An Endocrine-Disrupting Chemical, Bisphenol A Diglycidyl Ether (BADGE), Accelerates Neuritogenesis and Outgrowth of Cortical Neurons via the G-Protein-Coupled Estrogen Receptor
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Primary Neuronal Culture
2.3. Cell Treatments
2.4. Immunocytochemistry
2.5. RNA Extraction and Quantitative RT–PCR
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. BADGE Accelerates Neuronal Differentiation to Mature Neurons via ERs
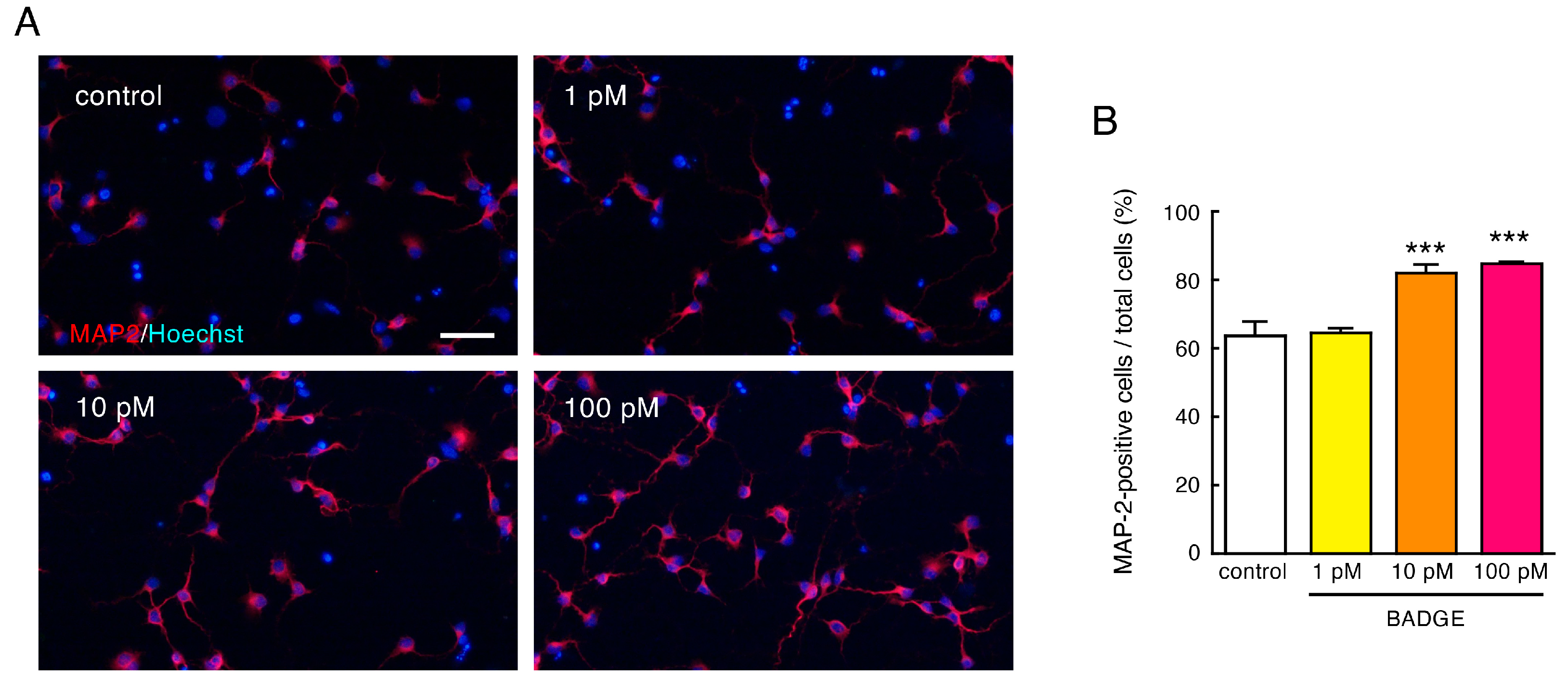
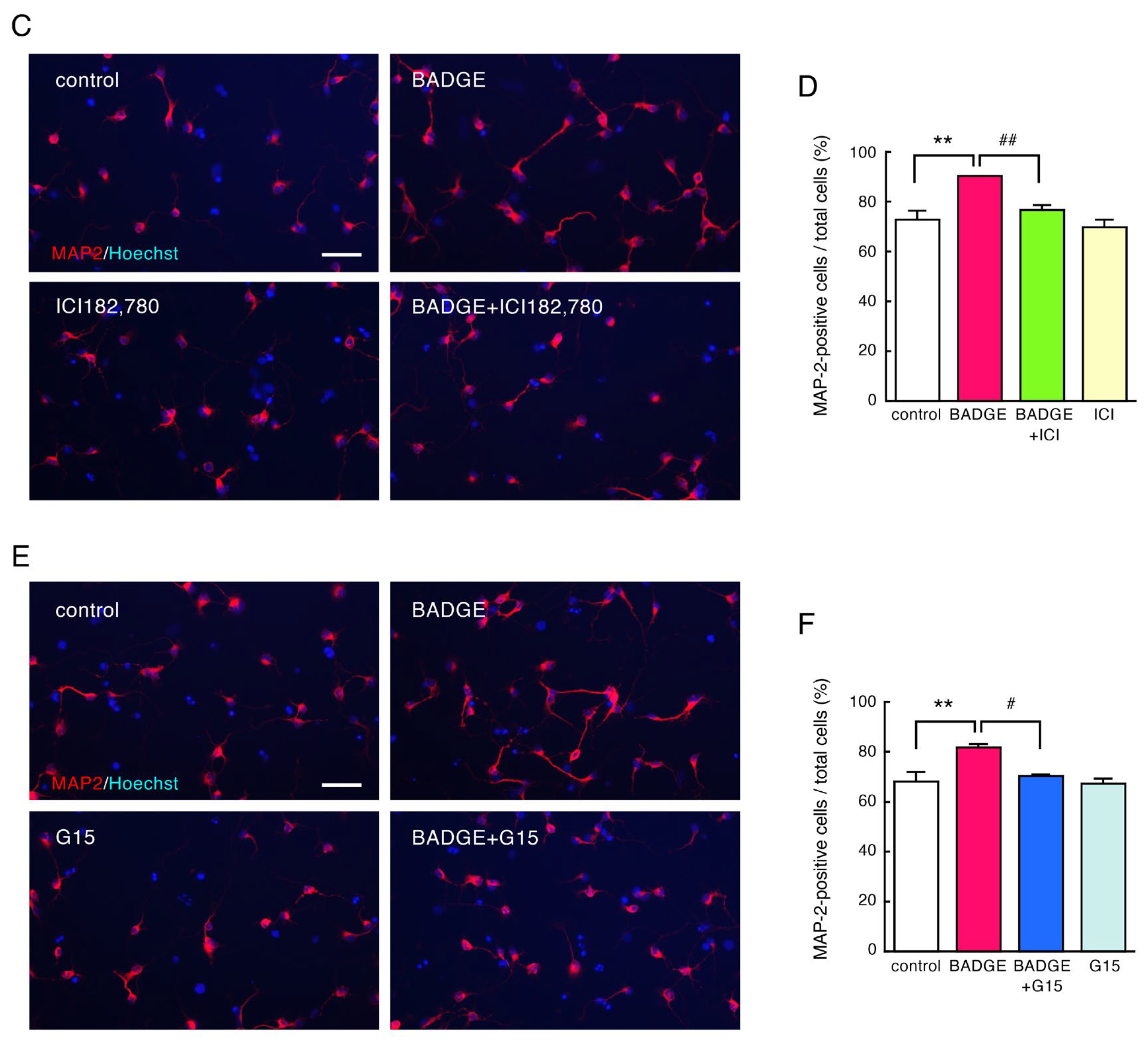
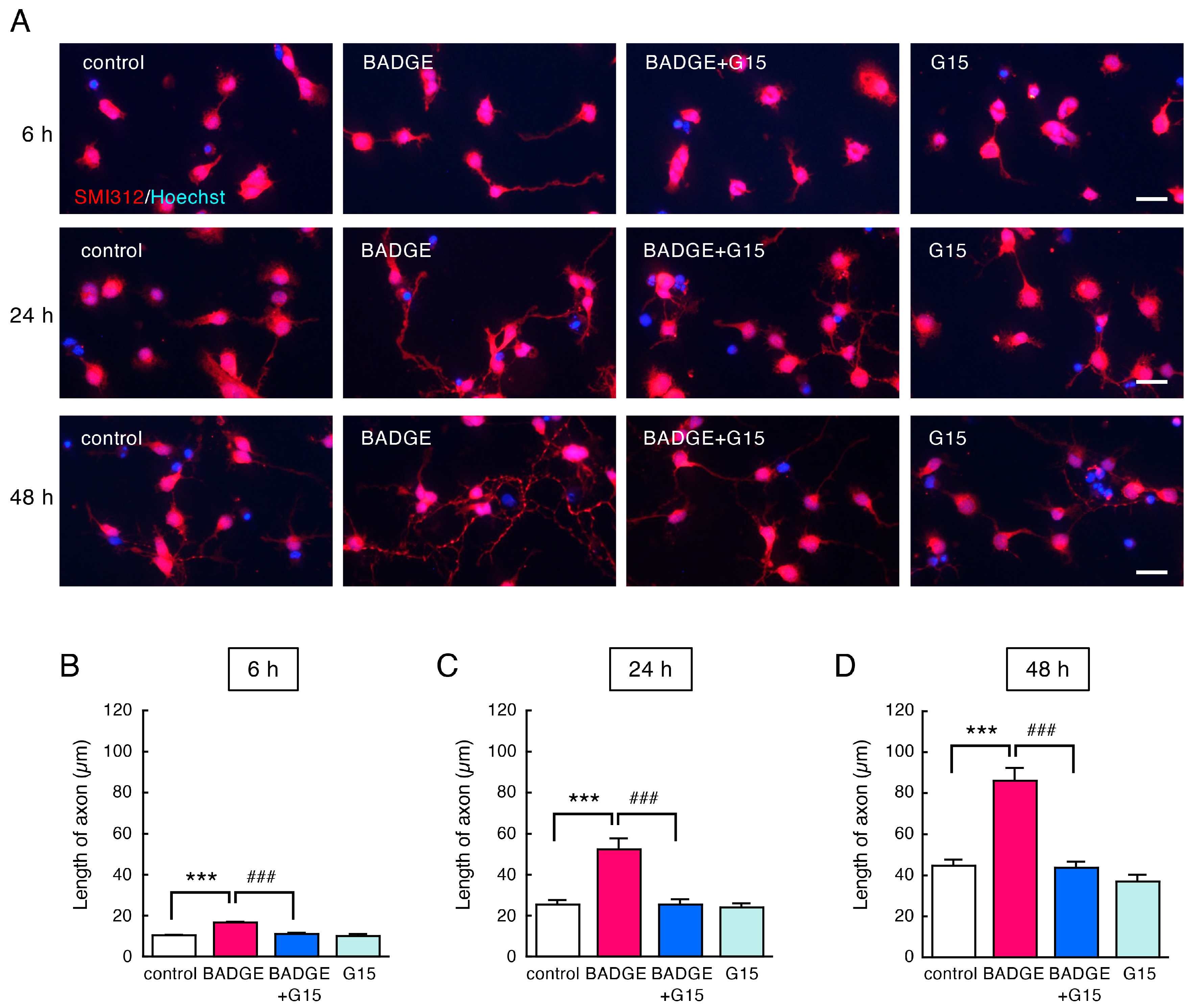
3.2. BADGE Accelerates Neurite Outgrowth via ERs
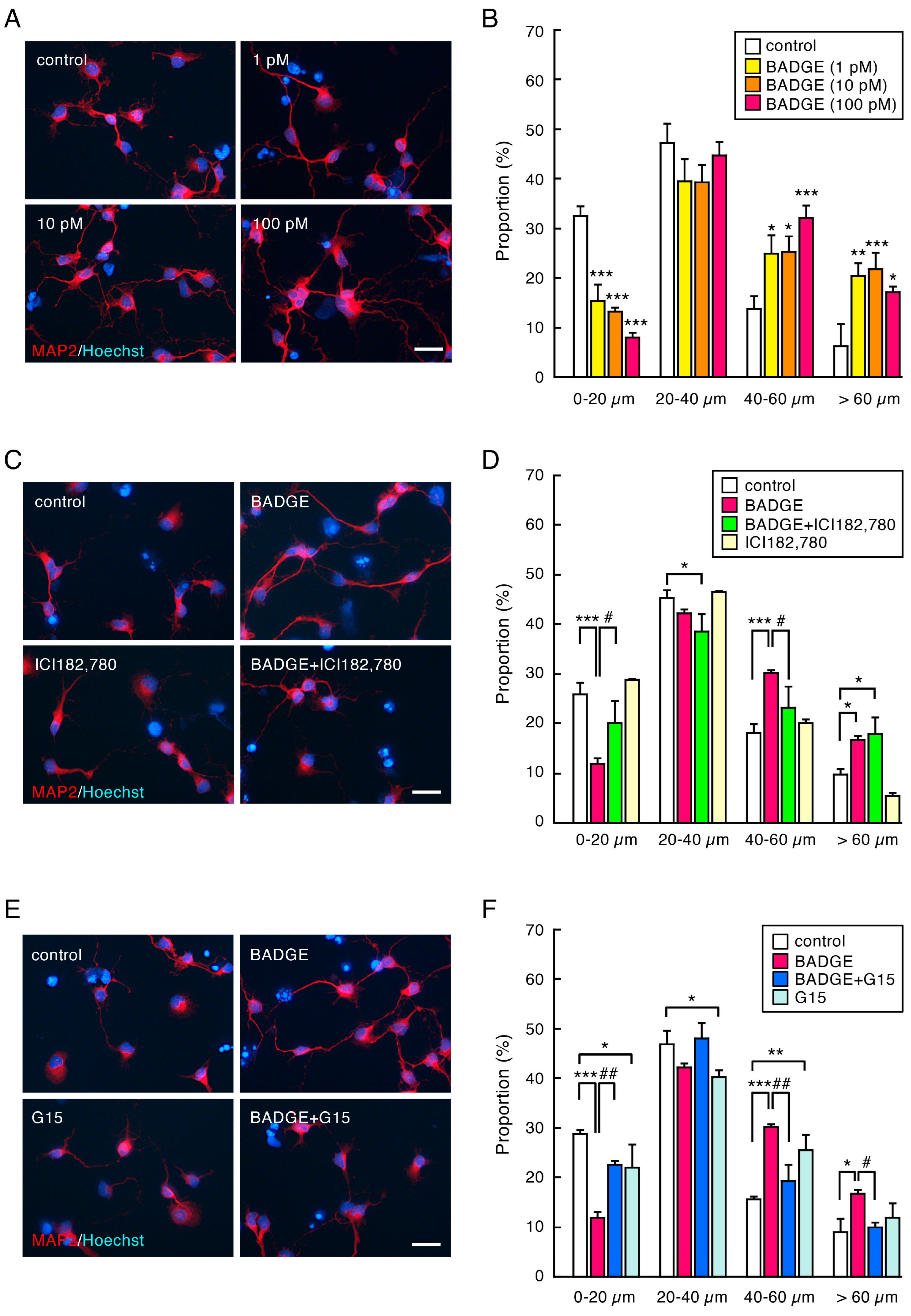
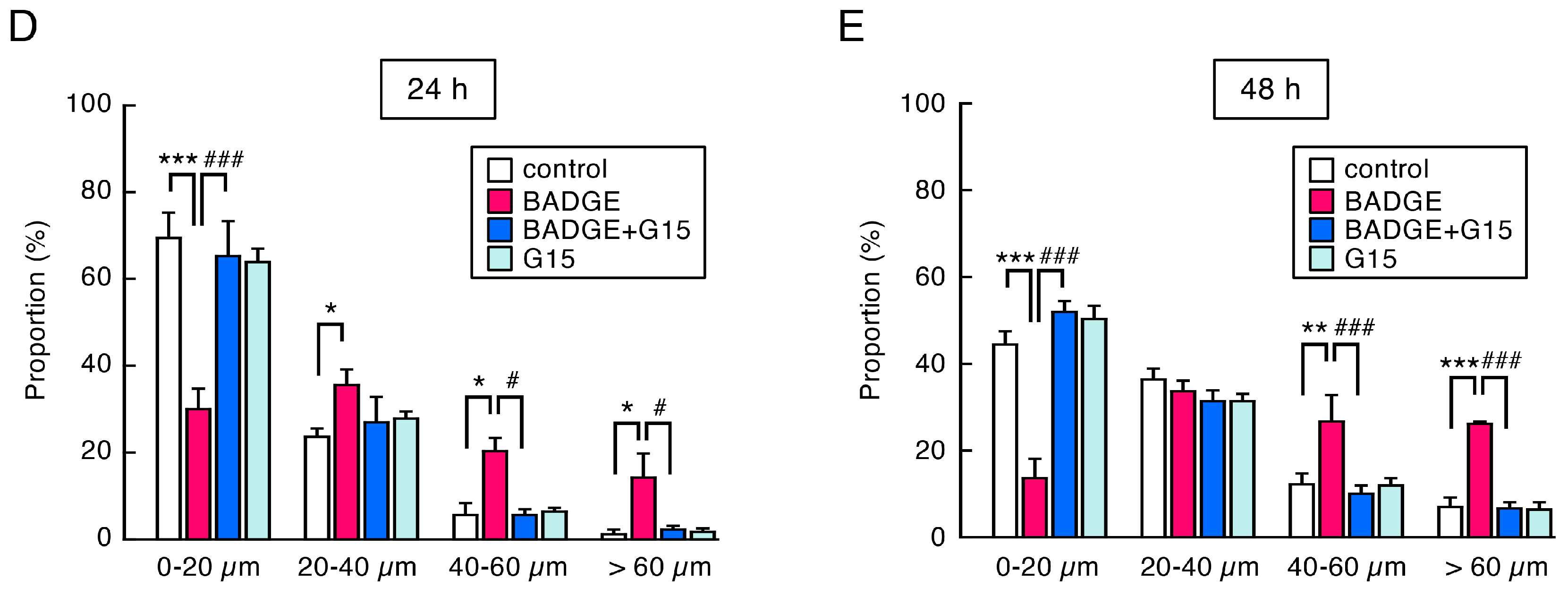
3.3. Time-Course Analysis of the Number and Length of Neurites After BADGE·2H2O Treatment


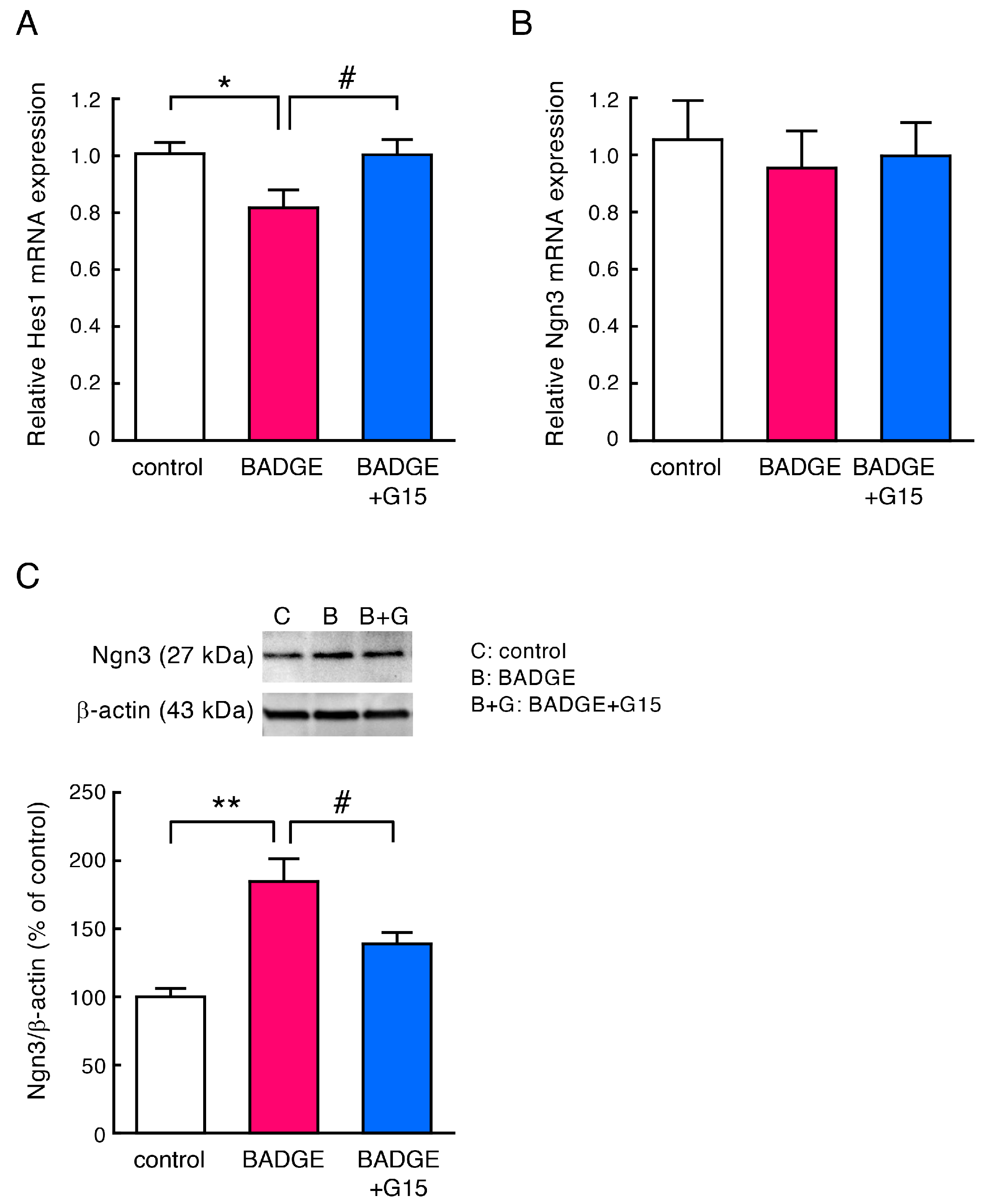
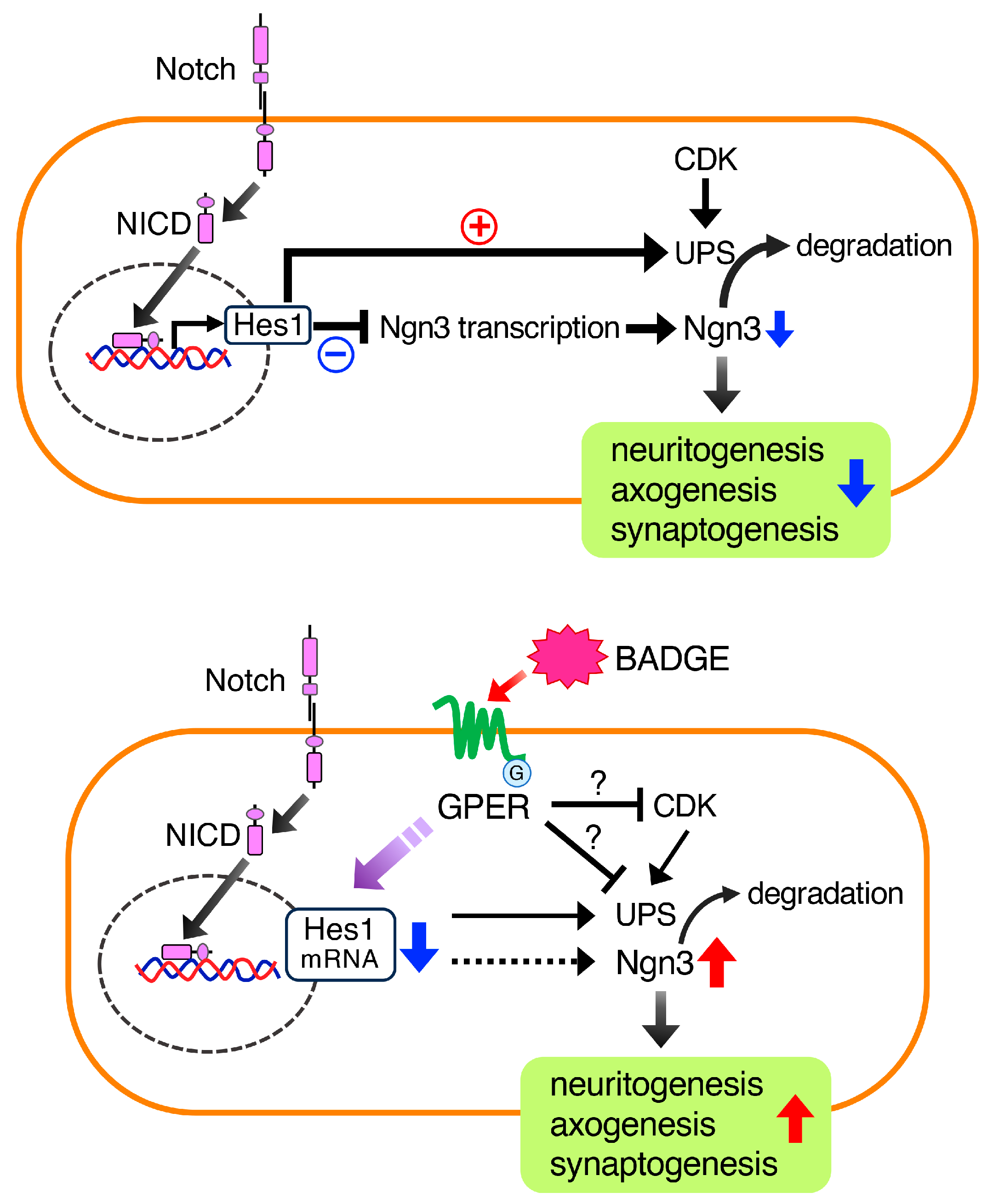
3.4. BADGE Down-Regulates Hes1 Expression and Increases Ngn3 Levels via GPER
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poole, A.; van Herwijnen, P.; Weideli, H.; Thomas, M.C.; Ransbotyn, G.; Vance, C. Review of the toxicology, human exposure and safety assessment for bisphenol A diglycidylether (BADGE). Food Addit. Contam. 2004, 21, 905–919. [Google Scholar] [CrossRef]
- Cabado, A.G.; Aldea, S.; Porro, C.; Ojea, G.; Lago, J.; Sobrado, C.; Vieites, J.M. Migration of BADGE (bisphenol A diglycidyl-ether) and BFDGE (bisphenol F diglycidyl-ether) in canned seafood. Food Chem. Toxicol. 2008, 46, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Kudlak, B.; Jatkowska, N.; Kubica, P.; Yotova, G.; Tsakovski, S. Influence of storage time and temperature on the toxicity, endocrine potential, and migration of epoxy resin precursors in extracts of food packaging materials. Molecules 2019, 24, 4396. [Google Scholar] [CrossRef] [PubMed]
