Efficacy of Segmental Muscle Vibration on Pain Modulation in Patients with Primary Cervical Dystonia Treated with Botulinum Type-A Toxin: A Protocol for a Randomized Controlled Trial
Abstract
1. Introduction
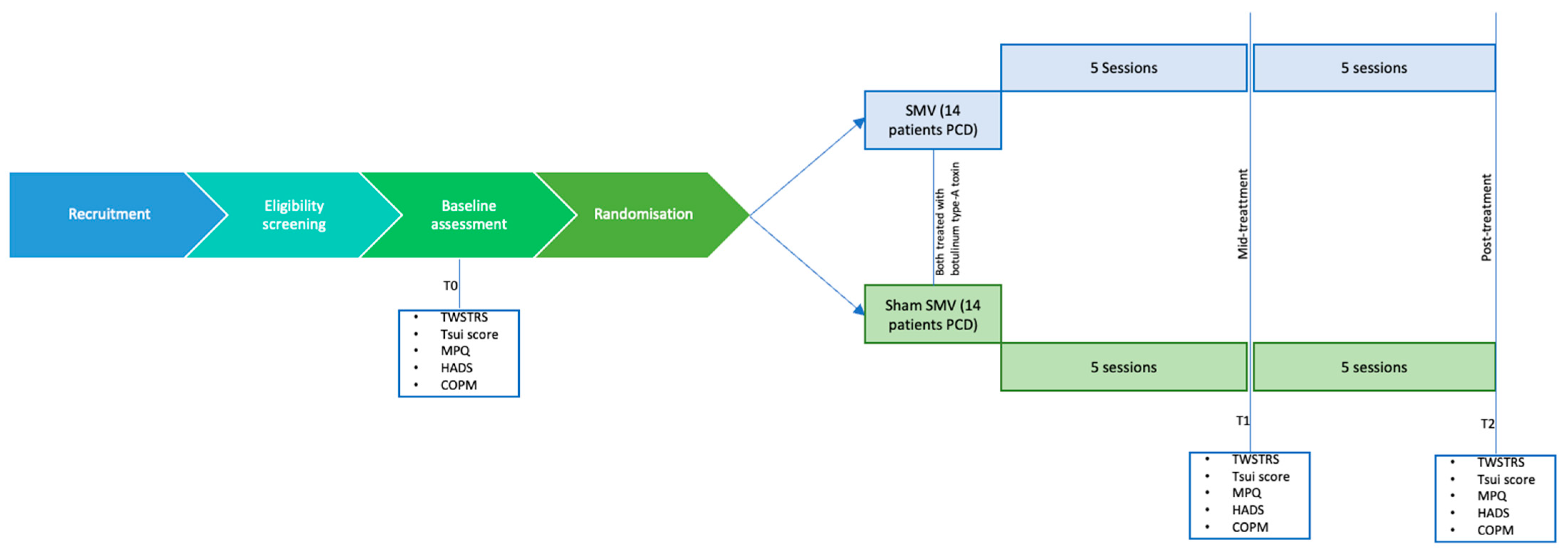
2. Materials and Methods
2.1. Study Design and Setting
2.2. Eligibility Criteria
- Inclusion criteria
- Adults aged 18 years old or older, both male and female.
- Clinical diagnosis of primary cervical dystonia with reported pain intensity scores greater than 3 on a numeric rating scale for pain (from 0 to 10).
- Clinical stability at the time of enrollment.
- Ability to provide informed consent.
- No contraindications to botulin toxin type-A treatment.
- Exclusion criteria
- Diagnosis of generalized dystonia or non-cervical focal dystonia.
- Comorbid conditions such as neurodegenerative, neuromuscular, or uncontrolled inflammatory diseases.
- Cognitive impairment with Mini-Mental State Examination score < 24 or language and cultural barriers.
- Prior treatment with botulin toxin type-A within 30 days before the study.
