A Case for Automated Segmentation of MRI Data in Neurodegenerative Diseases: Type II GM1 Gangliosidosis
Abstract
1. Introduction
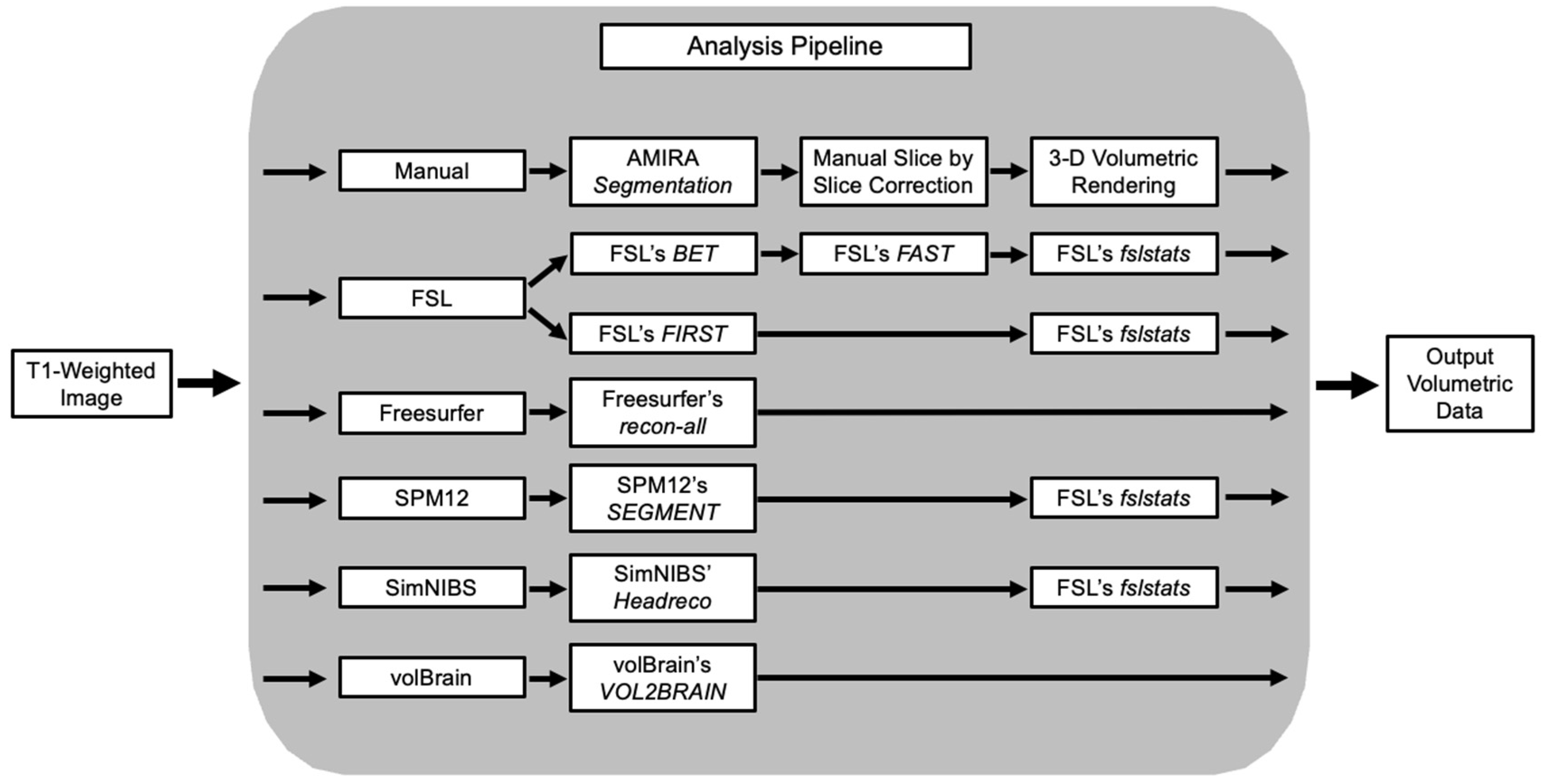
2. Methods
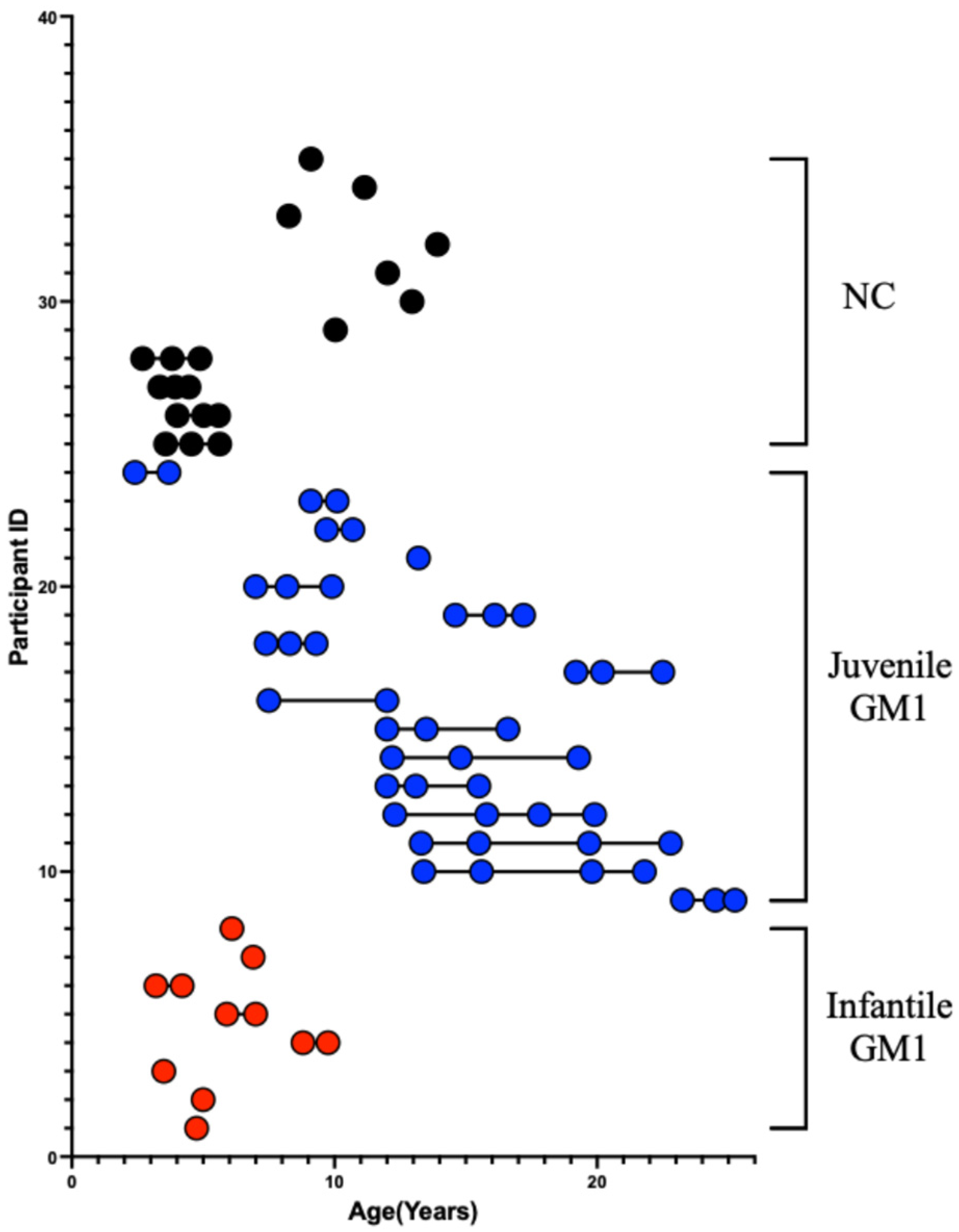
2.1. Type 2 GM1 Gangliosidosis Participants
2.2. Neurotypical Controls
2.3. T1-Weighted MRI Acquisition
2.4. Manual MRI Volumetric Segmentation
2.5. Freesurfer Volumetric Segmentation
2.6. FSL Volumetric Segmentation
2.7. volBrain Volumetric Segmentation
2.8. SPM Volumetric Segmentation
2.9. Headreco Volumetric Segmentation
2.10. Statistical Analysis
3. Results
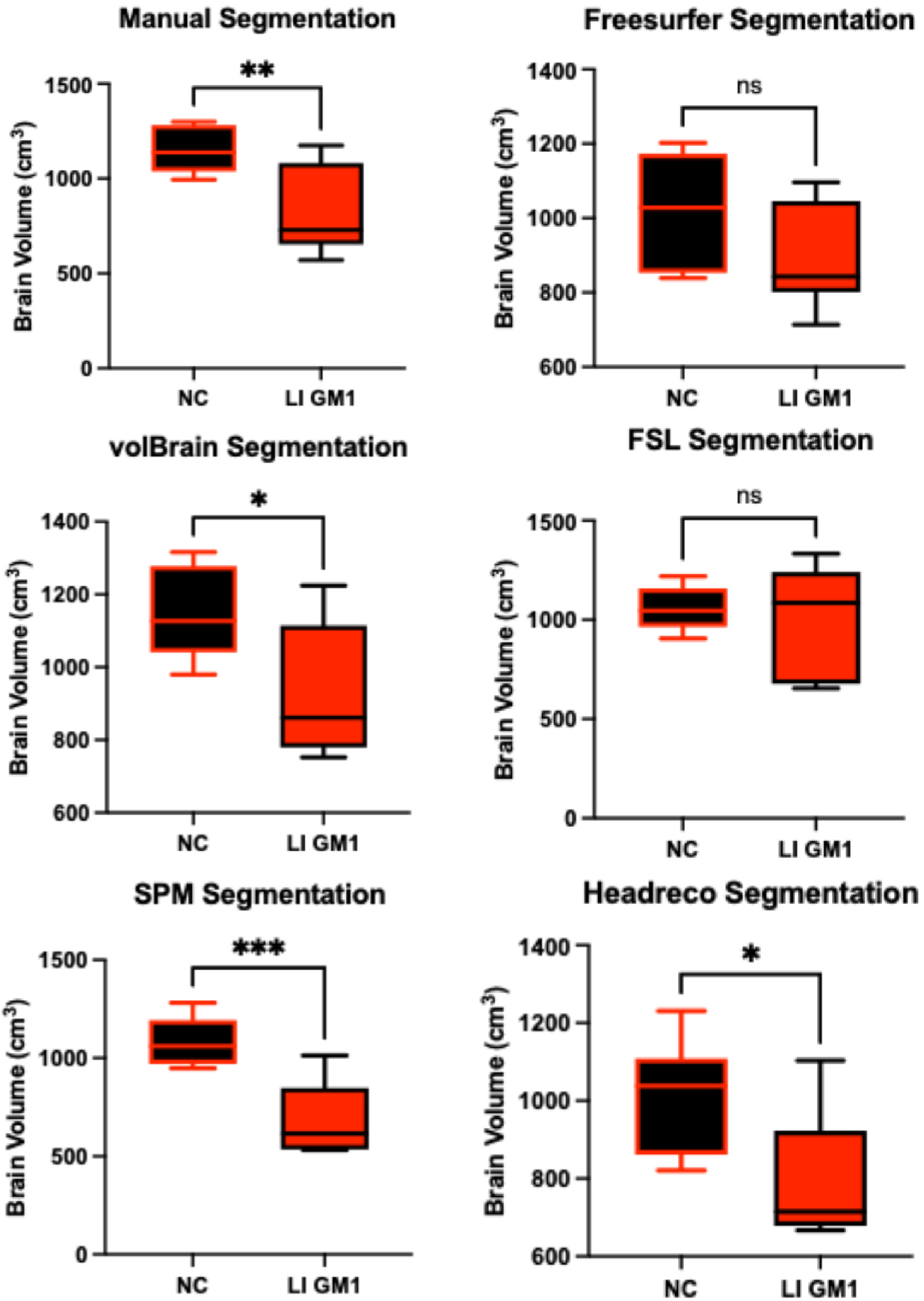
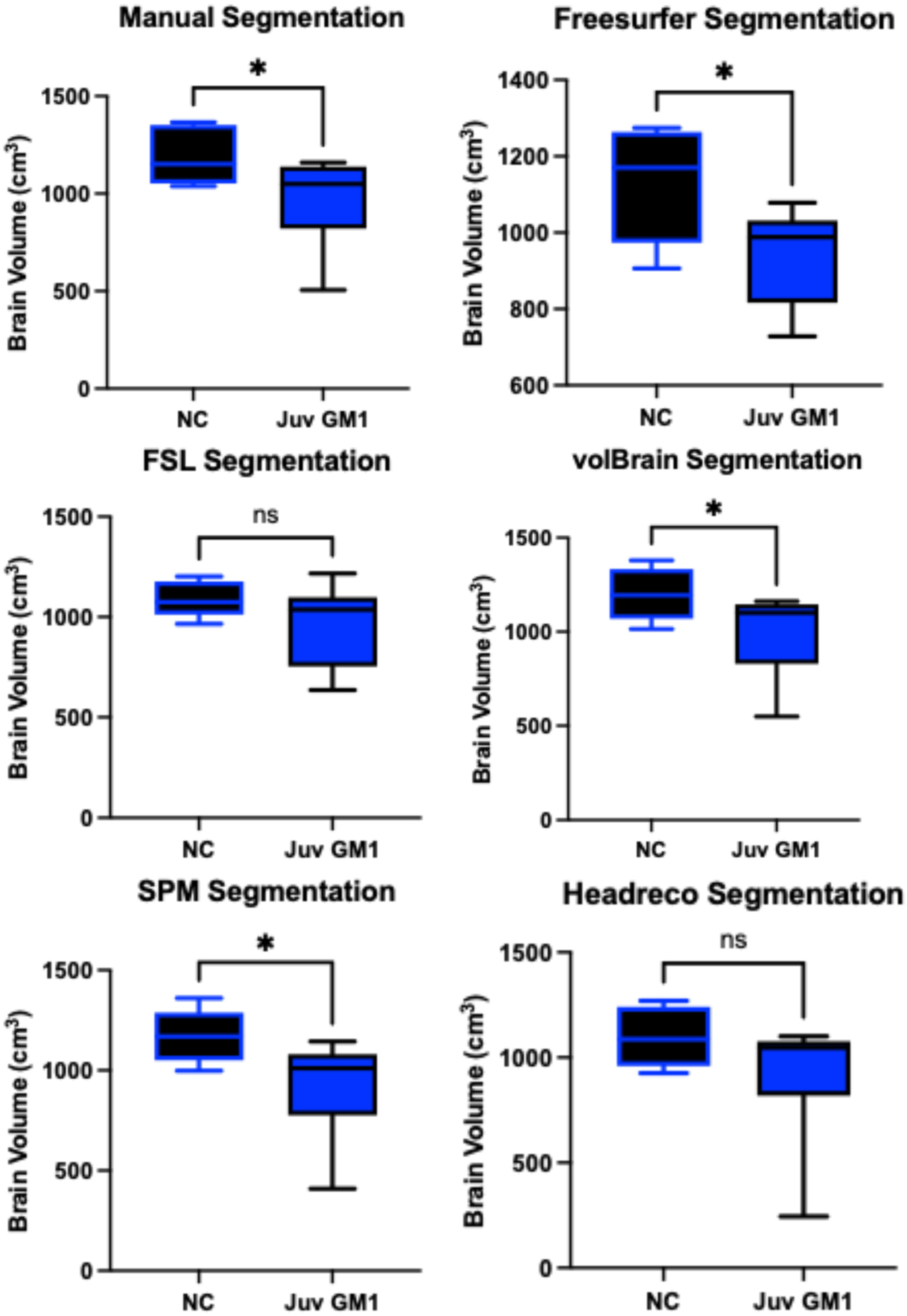
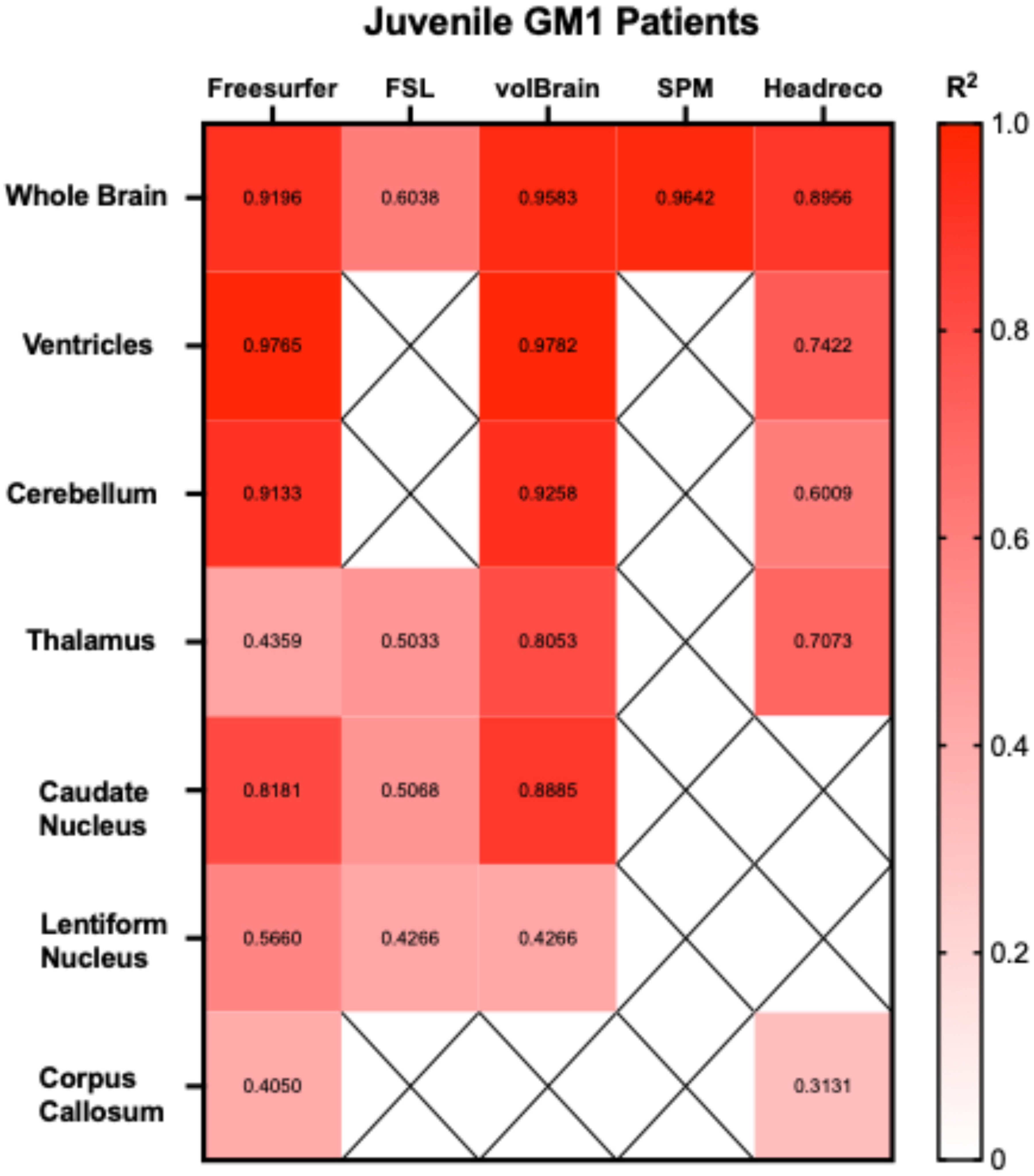
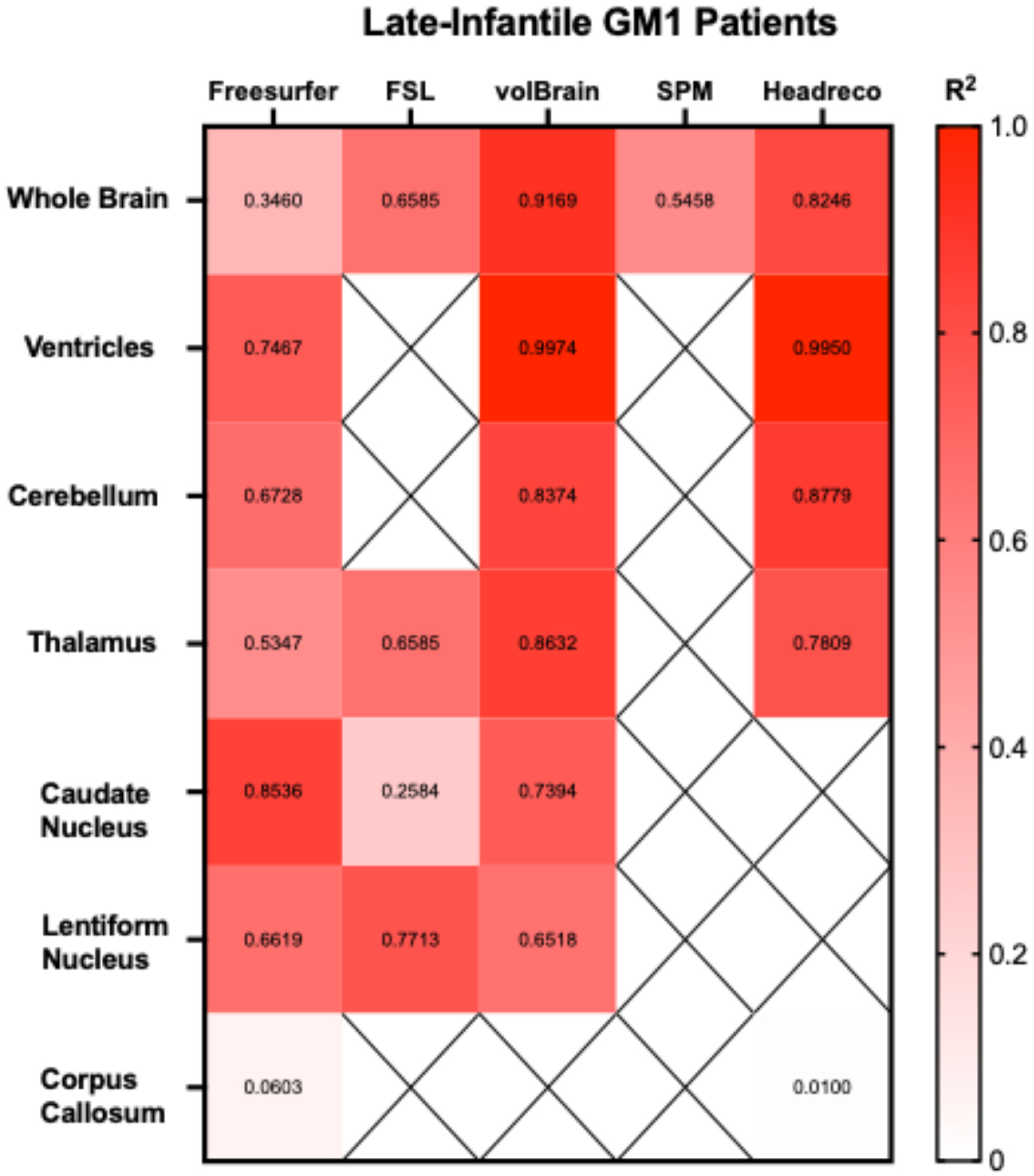
3.1. Whole Brain Volume
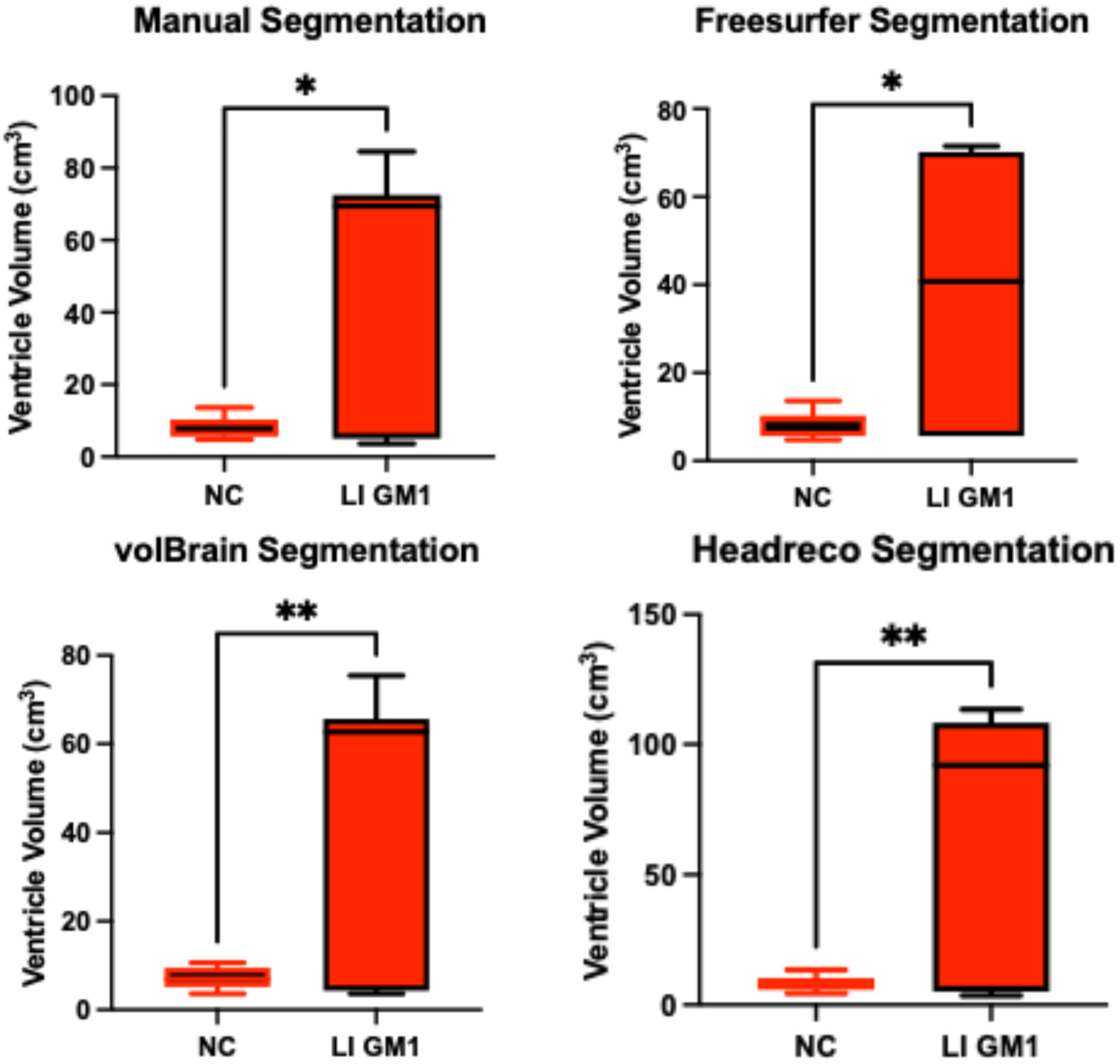
3.2. Ventricle Volume
3.3. Cerebellar Volume
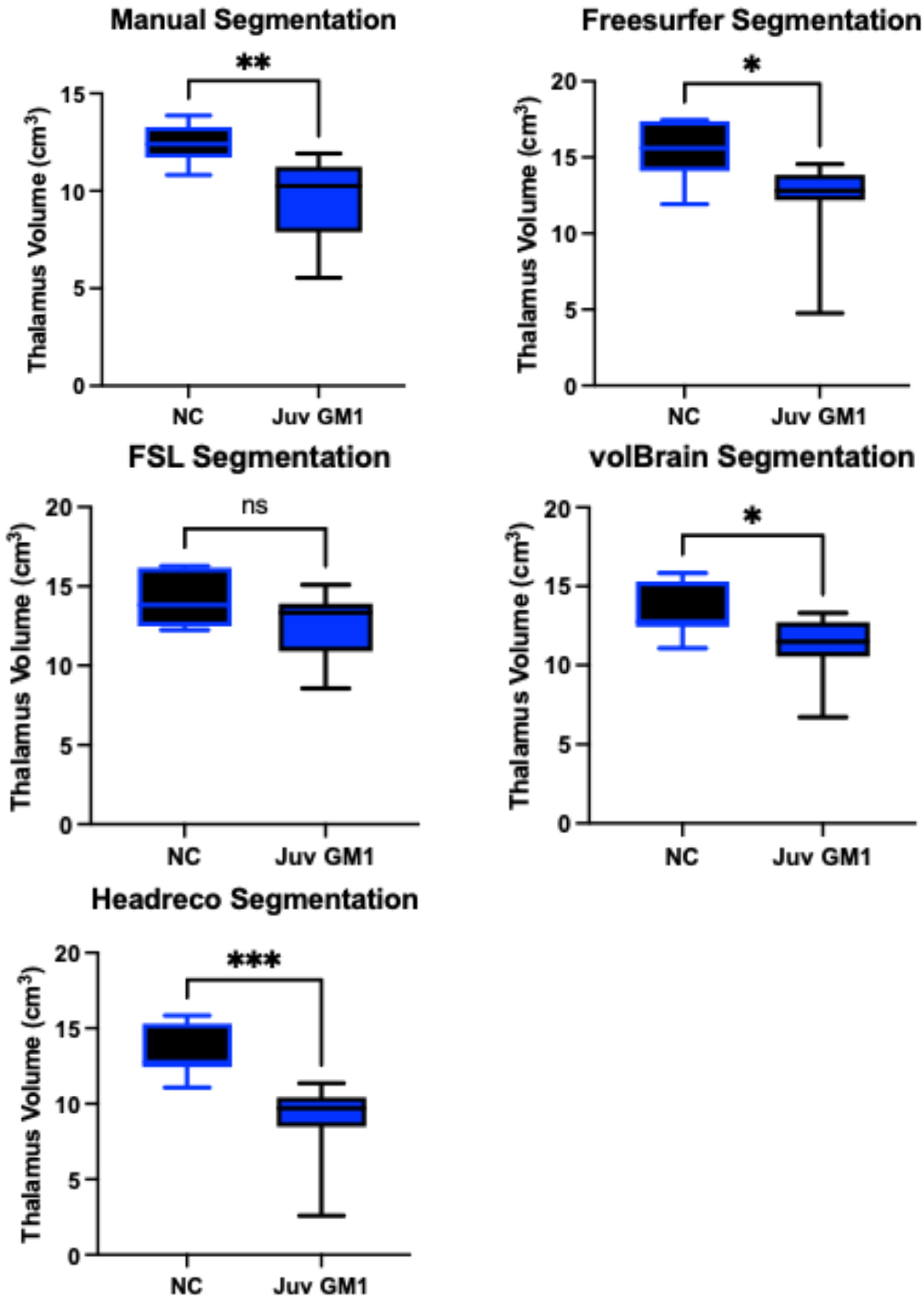
3.4. Thalamic Volume
3.5. Caudate Nucleus Volume
3.6. Lentiform Nucleus Volume
3.7. Corpus Callosum Volume
3.8. Extended Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Brien, L.M.; Ziegler, D.A.; Deutsch, C.K.; Frazier, J.A.; Herbert, M.R.; Locascio, J.J. Statistical adjustments for brain size in volumetric neuroimaging studies: Some practical implications in methods. Psychiatry Res. 2011, 193, 113–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bottino, C.M.; Castro, C.C.; Gomes, R.L.; Buchpiguel, C.A.; Marchetti, R.L.; Neto, M.R. Volumetric MRI measurements can differentiate Alzheimer’s disease, mild cognitive impairment, and normal aging. Int. Psychogeriatr. 2002, 14, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Pomponio, R.; Erus, G.; Habes, M.; Doshi, J.; Srinivasan, D.; Mamourian, E.; Bashyam, V.; Nasrallah, I.M.; Satterthwaite, T.D.; Fan, Y.; et al. Harmonization of large MRI datasets for the analysis of brain imaging patterns throughout the lifespan. NeuroImage 2020, 208, 116450. