Mittly, V.; Farkas-Kirov, C.; Zana, Á.; Szabó, K.; Ónodi-Szabó, V.; Purebl, G. The effect of animal-assisted interventions on the course of neurological diseases: A systematic review. Syst. Rev. 2023, 12, 224. [Google Scholar] [CrossRef]
- Souilm, N. Equine-assisted therapy effectiveness in improving emotion regulation, self-efficacy, and perceived self-esteem of patients suffering from substance use disorders. BMC Complement. Med. Ther. 2023, 23, 363. [Google Scholar] [CrossRef]
- Andriacchi, M.; Hopper, C.; Stein, A.; Nye, R.; Taylor, K. Animal-Assisted Interventions on a College Campus to Improve Wellness: Adventures With the Northern Michigan University Wildpups. J. Nurs. Educ. 2023, 62, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, B.F.; Adilbekov, E.; Zharmenov, S.; Akyol, A.; Yessirkepov, M. Evaluating the efficacy of hippotherapy: A promising intervention in rheumatology, pain medicine and geriatrics. Rheumatol. Int. 2023, 43, 2185–2191. [Google Scholar] [CrossRef]
- Villarreal-Zegarra, D.; Yllescas-Panta, T.; Malaquias-Obregon, S.; Dámaso-Román, A.; Mayo-Puchoc, N. Effectiveness of animal-assisted therapy and pet-robot interventions in reducing depressive symptoms among older adults: A systematic review and meta-analysis. Complement. Ther. Med. 2024, 80, 103023. [Google Scholar] [CrossRef]
- Muñoz Lasa, S.; Máximo Bocanegra, N.; Valero Alcaide, R.; Atín Arratibel, M.A.; Varela Donoso, E.; Ferriero, G. Animal assisted interventions in neurorehabilitation: A review of the most recent literature. Neurol. Engl. Ed. 2015, 30, 1–7. [Google Scholar] [CrossRef]
- Beavers, A.; Fleming, A.; Shahidullah, J.D. Animal-assisted therapies for autism. Curr. Probl. Pediatr. Adolesc. Health Care 2023, 53, 101478. [Google Scholar] [CrossRef] [PubMed]
- Kilmer, M.; Hong, M.; Randolph, D.; Reichel, A.; Huetter, S.; Bowden, M.; Kilmer, C. Animal-assisted therapy in pediatric autism spectrum disorder: A case report. Nurse Pract. 2024, 49, 31–39. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, E.; Palacios-Cuesta, A.; Rodríguez-Martínez, A.; Olmedilla-Jodar, M.; Fernández-Andrade, R.; Mediavilla-Fernández, R.; Sánchez-Díaz, J.I.; Máximo-Bocanegra, N. Implementation feasibility of animal-assisted therapy in a pediatric intensive care unit: Effectiveness on reduction of pain, fear, and anxiety. Eur. J. Pediatr. 2024, 183, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Y.; Saka, S.; Tuncer, D. Effect of hippotherapy on balance, functional mobility, and functional independence in children with Down syndrome: Randomized controlled trial. Eur. J. Pediatr. 2023, 182, 3147–3155. [Google Scholar] [CrossRef] [PubMed]
- Chubak, J.; Adler, A.; Bobb, J.F.; Hawkes, R.J.; Ziebell, R.A.; Pocobelli, G.; Ludman, E.J.; Zerr, D.M. A Randomized Controlled Trial of Animal-assisted Activities for Pediatric Oncology Patients: Psychosocial and Microbial Outcomes. J. Pediatr. Health Care 2024, 38, 354–364. [Google Scholar] [CrossRef]
- Mahoney, A.B.; Akard, T.F.; Cowfer, B.A.; Dietrich, M.S.; Newton, J.L.; Gilmer, M.J. Impact of Animal—Assisted Interaction on Anxiety in Children With Advanced Cancer and Their Caregivers. J. Palliat. Med. 2024, 27, 75–82. [Google Scholar] [CrossRef]
- Locher, C.; Petignat, M.; Wagner, C.; Hediger, K.; Roth, B.; Gaab, J.; Koechlin, H. Animal-Assisted Psychotherapy for Pediatric Chronic Pain: Case Series of an Open Pilot Study to Test Initial Feasibility and Potential Efficacy. J. Pain Res. 2023, 16, 1799–1811. [Google Scholar] [CrossRef]
