Therapeutic Effect of Padina arborescens Extract on a Cell System Model for Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagent Treatments
2.2. Rat Primary Microglia and Astrocyte Preparation and Culture
2.3. In Vitro Kinase Assay
2.4. Western Blot Analysis
2.5. Messenger RNA (mRNA) Isolation and Complementary DNA (cDNA) Synthesis
2.6. Quantitative-Polymerase Chain Reaction (qPCR) Analysis
2.7. Enzyme-Linked Immunosorbent Assays (ELISAs) of Cytokines and Neurotropic Factors
2.8. Measurement of Cellular NO Levels
2.9. Detection of Cellular ROS
2.10. Seed-Accelerated Fibrilization of α-Synuclein
2.11. Thioflavin T Assay
2.12. Sandwich ELISA for α-Synuclein
2.13. Analysis of Lysosomal Activity
2.14. Cathepsin D Activity
2.15. Culturing of Worms
2.16. Lifespan Assay of C. elegans
2.17. Data Estimation and Statistical Analyses
3. Results
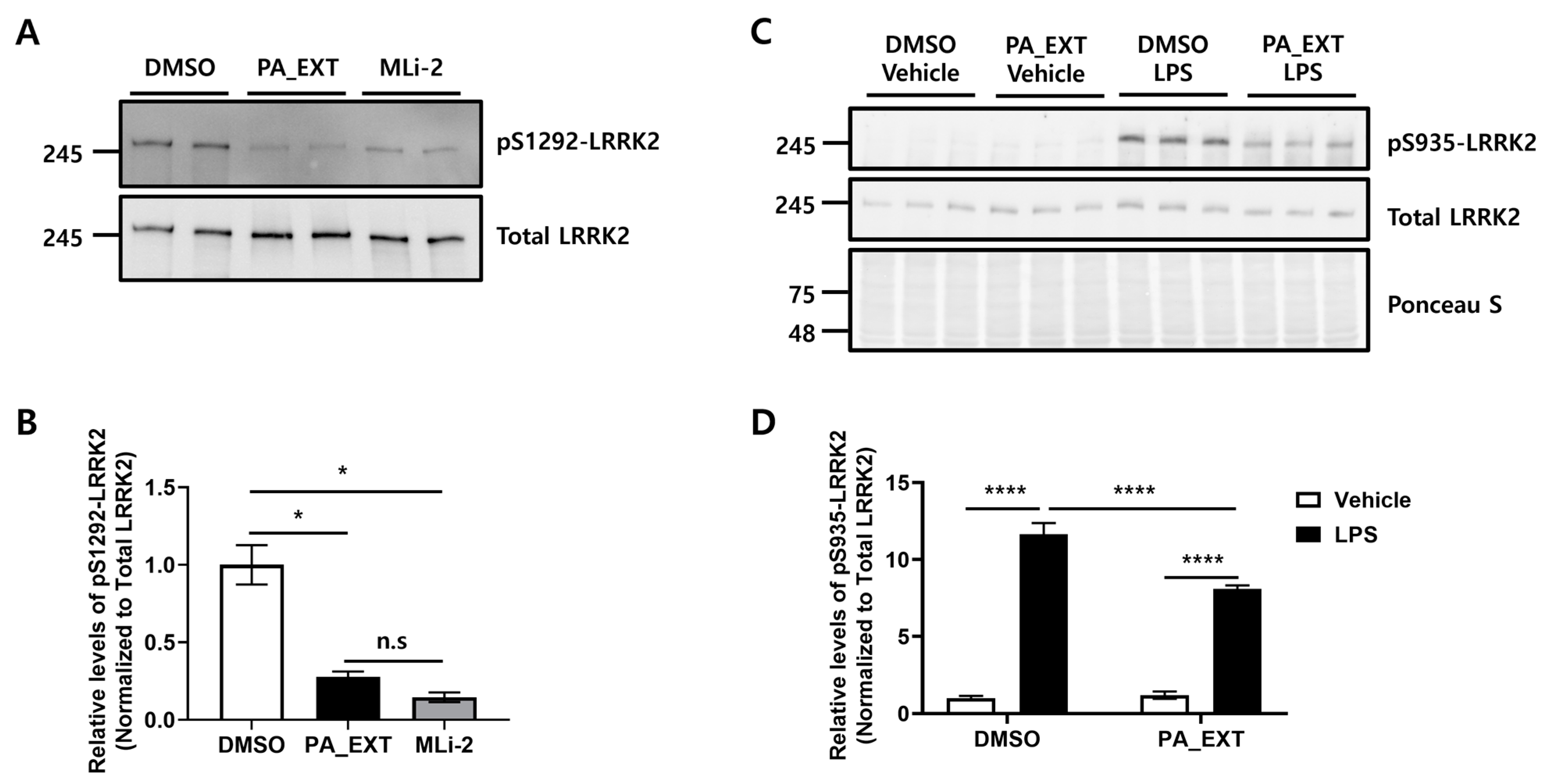
3.1. PA_EXT Inhibits LRRK2 Activity in BV2 Cells In Vitro
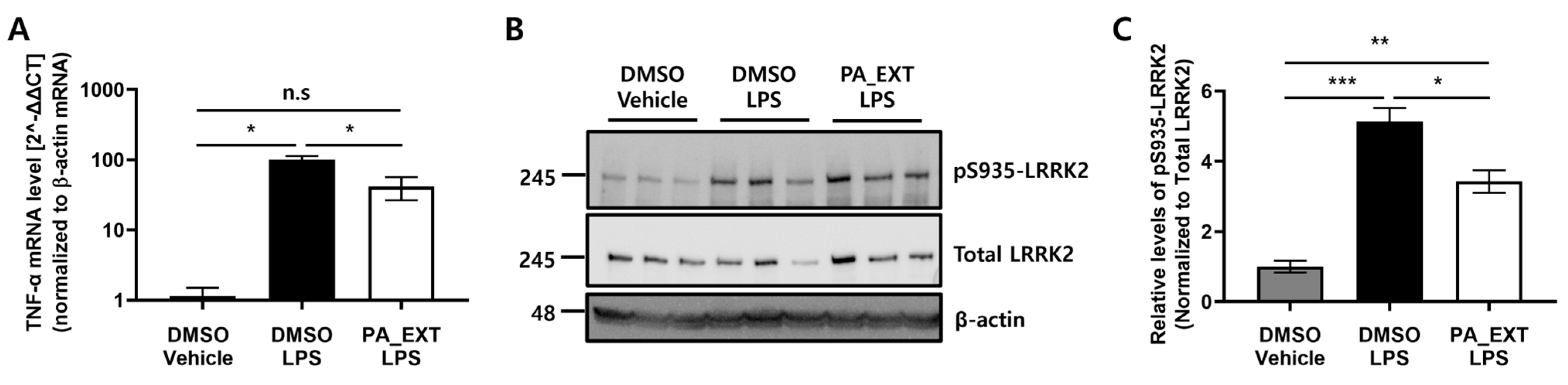