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, A.S.; Sun, S.; Taylor, C.; Ward, C.P.; Gamst, A.C.; Petersen, R.C.; Jack, C.R., Jr.; Aisen, P.S.; Thal, L.J. Volumetric MRI vs clinical predictors of Alzheimer disease in mild cognitive impairment. Neurology 2008, 70, 191–199. [Google Scholar] [CrossRef]
- Geng, D.Y.; Li, Y.X.; Zee, C.S. Magnetic resonance imaging-based volumetric analysis of basal ganglia nuclei and substantia nigra in patients with Parkinson’s disease. Neurosurgery 2006, 58, 256–262. [Google Scholar] [CrossRef]
- Wang, S.; Mautner, V.F.; Buchert, R.; Flibotte, S.; Suppa, P.; Friedman, J.M.; Heran, M.K.S. Alterations in brain morphology by MRI in adults with neurofibromatosis 1. Orphanet J. Rare Dis. 2021, 16, 462. [Google Scholar] [CrossRef]
- Hochstein, J.N.; Schulz, A.; Nickel, M.; Lezius, S.; Grosser, M.; Fiehler, J.; Sedlacik, J.; Löbel, U. Natural history of MRI brain volumes in patients with neuronal ceroid lipofuscinosis 3: A sensitive imaging biomarker. Neuroradiology 2022, 64, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Morey, R.A.; Petty, C.M.; Xu, Y.; Hayes, J.P.; Wagner, H.R., 2nd; Lewis, D.V.; LaBar, K.S.; Styner, M.; McCarthy, G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage 2009, 45, 855–866. [Google Scholar] [CrossRef]
- Antonopoulos, G.; More, S.; Raimondo, F.; Eickhoff, S.B.; Hoffstaedter, F.; Patil, K.R. A systematic comparison of VBM pipelines and their application to age prediction. NeuroImage 2023, 279, 120292. [Google Scholar] [CrossRef]
- Lenchik, L.; Heacock, L.; Weaver, A.A.; Boutin, R.D.; Cook, T.S.; Itri, J.; Filippi, C.G.; Gullapalli, R.P.; Lee, J.; Zagurovskaya, M.; et al. Automated Segmentation of Tissues Using CT and MRI: A Systematic Review. Acad. Radiol. 2019, 26, 1695–1706. [Google Scholar] [CrossRef]