- Marqueno, A.; Perez-Albaladejo, E.; Flores, C.; Moyano, E.; Porte, C. Toxic effects of bisphenol A diglycidyl ether and derivatives in human placental cells. Environ. Pollut. 2019, 244, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Noureddine El Moussawi, S.; Ouaini, R.; Matta, J.; Chebib, H.; Cladiere, M.; Camel, V. Simultaneous migration of bisphenol compounds and trace metals in canned vegetable food. Food Chem. 2019, 288, 228–238. [Google Scholar] [CrossRef]
- Toptanci, I. Risk assessment of bisphenol related compounds in canned convenience foods, olives, olive oil, and canned soft drinks in Turkey. Environ. Sci. Pollut. Res. Int. 2023, 30, 54177–54192. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Zhang, W.; Kannan, K. Widespread occurrence and distribution of bisphenol A diglycidyl ether (BADGE) and its derivatives in human urine from the United States and China. Environ. Sci. Technol. 2012, 46, 12968–12976. [Google Scholar] [CrossRef]
- Wang, L.; Xue, J.; Kannan, K. Widespread occurrence and accumulation of bisphenol A diglycidyl ether (BADGE), bisphenol F diglycidyl ether (BFDGE) and their derivatives in human blood and adipose fat. Environ. Sci. Technol. 2015, 49, 3150–3157. [Google Scholar] [CrossRef]
- Yang, R.; Duan, J.; Li, H.; Sun, Y.; Shao, B.; Niu, Y. Bisphenol-diglycidyl ethers in paired urine and serum samples from children and adolescents: Partitioning, clearance and exposure assessment. Environ. Pollut. 2022, 306, 119351. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Bai, X.; Zhang, T.; Xue, J.; Lu, S.; Kannan, K. Placental transfer of bisphenol diglycidyl ethers (BDGEs) and its association with maternal health in a population in South of China. Eco-Environ. Health 2022, 1, 244–250. [Google Scholar] [CrossRef]