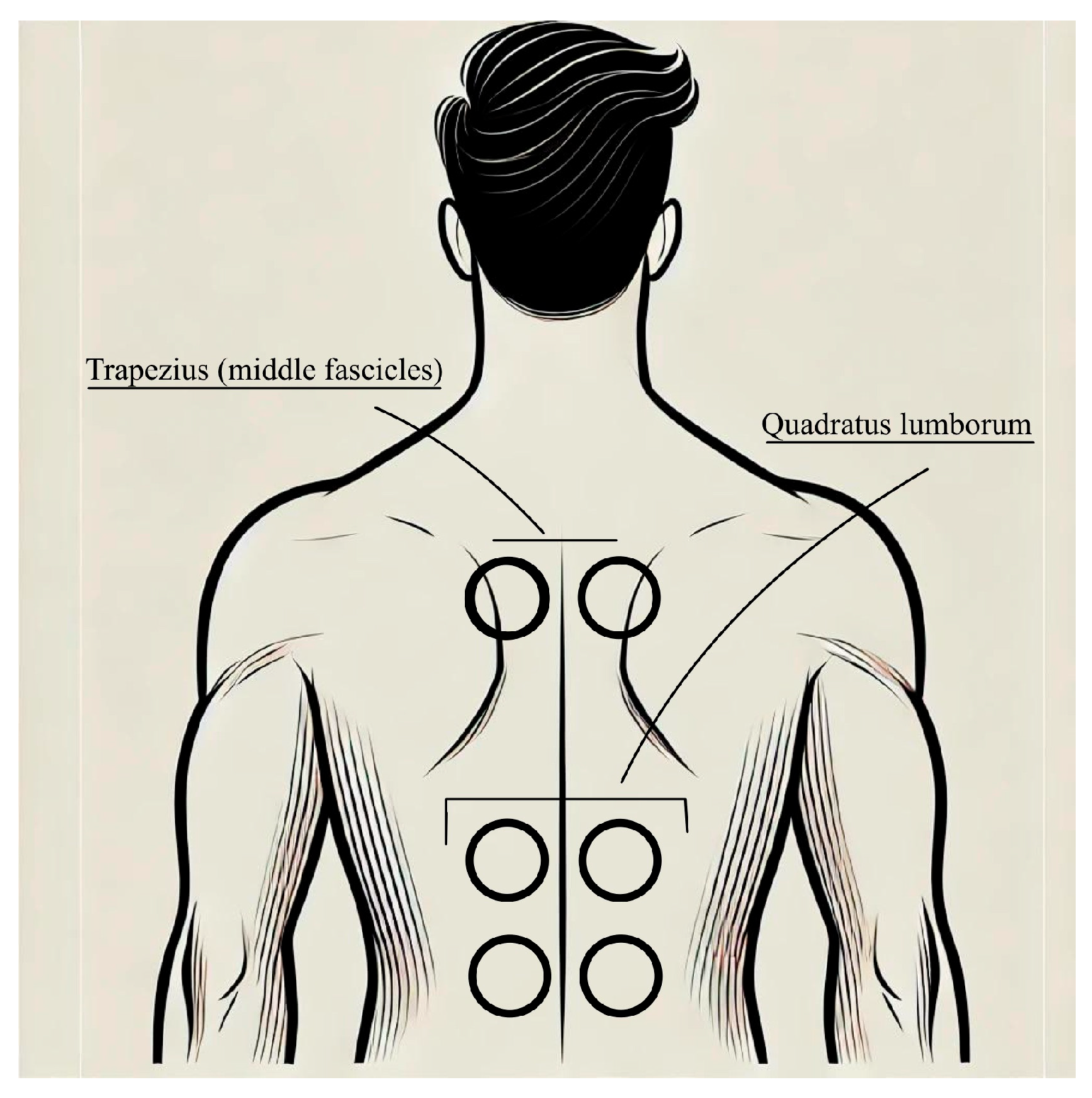
2.3. Interventions and Procedure
2.3.1. Segmental Muscle Vibration Intervention
2.3.2. Physical Therapy and Occupational Therapy
2.4. Outcomes and Timepoints
- The Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) is a widely recognized tool for assessing cervical dystonia. This scale evaluates the disorder across three distinct dimensions: severity, disability, and pain. The severity component measures the extent of abnormal postures, involuntary movements, and muscle hypertrophy, assessing the involvement of the neck and head in terms of rotation, tilt, flexion, or extension. The disability component examines the impact of the condition on daily activities, such as work, social interactions, and household tasks, highlighting the functional limitations caused by dystonia. Lastly, the pain dimension focuses on the intensity, frequency, and duration of pain, as well as its association with dystonic postures. Each dimension is scored independently, and the cumulative score provides a comprehensive picture of the condition’s burden, making the TWSTRS an essential tool for both clinical practice and research in cervical dystonia [20].