- Kelley, C.; Eller, C. Introduction to Animal Therapy and its Related Tax Benefits. J. Couns. Psychol. 2017, 1, 4. [Google Scholar]
- Kim, Y.W. Update on Stroke Rehabilitation in Motor Impairment. Brain Neurorehabil. 2022, 15, e12. [Google Scholar] [CrossRef]
- Putrino, D.; Zanders, H.; Hamilton, T.; Rykman, A.; Lee, P.; Edwards, D.J. Patient Engagement Is Related to Impairment Reduction During Digital Game-Based Therapy in Stroke. Games Health J. 2017, 6, 295–302. [Google Scholar] [CrossRef]
- Desai, K.; Prabhakaran, B.; Ifejika, N.; Annaswamy, T.M. Personalized 3D exergames for in-home rehabilitation after stroke: A pilot study. Disabil. Rehabil. Assist. Technol. 2023, 18, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Kumaran, D.S.; Unni, A.; Sardesai, S.; Prabhu, V.; Nirmal, P.; Pai, A.R.; Guddattu, V.; Arumugam, A. Effectiveness of an Intensive, Functional, and Gamified Rehabilitation Program on Upper Limb Function in People With Stroke (EnteRtain): A Multicenter Randomized Clinical Trial. Neurorehabil. Neural Repair 2024, 38, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Knutson, J.S.; Fu, M.J.; Cunningham, D.A.; Hisel, T.Z.; Friedl, A.S.; Gunzler, D.D.; Plow, E.B.; Busch, R.M.; Pundik, S. Contralaterally controlled functional electrical stimulation video game therapy for hand rehabilitation after stroke: A randomized controlled trial. Disabil. Rehabil. 2024, 46, 4466–4475. [Google Scholar] [CrossRef]
- Valdes, G. Microsoft is giving Xbox Adaptive Controllers, Consoles and Games to the VA [Internet] VentureBeat 2019. Available online: https://venturebeat.com/games/microsoft-is-giving-xbox-adaptive-controllers-consoles-and-games-to-the-va/ (accessed on 27 November 2024).
- Zuschnegg, J.; Schoberer, D.; Häussl, A.; Herzog, S.A.; Russegger, S.; Ploder, K.; Fellner, M.; Hofmarcher-Holzhacker, M.M.; Roller-Wirnsberger, R.; Paletta, L.; et al. Effectiveness of computer-based interventions for community-dwelling people with cognitive decline: A systematic review with meta-analyses. BMC Geriatr. 2023, 23, 229. [Google Scholar] [CrossRef] [PubMed]
- Nuic, D.; van de Weijer, S.; Cherif, S.; Skrzatek, A.; Zeeboer, E.; Olivier, C.; Corvol, J.-C.; Foulon, P.; Pastor, J.Z.; Mercier, G.; et al. Home-based exergaming to treat gait and balance disorders in patients with Parkinson’s disease: A phase II randomized controlled trial. Eur. J. Neurol. 2024, 31, e16055. [Google Scholar] [CrossRef]
- Çetin, B.; Kılınç, M.; Çakmaklı, G.Y. The effects of exergames on upper extremity performance, trunk mobility, gait, balance, and cognition in Parkinson’s disease: A randomized controlled study. Acta Neurol. Belg. 2024, 124, 853–863. [Google Scholar] [CrossRef]
- Gauthier, L.V.; Kane, C.; Borstad, A.; Strahl, N.; Uswatte, G.; Taub, E.; Morris, D.; Hall, A.; Arakelian, M.; Mark, V. Video Game Rehabilitation for Outpatient Stroke (VIGoROUS): Protocol for a multi-center comparative effectiveness trial of in-home gamified constraint-induced movement therapy for rehabilitation of chronic upper extremity hemiparesis. BMC Neurol. 2017, 17, 109. [Google Scholar] [CrossRef]
- Lenne, B.; Degraeve, B.; Davroux, J.; Norberciak, L.; Kwiatkowski, A.; Donze, C. Improving cognition in people with multiple sclerosis: Study protocol for a multiarm, randomised, blinded trial of multidomain cognitive rehabilitation using a video-serious game (E-SEP cognition). BMJ Neurol. Open 2023, 5, e000488. [Google Scholar] [CrossRef]