- Nakazawa, H.; Yamaguchi, A.; Inoue, K.; Yamazaki, T.; Kato, K.; Yoshimura, Y.; Makino, T. In Vitro assay of hydrolysis and chlorohydroxy derivatives of bisphenol A diglycidyl ether for estrogenic activity. Food Chem. Toxicol. 2002, 40, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Ohyama, K.; Aoki, N.; Iida, M.; Nagai, F. Study on anti-androgenic effects of bisphenol a diglycidyl ether (BADGE), bisphenol F diglycidyl ether (BFDGE) and their derivatives using cells stably transfected with human androgen receptor, AR-EcoScreen. Food Chem. Toxicol. 2004, 42, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Kazama, T.; Shiraishi, F.; Makino, M. Identification and estrogenic characterization of impurities in commercial bisphenol A diglycidyl ether (BADGE). Chemosphere 2006, 65, 873–880. [Google Scholar] [CrossRef]
- Chang, Y.; Nguyen, C.; Paranjpe, V.R.; Gilliland, F.; Zhang, J.J. Analysis of bisphenol A diglycidyl ether (BADGE) and its hydrolytic metabolites in biological specimens by high-performance liquid chromatography and tandem mass spectrometry. J. Chromatogr. B 2014, 965, 33–38. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, H.; Fei, X.; Synder, S.A.; Fang, M.; Liu, M. A comprehensive review on the analytical method, occurrence, transformation and toxicity of a reactive pollutant: BADGE. Environ. Int. 2021, 155, 106701. [Google Scholar] [CrossRef]
- Itoh, K.; Yaoi, T.; Fushiki, S. Bisphenol A, an endocrine-disrupting chemical, and brain development. Neuropathology 2012, 32, 447–457. [Google Scholar] [CrossRef]