- The Tsui score is a clinical rating scale used to assess the severity of cervical dystonia. It provides a quantitative evaluation of the disorder by examining several aspects of the condition, including the type and extent of abnormal neck movements, sustained postures, and tremor. The scoring system considers the degree of head deviation in different planes (e.g., rotation, tilt, or flexion–extension), the presence of sustained postures, and the frequency and intensity of dystonic tremor. Additional modifiers are applied for factors such as shoulder elevation or hypertrophy. The Tsui score offers a comprehensive yet concise evaluation of cervical dystonia, making it a valuable tool for tracking disease progression and evaluating treatment efficacy in both clinical and research settings [21].
- The McGill Pain Questionnaire (MPQ) is a multidimensional tool designed to assess the complex nature of pain by evaluating its sensory, affective, and evaluative components. This questionnaire provides a comprehensive framework for understanding how pain is experienced and perceived by individuals. The sensory dimension focuses on the physical qualities of pain, such as its intensity, location, and specific characteristics like sharpness, burning, throbbing, or stabbing. The affective dimension explores the emotional aspects of pain, such as distress, anxiety, or unpleasantness, evaluating how pain impacts the individual’s mood and emotional well-being. The evaluative dimension assesses the overall experience of pain by considering its severity and the individual’s subjective perception of its impact on daily life. The MPQ is widely used in clinical and research settings for its ability to provide a detailed understanding of pain, making it useful for tailoring pain management strategies [22].
- The Canadian Occupational Performance Measure (COPM) is a client-centered tool used to evaluate an individual’s self-perceived performance and satisfaction in daily activities, focusing on self-care, productivity, and leisure. Patients identify and prioritize meaningful activities, rate their importance (1–10), and assess their performance and satisfaction levels (1–10). Administered at baseline and post-intervention, the COPM measures changes over time, helping to evaluate treatment effectiveness and guide personalized care. Widely used in rehabilitation, it emphasizes patient collaboration and relevance to individual goals [23,24]
2.5. Sample Size
2.6. Allocation, Randomization, and Blinding
2.7. Statistical Methods
3. Conclusions
4. Ethics and Dissemination
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group. A Prevalence Study of Primary Dystonia in Eight European Countries. J. Neurol. 2000, 247, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Marras, C.; Van den Eeden, S.K.; Fross, R.D.; Benedict-Albers, K.S.; Klingman, J.; Leimpeter, A.D.; Nelson, L.M.; Risch, N.; Karter, A.J.; Bernstein, A.L.; et al. Minimum Incidence of Primary Cervical Dystonia in a Multiethnic Health Care Population. Neurology 2007, 69, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Tisch, S.; Limousin, P. Neurophysiological Insights in Dystonia and Its Response to Deep Brain Stimulation Treatment. Exp. Brain Res. 2020, 238, 1645–1657. [Google Scholar] [CrossRef]
- Kojovic, M.; Pareés, I.; Kassavetis, P.; Palomar, F.J.; Mir, P.; Teo, J.T.; Cordivari, C.; Rothwell, J.C.; Bhatia, K.P.; Edwards, M.J. Secondary and Primary Dystonia: Pathophysiological Differences. Brain 2013, 136, 2038–2049. [Google Scholar] [CrossRef]