- Luna-Oliva, L.; Ortiz-Gutiérrez, R.M.; Cano-de la Cuerda, R.; Piédrola, R.M.; Alguacil-Diego, I.M.; Sánchez-Camarero, C.; Martínez Culebras, M.D.C. Kinect Xbox 360 as a therapeutic modality for children with cerebral palsy in a school environment: A preliminary study. NeuroRehabilitation 2013, 33, 513–521. [Google Scholar] [CrossRef]
- Ilg, W.; Schatton, C.; Schicks, J.; Giese, M.A.; Schöls, L.; Synofzik, M. Video game-based coordinative training improves ataxia in children with degenerative ataxia. Neurology 2012, 79, 2056–2060. [Google Scholar] [CrossRef]
- Horne-Moyer, H.L.; Moyer, B.H.; Messer, D.C.; Messer, E.S. The use of electronic games in therapy: A review with clinical implications. Curr. Psychiatry Rep. 2014, 16, 520. [Google Scholar] [CrossRef]
- Lv, X.N.; Liu, Z.J.; Zhang, H.J.; Tzeng, C.M. Aromatherapy and the central nerve system (CNS): Therapeutic mechanism and its associated genes. Curr. Drug Targets 2013, 14, 872–879. [Google Scholar] [CrossRef]
- Fung, T.K.H.; Lau, B.W.M.; Ngai, S.P.C.; Tsang, H.W.H. Therapeutic Effect and Mechanisms of Essential Oils in Mood Disorders: Interaction between the Nervous and Respiratory Systems. Int. J. Mol. Sci. 2021, 22, 4844. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Quintero-Rincón, P.; Olivero-Verbel, J. Aromatherapy and Essential Oils: Holistic Strategies in Complementary and Alternative Medicine for Integral Wellbeing. Plants 2025, 14, 400. [Google Scholar] [CrossRef]
- Cui, J.; Li, M.; Wei, Y.; Li, H.; He, X.; Yang, Q.; Li, Z.; Duan, J.; Wu, Z.; Chen, Q.; et al. Inhalation Aromatherapy via Brain-Targeted Nasal Delivery: Natural Volatiles or Essential Oils on Mood Disorders. Front. Pharmacol. 2022, 13, 60043. [Google Scholar] [CrossRef]
- Qneibi, M.; Bdir, S.; Maayeh, C.; Bdair, M.; Sandouka, D.; Basit, D.; Hallak, M. A Comprehensive Review of Essential Oils and Their Pharmacological Activities in Neurological Disorders: Exploring Neuroprotective Potential. Neurochem. Res. 2024, 49, 258–289. [Google Scholar] [CrossRef]
- Zdrojewicz, Z.; Pypno, D.; Bugaj, B.; Cabała, K.; Waracki, M. Applications of salvia in treating cognitive disorders and Alzheimer’s disease. Postępy Fitoter. 2015, 4, s263–s267. [Google Scholar]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2003, 28, 53–59. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Verdin, A.; Mistrulli, L.; Laruelle, F.; Fourmentin, S.; Lounès-Hadj Sahraoui, A. Chemical Composition, Antioxidant and Anti-Inflammatory Activities of Clary Sage and Coriander Essential Oils Produced on Polluted and Amended Soils-Phytomanagement Approach. Molecules 2021, 26, 5321. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Miri, R.; Jassbi, A.R.; Erfani, N.; Asadollahi, M.; Ghasemi, M.; Saso, L.; Firuzi, O. Carthamus, Salvia and Stachys species protect neuronal cells against oxidative stress-induced apoptosis. Pharm. Biol. 2014, 52, 1550–1557. [Google Scholar] [CrossRef]
- Bavarsad, N.H.; Bagheri, S.; Kourosh-Arami, M.; Komaki, A. Aromatherapy for the brain: Lavender’s healing effect on epilepsy, depression, anxiety, migraine, and Alzheimer’s disease: A review article. Heliyon 2023, 9, e18492. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. 2—Essential oil composition. In Essential Oil Safety, 2nd ed.; Tisserand, R., Young, R., Eds.; Churchill Livingstone: St. Louis, MI, USA, 2014; pp. 5–22. ISBN 978-0-443-06241-4. [Google Scholar]