3.2. PA_EXT Decreases TNF-α Expression by Inhibiting LRRK2 Activity in BV2 Cells
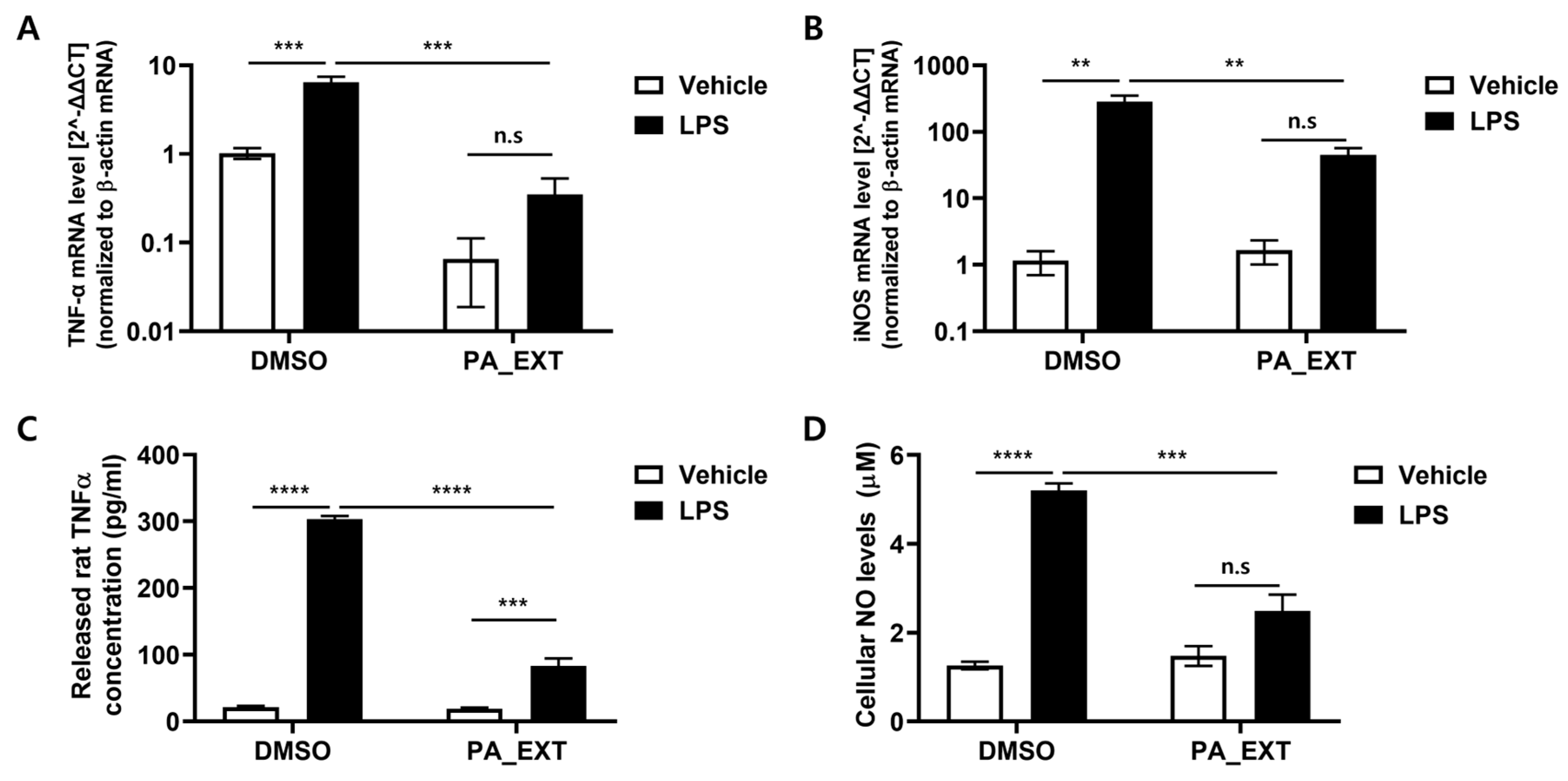
3.3. PA_EXT Reduces the Gene Expression of TNF-α and iNOS in Rat Primary Microglia
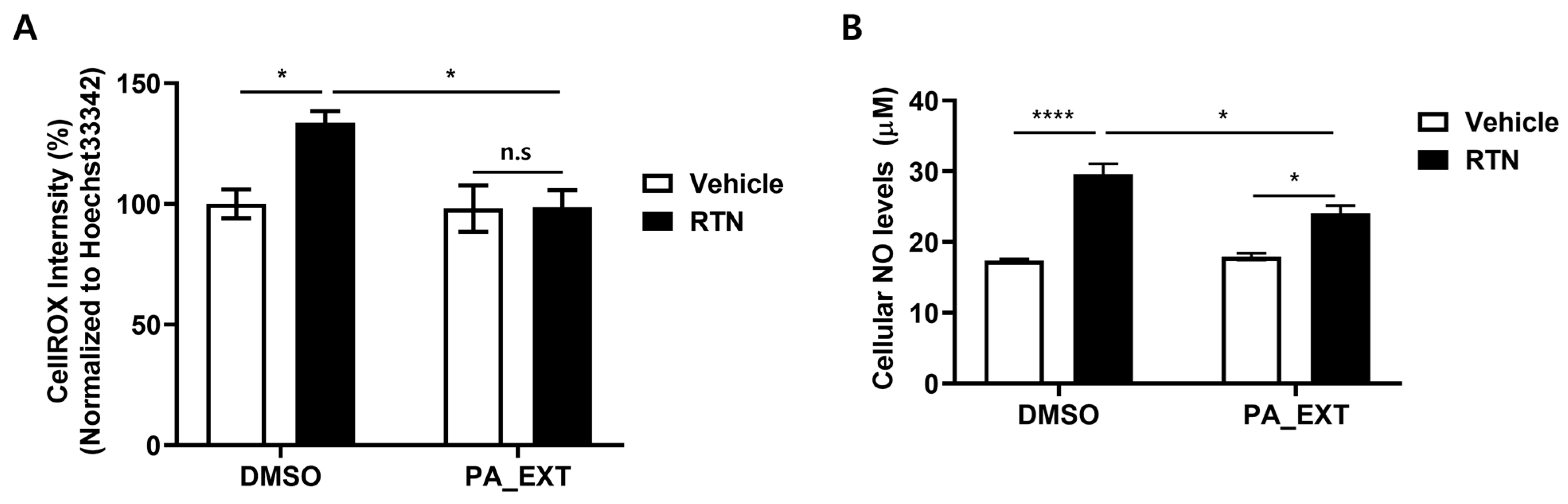
3.4. PA_EXT Alleviates Oxidative Stress in Rat Primary Astrocytes