- Giorgio, A.; De Stefano, N. Clinical use of brain volumetry. J. Magn. Reson. Imaging 2013, 37, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zoppo, C.; Kolstad, J.; Johnston, J.; D’Souza, P.; Kühn, A.L.; Vardar, Z.; Peker, A.; Lindsay, C.; Rentiya, Z.S.; King, R.; et al. Quantitative reliability assessment of brain MRI volumetric measurements in type II GM1 gangliosidosis patients. Front. Neuroimaging 2024, 3, 1410848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, M.K.; Singh, K.K. A Review of Publicly Available Automatic Brain Segmentation Methodologies, Machine Learning Models, Recent Advancements, and Their Comparison. Ann. Neurosci. 2021, 28, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Aggarwal, L.M. Automated medical image segmentation techniques. J. Med. Phys. 2010, 35, 3–14. [Google Scholar] [CrossRef]
- Bach Cuadra, M.; Duay, V.; Thiran, J.P. Atlas-based Segmentation. In Handbook of Biomedical Imaging; Paragios, N., Duncan, J., Ayache, N., Eds.; Springer: Boston, MA, USA, 2015. [Google Scholar] [CrossRef]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering 2023, 10, 1435. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pang, X.; de Keyzer, F.; Feng, Y.; Swinnen, J.V.; Yu, J.; Ni, Y. AI-based MRI auto-segmentation of brain tumor in rodents, a multicenter study. Acta Neuropathol. Commun. 2023, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Ikawa, F.; Nakazawa, S.; Koshino, S.; Ishii, D.; Kondo, H.; Hara, T.; Maeda, Y.; Sato, R.; Kaneko, T.; et al. Artificial intelligence for volumetric measurement of cerebral white matter hyperintensities on thick-slice fluid-attenuated inversion recovery (FLAIR) magnetic resonance images from multiple centers. Sci. Rep. 2024, 14, 10104. [Google Scholar] [CrossRef]