- Albanese, A.; Barnes, M.P.; Bhatia, K.P.; Fernandez-Alvarez, E.; Filippini, G.; Gasser, T.; Krauss, J.K.; Newton, A.; Rektor, I.; Savoiardo, M.; et al. A Systematic Review on the Diagnosis and Treatment of Primary (Idiopathic) Dystonia and Dystonia plus Syndromes: Report of an EFNS/MDS-ES Task Force. Eur. J. Neurol. 2006, 13, 433–444. [Google Scholar] [CrossRef]
- Costa, J.; Espírito-Santo, C.; Borges, A.; Ferreira, J.J.; Coelho, M.; Moore, P.; Sampaio, C. Botulinum Toxin Type A Therapy for Cervical Dystonia. Cochrane Database Syst. Rev. 2005, CD003633. [Google Scholar] [CrossRef]
- Camargo, C.H.F.; Cattai, L.; Teive, H.A.G. Pain Relief in Cervical Dystonia with Botulinum Toxin Treatment. Toxins 2015, 7, 2321–2335. [Google Scholar] [CrossRef]
- Rauch, F. Vibration Therapy. Dev. Med. Child. Neurol. 2009, 51, 166–168. [Google Scholar] [CrossRef]
- Roll, J.P.; Vedel, J.P. Kinaesthetic Role of Muscle Afferents in Man, Studied by Tendon Vibration and Microneurography. Exp. Brain Res. 1982, 47, 177–190. [Google Scholar] [CrossRef]
- Roll, J.P.; Vedel, J.P.; Ribot, E. Alteration of Proprioceptive Messages Induced by Tendon Vibration in Man: A Microneurographic Study. Exp. Brain Res. 1989, 76, 213–222. [Google Scholar] [CrossRef]
- Pope, Z.K.; DeFreitas, J.M. The Effects of Acute and Prolonged Muscle Vibration on the Function of the Muscle Spindle’s Reflex Arc. Somatosens. Mot. Res. 2015, 32, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Avanzino, L.; Fiorio, M. Proprioceptive Dysfunction in Focal Dystonia: From Experimental Evidence to Rehabilitation Strategies. Front. Hum. Neurosci. 2014, 8, 1000. [Google Scholar] [CrossRef]
- Annino, G.; Alashram, A.R.; Alghwiri, A.A.; Romagnoli, C.; Messina, G.; Tancredi, V.; Padua, E.; Mercuri, N.B. Effect of Segmental Muscle Vibration on Upper Extremity Functional Ability Poststroke: A Randomized Controlled Trial. Medicine 2019, 98, e14444. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Costanzo, M.; Avanzino, L.; Martino, D.; Salehi, P.; Standal, S.; Manzo, N.; Alizadeh, P.; Terranova, S.; Bonassi, G.; et al. Vibro-Tactile Stimulation of the Neck Reduces Pain in People with Cervical Dystonia: A Proof-of-Concept Study. Neurol. Sci. 2024, 45, 4847–4856. [Google Scholar] [CrossRef]
- Zhu, Y.; Mahnan, A.; Konczak, J. Vibro-Tactile Stimulation as a Non-Invasive Neuromodulation Therapy for Cervical Dystonia: A Case Study. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 6314–6317. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Rothwell, J.C. Differential Effect of Muscle Vibration on Intracortical Inhibitory Circuits in Humans. J. Physiol. 2003, 551, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, K.; Rothwell, J.C. The Effect of Sensory Input and Attention on the Sensorimotor Organization of the Hand Area of the Human Motor Cortex. J. Physiol. 2004, 561, 307–320. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Pesenti, A.; Paulus, W.; Tergau, F. Focal Reduction of Intracortical Inhibition in the Motor Cortex by Selective Proprioceptive Stimulation. Exp. Brain Res. 2003, 149, 9–16. [Google Scholar] [CrossRef]
- Miyara, K.; Etoh, S.; Kawamura, K.; Maruyama, A.; Kuronita, T.; Ohwatashi, A.; Shimodozono, M. Effects of Lower Limb Segmental Muscle Vibration on Primary Motor Cortex Short-Latency Intracortical Inhibition and Spinal Excitability in Healthy Humans. Exp. Brain Res. 2022, 240, 311–320. [Google Scholar] [CrossRef]