- Chen, M.; Fan, Z.; Zhao, F.; Gao, F.; Mu, D.; Zhou, Y.; Shen, H.; Hu, J. Occurrence and maternal transfer of chlorinated bisphenol A and nonylphenol in pregnant women and their matching embryos. Environ. Sci. Technol. 2016, 50, 970–977. [Google Scholar] [CrossRef]
- Nakamura, K.; Itoh, K.; Yaoi, T.; Fujiwara, Y.; Sugimoto, T.; Fushiki, S. Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of Bisphenol A. J. Neurosci. Res. 2006, 84, 1197–1205. [Google Scholar] [CrossRef]
- Wan, Y.; Choi, K.; Kim, S.; Ji, K.; Chang, H.; Wiseman, S.; Jones, P.D.; Khim, J.S.; Park, S.; Park, J.; et al. Hydroxylated polybrominated diphenyl ethers and bisphenol A in pregnant women and their matching fetuses: Placental transfer and potential risks. Environ. Sci. Technol. 2010, 44, 5233–5239. [Google Scholar] [CrossRef]
- Miyazaki, I.; Kikuoka, R.; Isooka, N.; Takeshima, M.; Sonobe, K.; Arai, R.; Funakoshi, H.; Quin, K.E.; Smart, J.; Zensho, K.; et al. Effects of maternal bisphenol A diglycidyl ether exposure during gestation and lactation on behavior and brain development of the offspring. Food Chem. Toxicol. 2020, 138, 111235. [Google Scholar] [CrossRef]
- Costa, H.E.; Cairrao, E. Effect of bisphenol A on the neurological system: A review update. Arch. Toxicol. 2024, 98, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Varshney, M.K.; Inzunza, J.; Lupu, D.; Ganapathy, V.; Antonson, P.; Ruegg, J.; Nalvarte, I.; Gustafsson, J.A. Role of estrogen receptor beta in neural differentiation of mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 2017, 114, E10428–E10437. [Google Scholar] [CrossRef] [PubMed]
- Perian, S.; Vanacker, J.M. GPER as a Receptor for Endocrine-Disrupting Chemicals (EDCs). Front. Endocrinol. 2020, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palmero, I.; Simon-Areces, J.; Garcia-Segura, L.M.; Arevalo, M.A. Notch/neurogenin 3 signalling is involved in the neuritogenic actions of oestradiol in developing hippocampal neurones. J. Neuroendocrinol. 2011, 23, 355–364. [Google Scholar] [CrossRef]