- Heinen, R.; Steenwijk, M.D.; Barkhof, F.; Biesbroek, J.M.; van der Flier, W.M.; Kuijf, H.J.; Prins, N.D.; Vrenken, H.; Biessels, G.J.; de Bresser, J. Performance of five automated white matter hyperintensity segmentation methods in a multicenter dataset. Sci. Rep. 2019, 9, 16742. [Google Scholar] [CrossRef]
- Schell, M.; Foltyn-Dumitru, M.; Bendszus, M.; Vollmuth, P. Automated hippocampal segmentation algorithms evaluated in stroke patients. Sci. Rep. 2023, 13, 11712. [Google Scholar] [CrossRef]
- Opfer, R.; Krüger, J.; Spies, L.; Ostwaldt, A.C.; Kitzler, H.H.; Schippling, S.; Buchert, R. Automatic segmentation of the thalamus using a massively trained 3D convolutional neural network: Higher sensitivity for the detection of reduced thalamus volume by improved inter-scanner stability. Eur. Radiol. 2023, 33, 1852–1861. [Google Scholar] [CrossRef]
- Yamanakkanavar, N.; Choi, J.Y.; Lee, B. MRI Segmentation and Classification of Human Brain Using Deep Learning for Diagnosis of Alzheimer’s Disease: A Survey. Sensors 2020, 20, 3243. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Hong, E.; Yum, M.S.; Lee, Y.-J.; Kim, J.; Ko, T.-S. Deep learning-based, fully automated, pediatric brain segmentation. Sci. Rep. 2024, 14, 4344. [Google Scholar] [CrossRef]
- Billot, B.; Bocchetta, M.; Todd, E.; Dalca, A.V.; Rohrer, J.D.; Iglesias, J.E. Automated segmentation of the hypothalamus and associated subunits in brain MRI. NeuroImage 2020, 223, 117287. [Google Scholar] [CrossRef]
- Nigro, S.; Cerasa, A.; Zito, G.; Perrotta, P.; Chiaravalloti, F.; Donzuso, G.; Fera, F.; Bilotta, E.; Pantano, P.; Quattrone, A.; et al. Fully automated segmentation of the pons and midbrain using human T1 MR brain images. PLoS ONE 2014, 9, e85618. [Google Scholar] [CrossRef]