- Posadzki, P.; Alotaibi, A.; Ernst, E. Adverse effects of aromatherapy: A systematic review of case reports and case series. Int. J. Risk Saf. Med. 2012, 24, 147–161. [Google Scholar] [CrossRef]
- Henley, D.V.; Lipson, N.; Korach, K.S.; Bloch, C.A. Prepubertal Gynecomastia Linked to Lavender and Tea Tree Oils. N. Engl. J. Med. 2007, 356, 5. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa064725 (accessed on 10 May 2025). [CrossRef]
- Hokkanen, L.; Rantala, L.; Remes, A.M.; Härkönen, B.; Viramo, P.; Winblad, I. Dance and movement therapeutic methods in management of dementia: A randomized, controlled study. J. Am. Geriatr. Soc. 2008, 56, 771–772. [Google Scholar] [CrossRef]
- Aguiar, L.P.C.; da Rocha, P.A.; Morris, M. Therapeutic Dancing for Parkinson’s Disease. Int. J. Gerontol. 2016, 10, 64–70. [Google Scholar] [CrossRef]
- Ruiz-Muelle, A.; López-Rodríguez, M.M. Dance for People with Alzheimer’s Disease: A Systematic Review. Curr. Alzheimer Res. 2019, 16, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Biondo, J. Dance/Movement Therapy as a Holistic Approach to Diminish Health Discrepancies and Promote Wellness for People with Schizophrenia: A Review of the Literature; PubMed Central: Bethesda, MD, USA, 2023; Available online: https://f1000research.com/articles/12-33 (accessed on 27 November 2024).
- Guan, H.; Zhou, Z.; Li, X.; Pan, Y.; Zou, Z.; Meng, X.; Guan, K.; Zhang, L.; Li, Z.; Li, X.; et al. Dance/movement therapy for improving balance ability and bone mineral density in long-term patients with schizophrenia: A randomized controlled trial. Schizophrenia 2023, 9, 1–6. [Google Scholar] [CrossRef]
- Aleksander-Szymanowicz, P.; Filar-Mierzwa, K.; Skiba, A. Effect of dance movement therapy on balance in adults with Down Syndrome. A pilot study. J. Intellect. Disabil. JOID 2025, 29, 40–49. [Google Scholar] [CrossRef]
- Moo, J.T.; Ho, R.T. Benefits and challenges of tele-dance movement psychotherapy with children with autism and their parents. Digit. Health 2023, 9, 20552076231171233. [Google Scholar] [CrossRef]
- Ladwig, J.C.; Broeckelmann, E.M.; Sibley, K.M.; Ripat, J.; Glazebrook, C.M. A synthesis of the characteristics of dance interventions engaging adults with neurodevelopmental disabilities: A scoping review. Disabil. Rehabil. 2024, 46, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Bryl, K.; Tortora, S.; Whitley, J.; Kim, S.-D.; Raghunathan, N.J.; Mao, J.J.; Chimonas, S. Utilization, Delivery, and Outcomes of Dance/Movement Therapy for Pediatric Oncology Patients and their Caregivers: A Retrospective Chart Review. Curr. Oncol. 2023, 30, 6497–6507. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Y.; Zhang, B.; Xiong, Q.; Zhao, H.; Li, S.; Liu, J.; Bian, Y. Self-Guided DMT: Exploring a Novel Paradigm of Dance Movement Therapy in Mixed Reality for Children with ASD. IEEE Trans. Vis. Comput. Graph. 2024, 30, 2119–2128. [Google Scholar] [CrossRef]

| Study | Type of Therapy | Neurological Condition | Tests/Scales | Quantitative Improvement (Experimental) | Quantitative Improvement (Control) |
|---|---|---|---|---|---|
| Putrino et al. (2017) [73] | GT | Stroke/UL rehabilitation | FMA-UL, SUS, PACES | 2.8 ± 2.1 pts (FMA-UL), 72 ± 7.9 pts (SUS), 65.8 ± 10.6 pts (PACES) | N/A |