- Arevalo, M.A.; Ruiz-Palmero, I.; Simon-Areces, J.; Acaz-Fonseca, E.; Azcoitia, I.; Garcia-Segura, L.M. Estradiol meets notch signaling in developing neurons. Front. Endocrinol. 2011, 2, 21. [Google Scholar] [CrossRef]
- Harrill, J.A.; Robinette, B.L.; Freudenrich, T.; Mundy, W.R. Use of high content image analyses to detect chemical-mediated effects on neurite sub-populations in primary rat cortical neurons. Neurotoxicology 2013, 34, 61–73. [Google Scholar] [CrossRef]
- Baba, K.; Okada, K.; Kinoshita, T.; Imaoka, S. Bisphenol A disrupts Notch signaling by inhibiting gamma-secretase activity and causes eye dysplasia of Xenopus laevis. Toxicol. Sci. 2009, 108, 344–355. [Google Scholar] [CrossRef]
- Gupta, A.; Wang, Y.; Browne, C.; Kim, S.; Case, T.; Paul, M.; Wills, M.L.; Matusik, R.J. Neuroendocrine differentiation in the 12T-10 transgenic prostate mouse model mimics endocrine differentiation of pancreatic beta cells. Prostate 2008, 68, 50–60. [Google Scholar] [CrossRef]
- Denley, M.C.S.; Gatford, N.J.F.; Sellers, K.J.; Srivastava, D.P. Estradiol and the Development of the Cerebral Cortex: An Unexpected Role? Front. Neurosci. 2018, 12, 245. [Google Scholar] [CrossRef]
- McCarthy, M.M. Estradiol and the developing brain. Physiol. Rev. 2008, 88, 91–124. [Google Scholar] [CrossRef]
- Cisternas, C.D.; Cabrera Zapata, L.E.; Mir, F.R.; Scerbo, M.J.; Arevalo, M.A.; Garcia-Segura, L.M.; Cambiasso, M.J. Estradiol-dependent axogenesis and Ngn3 expression are determined by XY sex chromosome complement in hypothalamic neurons. Sci. Rep. 2020, 10, 8223. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Z.; Su, J.D.; Sun, Q.W.; Jiao, B.H. Expression of estrogen receptor (ER) -alpha and -beta transcripts in the neonatal and adult rat cerebral cortex, cerebellum, and olfactory bulb. Cell Res. 2001, 11, 321–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raz, L.; Khan, M.M.; Mahesh, V.B.; Vadlamudi, R.K.; Brann, D.W. Rapid estrogen signaling in the brain. Neurosignals 2008, 16, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Bubb, M.; Beyer, A.L.; Dasgupta, P.; Kaemmerer, D.; Sanger, J.; Evert, K.; Wirtz, R.M.; Schulz, S.; Lupp, A. Assessment of G Protein-Coupled Oestrogen Receptor Expression in Normal and Neoplastic Human Tissues Using a Novel Rabbit Monoclonal Antibody. Int. J. Mol. Sci. 2022, 23, 5191. [Google Scholar] [CrossRef]
- Ruiz-Palmero, I.; Hernando, M.; Garcia-Segura, L.M.; Arevalo, M.A. G protein-coupled estrogen receptor is required for the neuritogenic mechanism of 17beta-estradiol in developing hippocampal neurons. Mol. Cell. Endocrinol. 2013, 372, 105–115. [Google Scholar] [CrossRef]
- Romano, S.N.; Gorelick, D.A. Crosstalk between nuclear and G protein-coupled estrogen receptors. Gen. Comp. Endocrinol. 2018, 261, 190–197. [Google Scholar] [CrossRef]
- Salama-Cohen, P.; Arevalo, M.A.; Grantyn, R.; Rodriguez-Tebar, A. Notch and NGF/p75NTR control dendrite morphology and the balance of excitatory/inhibitory synaptic input to hippocampal neurones through Neurogenin 3. J. Neurochem. 2006, 97, 1269–1278. [Google Scholar] [CrossRef]
- Bender, R.A.; Zhou, L.; Wilkars, W.; Fester, L.; Lanowski, J.S.; Paysen, D.; Konig, A.; Rune, G.M. Roles of 17ss-estradiol involve regulation of reelin expression and synaptogenesis in the dentate gyrus. Cereb. Cortex 2010, 20, 2985–2995. [Google Scholar] [CrossRef][Green Version]