- Choi, U.S.; Sung, Y.W.; Ogawa, S. deepPGSegNet: MRI-based pituitary gland segmentation using deep learning. Front. Endocrinol. 2024, 15, 1338743. [Google Scholar] [CrossRef]
- Van Elst, S.; de Bloeme, C.M.; Noteboom, S.; de Jong, M.C.; Moll, A.C.; Göricke, S.; de Graaf, P.; Caan, M.W.A. Automatic segmentation and quantification of the optic nerve on MRI using a 3D U-Net. J. Med. Imaging 2023, 10, 034501. [Google Scholar] [CrossRef]
- D’Souza, P.; Farmer, C.; Johnston, J.M.; Han, S.T.; Adams, D.; Hartman, A.L.; Zein, W.; Huryn, L.A.; Solomon, B.; King, K.; et al. GM1 gangliosidosis type II: Results of a 10-year prospective study. Genet. Med. Off. J. Am. Coll. Med. Genet. 2024, 26, 101144. [Google Scholar] [CrossRef]
- Laur, D.; Pichard, S.; Bekri, S.; Caillaud, C.; Froissart, R.; Levade, T.; Roubertie, A.; Desguerre, I.; Héron, B.; Auvin, S. Natural history of GM1 gangliosidosis—Retrospective cohort study of 61 French patients from 1998 to 2019. J. Inherit. Metab. Dis. 2023, 46, 972–981. [Google Scholar] [CrossRef]
- Lang, F.M.; Korner, P.; Harnett, M.; Karunakara, A.; Tifft, C.J. The natural history of Type 1 infantile GM1 gangliosidosis: A literature-based meta-analysis. Mol. Genet. Metab. 2020, 129, 228–235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nicoli, E.-R.; Annunziata, I.; d’Azzo, A.; Platt, F.M.; Tifft, C.J.; Stepien, K.M. GM1 Gangliosidosis—A Mini-Review. Front. Genet. 2021, 12, 734878. [Google Scholar] [CrossRef]
- Liu, S.; Ma, W.; Feng, Y.; Zhang, Y.; Jia, X.; Tang, C.; Tang, F.; Wu, X.; Huang, Y. AAV9-coGLB1 Improves Lysosomal Storage and Rescues Central Nervous System Inflammation in a Mutant Mouse Model of GM1 Gangliosidosis. Curr. Gene Ther. 2022, 22, 352–365. [Google Scholar]