- Jen, M.-H.; Kurth, H.; Iheanacho, I.; Dinet, J.; Gabriel, S.; Wasiak, R.; Jost, W.H. Assessing the Burden of Illness from Cervical Dystonia Using the Toronto Western Spasmodic Torticollis Rating Scale Scores and Health Utility: A Meta-Analysis of Baseline Patient-Level Clinical Trial Data. J. Med. Econ. 2014, 17, 803–809. [Google Scholar] [CrossRef]
- Jost, W.H.; Hefter, H.; Stenner, A.; Reichel, G. Rating Scales for Cervical Dystonia: A Critical Evaluation of Tools for Outcome Assessment of Botulinum Toxin Therapy. J. Neural Transm. 2013, 120, 487–496. [Google Scholar] [CrossRef]
- Main, C.J. Pain Assessment in Context: A State of the Science Review of the McGill Pain Questionnaire 40 Years On. Pain 2016, 157, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, G.J.Q.; Wolf, M.J.M.A.G.; Louwers, A.M.; Meester-Delver, A.; Nollet, F. The Reproducibility and Validity of the Canadian Occupational Performance Measure in Parents of Children with Disabilities. Clin. Rehabil. 2006, 20, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Dedding, C.; Cardol, M.; Eyssen, I.C.J.M.; Beelen, A. Validity of the Canadian Occupational Performance Measure: A Client-Centred Outcome Measurement. Clin. Rehabil. 2004, 18, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Quagliato, E.M.A.B.; Carelli, E.F.; Viana, M.A. A Prospective, Randomized, Double-Blind Study Comparing the Efficacy and Safety of Type a Botulinum Toxins Botox and Prosigne in the Treatment of Cervical Dystonia. Clin. Neuropharmacol. 2010, 33, 22–26. [Google Scholar] [CrossRef]
- Boyce, M.J.; Canning, C.G.; Mahant, N.; Morris, J.; Latimer, J.; Fung, V.S. Active Exercise for Individuals with Cervical Dystonia: A Pilot Randomized Controlled Trial. Clin. Rehabil. 2013, 27, 226–235. [Google Scholar] [CrossRef]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buraschi, R.; Pedersini, P.; Redegalli, G.; Pullara, R.; Pollet, J.; Rossi, M.; Gobbo, M.; Gueli, S.; Falso, M. Efficacy of Segmental Muscle Vibration on Pain Modulation in Patients with Primary Cervical Dystonia Treated with Botulinum Type-A Toxin: A Protocol for a Randomized Controlled Trial. NeuroSci 2025, 6, 30. https://doi.org/10.3390/neurosci6020030
Buraschi R, Pedersini P, Redegalli G, Pullara R, Pollet J, Rossi M, Gobbo M, Gueli S, Falso M. Efficacy of Segmental Muscle Vibration on Pain Modulation in Patients with Primary Cervical Dystonia Treated with Botulinum Type-A Toxin: A Protocol for a Randomized Controlled Trial. NeuroSci. 2025; 6(2):30. https://doi.org/10.3390/neurosci6020030
Chicago/Turabian StyleBuraschi, Riccardo, Paolo Pedersini, Giacomo Redegalli, Rosa Pullara, Joel Pollet, Marina Rossi, Massimiliano Gobbo, Sara Gueli, and Maurizio Falso. 2025. "Efficacy of Segmental Muscle Vibration on Pain Modulation in Patients with Primary Cervical Dystonia Treated with Botulinum Type-A Toxin: A Protocol for a Randomized Controlled Trial" NeuroSci 6, no. 2: 30. https://doi.org/10.3390/neurosci6020030
APA StyleBuraschi, R., Pedersini, P., Redegalli, G., Pullara, R., Pollet, J., Rossi, M., Gobbo, M., Gueli, S., & Falso, M. (2025). Efficacy of Segmental Muscle Vibration on Pain Modulation in Patients with Primary Cervical Dystonia Treated with Botulinum Type-A Toxin: A Protocol for a Randomized Controlled Trial. NeuroSci, 6(2), 30. https://doi.org/10.3390/neurosci6020030