| Luna-Oliva et al. (2013) [83] | GT | Cerebral palsy | GMFM, AMPS | GMFM: 85.56 ± 13.62 pts (p = 0.001); AMPS motor: 1.15 ± 0.28 pts (p = 0.001) | GMFM: 93.30 ± 13.99 pts (p = 0.001); AMPS motor: 2.04 ± 0.61 pts (p = 0.001) |
| López-Fernández et al. (2024) [66] | AAT | Fear, pain, anxiety | Wong–Baker scale, Child Medical Fear Scale, modified Yale Preoperatory Anxiety Scale | Wong–Baker: 2 to 0; CMFS: 1 to 0; M-YPAS: 40 to 23 | CMFS: 1 to 0; M-YPAS: 32 to 23 (pre vs. post intervention) |
| Guan et al. (2023) [103] | DMT | Schizophrenia | BMD and BBS | BMD: 0.4 ± 0.02 to 0.5 ± 0.06; BBS: 43.1 ± 5.2 to 50.4 ± 4.5 | BMD: 0.4 ± 0.03 to 0.4 ± 0.4; BBS: 44.5 ± 6.3 to 44.8 ± 7.7 |
| Hokkanen et al. (2008) [99] | DMT | Dementia | MMSE, Word List savings score, clock drawing test, Cookie Theft picture description task from Boston Diagnostic Aphasia Test | MMSE: 12.08 ± 5.53 to 14.16 ± 6.67; Clock drawing: 1.05 ± 1.27; Word list savings: no difference compared to control; Picture description test: 14.32 ± 13.62 to 19.76 ± 8.02 | MMSE: no improvement; Clock drawing test: 0.30 ± 0.48; Word list savings: no difference compared to experimental; Picture description test: 13.75 ± 12.68 to 13.4 ± 10.15 |
| Ettinger et al. (2023) [31] | ART | Parkinson’s disease | HTP-PDS | Motor control: +1.0; visual/spatial functioning: +0.5; cognition: +1.0; motivation: +1.1; emotion: +1.5; self: +0.9; interpersonal relatedness: +0.8; creativity: +1.0 | N/A |
| Thaut et al. (2018) [19] | MT | Parkinson’s disease | BBS, TUG | BBS: 46.6 to 51.8; TUG: 12 to 12.1 s (pre vs. post intervention at 24 weeks) | BBS: 47.8 to 51.7; TUG: 11.5 to 12 s (pre vs. post intervention at 24 wks) |
| Wittwer et al. (2019) [26] | MT | Supranuclear palsy | ACE-III, GDS, QS | Median ACE-III: 91; median ACE III fluency: 9; median GDS: 7; median QS: 24 | Median ACE-III: 89; median ACE III fluency: 10; median GDS: 7; median QS: 22 |
| Akhondzadeh et al. (2003) [92] | AT | Alzheimer’s disease | ADAS-cog and/or CDR | ADAS-cog: F = 4.77, d.f. = 1, p = 0.03; CDR-SB: F = 10.84, p < 0.003 | N/A |
| Park et al. (2009) [45] | SY | Stress | SCC, PR, SBP, DBP, HF (after walking) | SCC: 15.8% decrease; PR: 3.9% decrease; SBP: 1.9% decrease; DBP: 2.1% decrease; HF: 102.0% enhancement | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.W.-C.; Hirani, R.; Ogulnick, J.; Tiwari, R.K.; Etienne, M. Emerging Therapies for Neurological Disorders: A Clinical Review of MANAGED (Music, Art, Nature-Based, Animal-Assisted, Game, Essential Oil, Dance) Care. NeuroSci 2025, 6, 51. https://doi.org/10.3390/neurosci6020051
Lee AW-C, Hirani R, Ogulnick J, Tiwari RK, Etienne M. Emerging Therapies for Neurological Disorders: A Clinical Review of MANAGED (Music, Art, Nature-Based, Animal-Assisted, Game, Essential Oil, Dance) Care. NeuroSci. 2025; 6(2):51. https://doi.org/10.3390/neurosci6020051
Chicago/Turabian StyleLee, Alyssa Wan-Chei, Rahim Hirani, Jonathan Ogulnick, Raj K. Tiwari, and Mill Etienne. 2025. "Emerging Therapies for Neurological Disorders: A Clinical Review of MANAGED (Music, Art, Nature-Based, Animal-Assisted, Game, Essential Oil, Dance) Care" NeuroSci 6, no. 2: 51. https://doi.org/10.3390/neurosci6020051
APA StyleLee, A. W.-C., Hirani, R., Ogulnick, J., Tiwari, R. K., & Etienne, M. (2025). Emerging Therapies for Neurological Disorders: A Clinical Review of MANAGED (Music, Art, Nature-Based, Animal-Assisted, Game, Essential Oil, Dance) Care. NeuroSci, 6(2), 51. https://doi.org/10.3390/neurosci6020051