- Gray-Edwards, H.L.; Maguire, A.S.; Salibi, N.; Ellis, L.E.; Voss, T.L.; Diffie, E.B.; Koehler, J.; Randle, A.N.; Taylor, A.R.; Brunson, B.L.; et al. 7T MRI Predicts Amelioration of Neurodegeneration in the Brain after AAV Gene Therapy. Mol. Ther. Methods Clin. Dev. 2019, 17, 258–270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lewis, C.J.; Johnston, J.M.; Zaragoza Domingo, S.; Vezina, G.; D’souza, P.; Gahl, W.A.; Adams, D.A.; Tifft, C.J.; Acosta, M.T. Retrospective assessment of clinical global impression of severity and change in GM1 gangliosidosis: A tool to score natural history data in rare disease cohorts. Orphanet J. Rare Dis. 2025, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Regier, D.S.; Kwon, H.J.; Johnston, J.; Golas, G.; Yang, S.; Wiggs, E.; Latour, Y.; Thomas, S.; Portner, C.; Adams, D.; et al. MRI/MRS as a surrogate marker for clinical progression in GM1 gangliosidosis. Am. J. Med. Genet. Part A 2016, 170, 634–644. [Google Scholar] [CrossRef]
- Nestrasil, I.; Ahmed, A.; Utz, J.M.; Rudser, K.; Whitley, C.B.; Jarnes-Utz, J.R. Distinct progression patterns of brain disease in infantile and juvenile gangliosidoses: Volumetric quantitative MRI study. Mol. Genet. Metab. 2018, 123, 97–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kolstad, J.; Zoppo, C.; Johnston, J.M.; D’souza, P.; Kühn, A.L.; Vardar, Z.; Peker, A.; Hader, A.; Celik, H.; Lewis, C.J.; et al. Natural history progression of MRI brain volumetrics in type II late-infantile and juvenile GM1 gangliosidosis patients. Mol. Genet. Metab. 2025, 144, 109025. [Google Scholar] [CrossRef]
- National Human Genome Research Institute. Natural History of Glycosphingolipid Storage Disorders and Glycoprotein Disorders ClinicalTrials.gov Identifier: NCT00029965. Updated 7 August 2024. Available online: https://clinicaltrials.gov/study/NCT00029965 (accessed on 12 August 2024).