- Roark, R.; Itzhaki, L.; Philpott, A. Complex regulation controls Neurogenin3 proteolysis. Biol. Open 2012, 1, 1264–1272. [Google Scholar] [CrossRef]
- Qu, X.; Afelik, S.; Jensen, J.N.; Bukys, M.A.; Kobberup, S.; Schmerr, M.; Xiao, F.; Nyeng, P.; Veronica Albertoni, M.; Grapin-Botton, A.; et al. Notch-mediated post-translational control of Ngn3 protein stability regulates pancreatic patterning and cell fate commitment. Dev. Biol. 2013, 376, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boscaro, C.; Carotti, M.; Albiero, M.; Trenti, A.; Fadini, G.P.; Trevisi, L.; Sandona, D.; Cignarella, A.; Bolego, C. Non-genomic mechanisms in the estrogen regulation of glycolytic protein levels in endothelial cells. FASEB J. 2020, 34, 12768–12784. [Google Scholar] [CrossRef] [PubMed]
- Brannvall, K.; Korhonen, L.; Lindholm, D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol. Cell. Neurosci. 2002, 21, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Z. The Impact of Microglia on Neurodevelopment and Brain Function in Autism. Biomedicines 2024, 12, 210. [Google Scholar] [CrossRef]
- Yang, R.; Niu, Y.; Wang, B.; Zhang, J.; Shao, B. Determination of Nine Bisphenol-Diglycidyl Ethers in Human Breast Milk by Ultrahigh-Performance Liquid Chromatography Tandem Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 9810–9818. [Google Scholar] [CrossRef]
- Kuwamura, M.; Tanaka, K.; Onoda, A.; Taki, K.; Koriyama, C.; Kitagawa, K.; Kawamoto, T.; Tsuji, M. Measurement of Bisphenol A Diglycidyl Ether (BADGE), BADGE derivatives, and Bisphenol F Diglycidyl Ether (BFDGE) in Japanese infants with NICU hospitalization history. BMC Pediatr. 2024, 24, 26. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request from the Commission related to 2,2-bis(4 hydroxyphenyl)propane bis(2,3-epoxypropyl)ether (Bisphenol A diglycidyl ether, BADGE). EFSA J. 2004, 2, 86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, I.; Nishiyama, C.; Nagoshi, T.; Miyako, A.; Ono, S.; Misawa, I.; Isse, A.; Tomimoto, K.; Masai, K.; Zensho, K.; et al. An Endocrine-Disrupting Chemical, Bisphenol A Diglycidyl Ether (BADGE), Accelerates Neuritogenesis and Outgrowth of Cortical Neurons via the G-Protein-Coupled Estrogen Receptor. NeuroSci 2025, 6, 53. https://doi.org/10.3390/neurosci6020053
Miyazaki I, Nishiyama C, Nagoshi T, Miyako A, Ono S, Misawa I, Isse A, Tomimoto K, Masai K, Zensho K, et al. An Endocrine-Disrupting Chemical, Bisphenol A Diglycidyl Ether (BADGE), Accelerates Neuritogenesis and Outgrowth of Cortical Neurons via the G-Protein-Coupled Estrogen Receptor. NeuroSci. 2025; 6(2):53. https://doi.org/10.3390/neurosci6020053
Chicago/Turabian StyleMiyazaki, Ikuko, Chiharu Nishiyama, Takeru Nagoshi, Akane Miyako, Suzuka Ono, Ichika Misawa, Aika Isse, Kana Tomimoto, Kaori Masai, Kazumasa Zensho, and et al. 2025. "An Endocrine-Disrupting Chemical, Bisphenol A Diglycidyl Ether (BADGE), Accelerates Neuritogenesis and Outgrowth of Cortical Neurons via the G-Protein-Coupled Estrogen Receptor" NeuroSci 6, no. 2: 53. https://doi.org/10.3390/neurosci6020053
APA StyleMiyazaki, I., Nishiyama, C., Nagoshi, T., Miyako, A., Ono, S., Misawa, I., Isse, A., Tomimoto, K., Masai, K., Zensho, K., & Asanuma, M. (2025). An Endocrine-Disrupting Chemical, Bisphenol A Diglycidyl Ether (BADGE), Accelerates Neuritogenesis and Outgrowth of Cortical Neurons via the G-Protein-Coupled Estrogen Receptor. NeuroSci, 6(2), 53. https://doi.org/10.3390/neurosci6020053