- Reynolds, J.E.; Long, X.; Paniukov, D.; Bagshawe, M.; Lebel, C. Calgary Preschool magnetic resonance imaging (MRI) dataset. Data Brief. 2020, 29, 105224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mah, A.; Geeraert, B.; Lebel, C. Detailing neuroanatomical development in late childhood and early adolescence using NODDI. PLoS ONE 2017, 12, e0182340. [Google Scholar] [CrossRef]
- Li, X.; Morgan, P.S.; Ashburner, J.; Smith, J.; Rorden, C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J. Neurosci. Methods 2016, 264, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.L.; Neelin, P.; Peters, T.M.; Evans, A.C. Automatic 3D intersubject registration of MR volumetric data in standardized talairach space. J. Comput. Assist. Tomogr. 1994, 18, 192–205. [Google Scholar] [CrossRef]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis: I. segmentation and surface reconstruction. Neuroimage 1999, 9, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical surface-based analysis: Ii: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999, 9, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Sereno, M.I.; Tootell, R.B.; Dale, A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999, 8, 272–284. [Google Scholar] [CrossRef]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Liu, A.; Dale, A.M. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging 2001, 20, 70–80. [Google Scholar] [CrossRef]
- Non-Uniform Intensity Correction. Available online: http://www.bic.mni.mcgill.ca/software/N3/node6.html (accessed on 7 July 2024).
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; Van Der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Fischl, B.; Van Der Kouwe, A.; Destrieux, C.; Halgren, E.; Ségonne, F.; Salat, D.H.; Busa, E.; Seidman, L.J.; Goldstein, J.; Kennedy, D.; et al. Automatically parcellating the human cerebral cortex. Cereb. Cortex 2004, 14, 11–22. [Google Scholar] [CrossRef]
- Woolrich, M.W.; Jbabdi, S.; Patenaude, B.; Chappell, M.; Makni, S.; Behrens, T.; Beckmann, C.; Jenkinson, M.; Smith, S.M. Bayesian analysis of neuroimaging data in FSL. NeuroImage 2009, 45, S173–S186. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004, 23 (Suppl. S1), S208–S219. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Manjón, J.V.; Coupé, P. volBrain: An Online MRI Brain Volumetry System. Front. Neuroinformatics 2016, 10, 30. [Google Scholar] [CrossRef]
- Manjón, J.V.; Romero, J.E.; Vivo-Hernando, R.; Rubio, G.; Aparici, F.; de la Iglesia-Vaya, M.; Coupé, P. vol2Brain: A New Online Pipeline for Whole Brain MRI Analysis. Front. Neuroinformatics 2022, 16, 862805. [Google Scholar] [CrossRef]
- Penny, W.D.; Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E. (Eds.) Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Nielsen, J.D.; Madsen, K.H.; Puonti, O.; Siebner, H.R.; Bauer, C.; Madsen, C.G.; Saturnino, G.B.; Thielscher, A. Automatic skull segmentation from MR images for realistic volume conductor models of the head: Assessment of the state-of-the-art. NeuroImage 2018, 174, 587–598. [Google Scholar] [CrossRef]
- Dahnke, R.; Yotter, R.A.; Gaser, C. Cortical thickness and central surface estimation. NeuroImage 2013, 65, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Stolte, S.E.; Indahlastari, A.; Chen, J.; Albizu, A.; Dunn, A.; Pedersen, S.; See, K.B.; Woods, A.J.; Fang, R. Precise and Rapid Whole-Head Segmentation from Magnetic Resonance Images of Older Adults using Deep Learning. Imaging Neurosci. 2024, 2, 1–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tavares, V.; Prata, D.; Ferreira, H.A. Comparing SPM12 and CAT12 segmentation pipelines: A brain tissue volume-based age and Alzheimer’s disease study. J. Neurosci. Methods 2019, 334, 108565. [Google Scholar] [CrossRef]
- Hammers, A.; Allom, R.; Koepp, M.J.; Free, S.L.; Myers, R.; Lemieux, L.; Mitchell, T.N.; Brooks, D.J.; Duncan, J.S. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 2003, 19, 224–247. [Google Scholar] [CrossRef]
- Khan, A.F.; Drozd, J.J.; Moreland, R.K.; Ta, R.M.; Borrie, M.J.; Bartha, R. A novel MRI-compatible brain ventricle phantom for validation of segmentation and volumetry methods. J. Magn. Reson. Imaging 2012, 36, 476–482. [Google Scholar] [CrossRef]
- Strittmatter, W.J. Bathing the brain. J. Clin. Investig. 2013, 123, 1013–1015. [Google Scholar] [CrossRef]
- Driscoll, M.E.; Bollu, P.C.; Tadi, P. Neuroanatomy, Nucleus Caudate. [Updated 24 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557407/ (accessed on 19 February 2025).
- Javed, N.; Cascella, M. Neuroanatomy, Globus Pallidus. [Updated 20 February 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557755/ (accessed on 19 February 2025).
- Torrico, T.J.; Munakomi, S. Neuroanatomy, Thalamus. [Updated 24 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542184/ (accessed on 19 February 2025).
- Ghandili, M.; Munakomi, S. Neuroanatomy, Putamen. [Updated 30 January 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542170/ (accessed on 19 February 2025).
- Makowski, C.; Béland, S.; Kostopoulos, P.; Bhagwat, N.; Devenyi, G.A.; Malla, A.K.; Joober, R.; Lepage, M.; Chakravarty, M.M. Evaluating accuracy of striatal, pallidal, and thalamic segmentation methods: Comparing automated approaches to manual delineation. Neuroimage 2018, 170, 182–198. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, E.I.; Bidesi, A.S.; Thomas, B.P.; Meda, S.A.; Francis, A.; Moates, A.F.; Witte, B.; Keshavan, M.S.; Tamminga, C.A. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res. 2012, 204, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Brusini, I.; Andersson, O.; Ouellette, R.; Piehl, F.; Wang, C.; Granberg, T. Deep Learning Corpus Callosum Segmentation as a Neurodegenerative Marker in Multiple Sclerosis. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2021, 31, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Brusini, I.; Platten, M.; Ouellette, R.; Piehl, F.; Wang, C.; Granberg, T. Automatic deep learning multicontrast corpus callosum segmentation in multiple sclerosis. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2022, 32, 459–470. [Google Scholar] [CrossRef]
- Vachet, C.; Yvernault, B.; Bhatt, K.; Smith, R.G.; Gerig, G.; Hazlett, H.C.; Styner, M. Automatic corpus callosum segmentation using a deformable active Fourier contour model. Proc. SPIE-Int. Soc. Opt. Eng. 2012, 8317, 831707. [Google Scholar] [CrossRef]
- Gerrits, N.J.; van Loenhoud, A.C.; van den Berg, S.F.; Berendse, H.W.; Foncke, E.M.; Klein, M.; Stoffers, D.; van der Werf, Y.D.; van den Heuvel, O.A. Cortical Thickness, Surface Area and Subcortical Volume Differentially Contribute to Cognitive Heterogeneity in Parkinson’s Disease. PLoS ONE 2016, 11, e0148852. [Google Scholar] [CrossRef]
- Matuskey, D.; Tinaz, S.; Wilcox, K.C.; Naganawa, M.; Toyonaga, T.; Dias, M.; Henry, S.; Pittman, B.; Ropchan, J.; Nabulsi, N.; et al. Synaptic Changes in Parkinson Disease Assessed with in vivo Imaging. Ann. Neurol. 2020, 87, 329–338. [Google Scholar] [CrossRef]
- Vasconcellos, L.F.; Pereira, J.S.; Adachi, M.; Greca, D.; Cruz, M.; Malak, A.L.; Charchat-Fichman, H. Volumetric brain analysis as a predictor of a worse cognitive outcome in Parkinson’s disease. J. Psychiatr. Res. 2018, 102, 254–260. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, M.; Ouyang, M.; Guo, R.; Yu, Q.; Peng, Q.; Zhang, N.; Zhang, Y.; Duan, Y.; Tang, X.; et al. Brain white matter microstructural alterations in children of type I Gaucher disease characterized with diffusion tensor MR imaging. Eur. J. Radiol. 2018, 102, 22–29. [Google Scholar] [CrossRef]
- Lidove, O.; Klein, I.; Lelièvre, J.D.; Lavallée, P.; Serfaty, J.-M.; Dupuis, E.; Papo, T.; Laissy, J.-P. Imaging features of Fabry disease. AJR Am. J. Roentgenol. 2006, 186, 1184–1191. [Google Scholar] [CrossRef]
- Crain, A.M.; Kitchen, D.L.; Godiyal, N.; Pfeifer, C.M. MRI findings in neuronal ceroid lipofuscinosis. Radiol. Case Rep. 2020, 15, 2375–2377. [Google Scholar] [CrossRef] [PubMed]
- Knoernschild, K.; Johnson, H.J.; Schroeder, K.E.; Swier, V.J.; White, K.A.; Sato, T.S.; Rogers, C.S.; Weimer, J.M.; Sieren, J.C. Magnetic resonance brain volumetry biomarkers of CLN2 Batten disease identified with miniswine model. Sci. Rep. 2023, 13, 5146. [Google Scholar] [CrossRef]
- Matilla-Dueñas, A.; Corral-Juan, M.; Rodríguez-Palmero Seuma, A.; Vilas, D.; Ispierto, L.; Morais, S.; Sequeiros, J.; Alonso, I.; Volpini, V.; Serrano-Munuera, C.; et al. Rare Neurodegenerative Diseases: Clinical and Genetic Update. In Rare Diseases Epidemiology: Update and Overview; Posada de la Paz, M., Taruscio, D., Groft, S., Eds.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 1031. [Google Scholar] [CrossRef]
- Knickmeyer, R.C.; Gouttard, S.; Kang, C.; Evans, D.; Wilber, K.; Smith, J.K.; Hamer, R.M.; Lin, W.; Gerig, G.; Gilmore, J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008, 28, 12176–12182. [Google Scholar] [CrossRef]
- Zöllei, L.; Iglesias, J.E.; Ou, Y.; Grant, P.E.; Fischl, B. Infant FreeSurfer: An automated segmentation and surface extraction pipeline for T1-weighted neuroimaging data of infants 0-2 years. Neuroimage 2020, 218, 116946. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.H.; Warfield, S.K.; Bharatha, A.; Tempany, C.M.; Kaus, M.R.; Haker, S.J.; Wells, W.M., 3rd; Jolesz, F.A.; Kikinis, R. Statistical validation of image segmentation quality based on a spatial overlap index. Acad. Radiol. 2004, 11, 178–189. [Google Scholar] [CrossRef]
- Deeley, M.A.; Chen, A.; Datteri, R.; Noble, J.H.; Cmelak, A.J.; Donnelly, E.F.; Malcolm, A.W.; Moretti, L.; Jaboin, J.; Niermann, K.; et al. Comparison of manual and automatic segmentation methods for brain structures in the presence of space-occupying lesions: A multi-expert study. Phys. Med. Biol. 2011, 56, 4557–4577. [Google Scholar] [CrossRef]
- Aydin, O.U.; Taha, A.A.; Hilbert, A.; Khalil, A.A.; Galinovic, I.; Fiebach, J.B.; Frey, D.; Madai, V.I. On the usage of average Hausdorff distance for segmentation performance assessment: Hidden error when used for ranking. Eur. Radiol. Exp. 2021, 5, 4. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, C.J.; Johnston, J.M.; D’Souza, P.; Kolstad, J.; Zoppo, C.; Vardar, Z.; Kühn, A.L.; Peker, A.; Rentiya, Z.S.; Yousef, M.H.; et al. A Case for Automated Segmentation of MRI Data in Neurodegenerative Diseases: Type II GM1 Gangliosidosis. NeuroSci 2025, 6, 31. https://doi.org/10.3390/neurosci6020031
Lewis CJ, Johnston JM, D’Souza P, Kolstad J, Zoppo C, Vardar Z, Kühn AL, Peker A, Rentiya ZS, Yousef MH, et al. A Case for Automated Segmentation of MRI Data in Neurodegenerative Diseases: Type II GM1 Gangliosidosis. NeuroSci. 2025; 6(2):31. https://doi.org/10.3390/neurosci6020031
Chicago/Turabian StyleLewis, Connor J., Jean M. Johnston, Precilla D’Souza, Josephine Kolstad, Christopher Zoppo, Zeynep Vardar, Anna Luisa Kühn, Ahmet Peker, Zubir S. Rentiya, Muhammad H. Yousef, and et al. 2025. "A Case for Automated Segmentation of MRI Data in Neurodegenerative Diseases: Type II GM1 Gangliosidosis" NeuroSci 6, no. 2: 31. https://doi.org/10.3390/neurosci6020031
APA StyleLewis, C. J., Johnston, J. M., D’Souza, P., Kolstad, J., Zoppo, C., Vardar, Z., Kühn, A. L., Peker, A., Rentiya, Z. S., Yousef, M. H., Gahl, W. A., Shazeeb, M. S., Tifft, C. J., & Acosta, M. T. (2025). A Case for Automated Segmentation of MRI Data in Neurodegenerative Diseases: Type II GM1 Gangliosidosis. NeuroSci, 6(2), 31. https://doi.org/10.3390/neurosci6020031