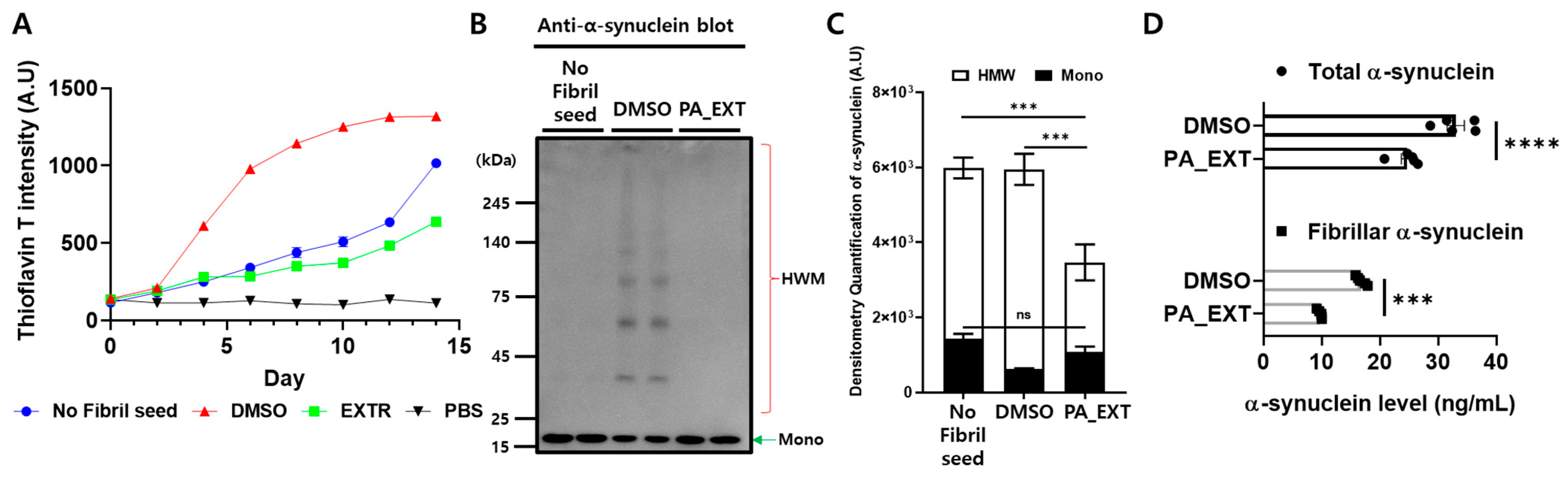
3.5. PA_EXT Blocks α-Synuclein Aggregation and Promotes Its Clearance in Differentiated SH-SY5Y (dSH) Cells
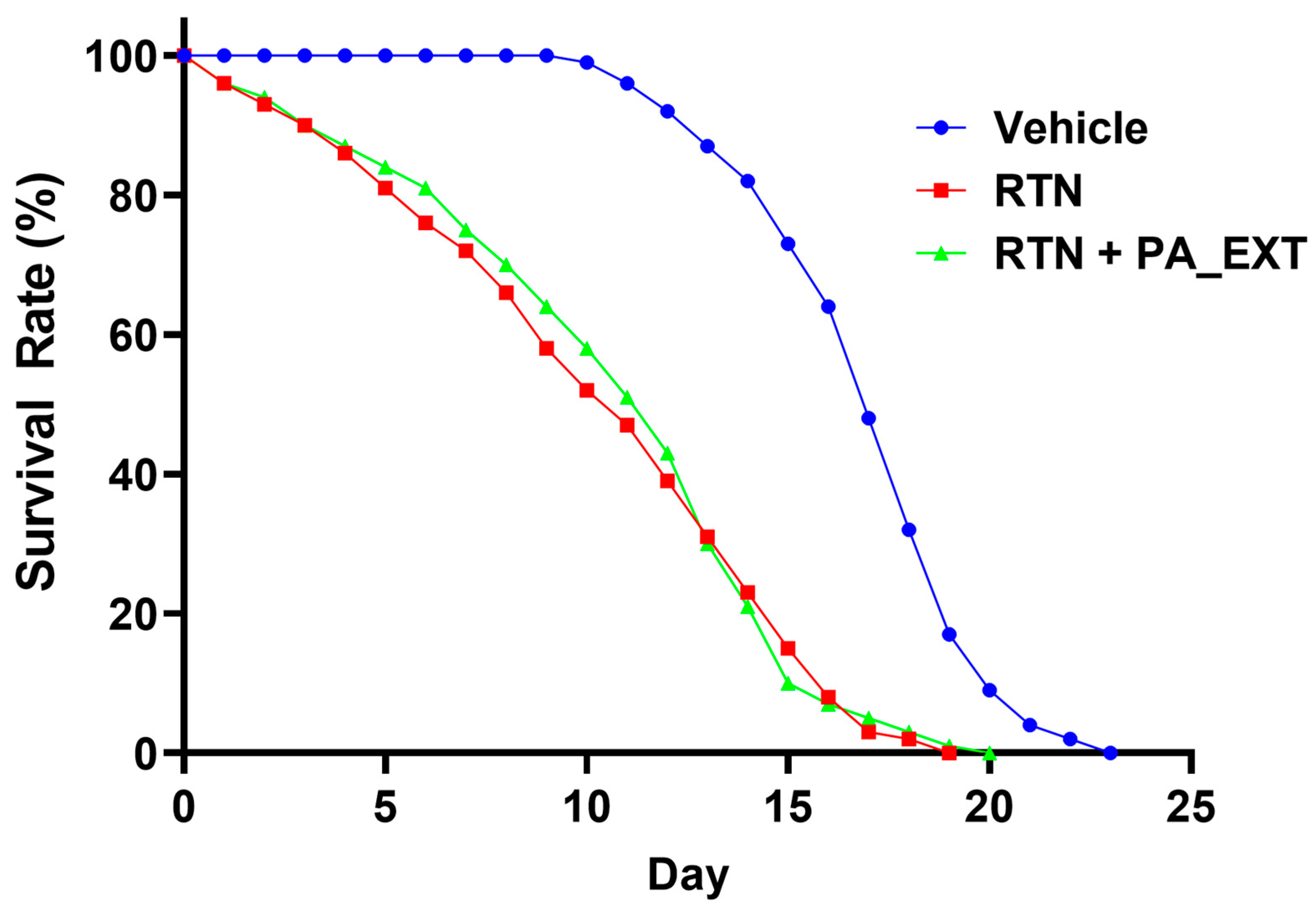
3.6. PA_EXT Prolongs the Lifespan of C. elegans Damaged by RTN
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Opara, J.; Małecki, A.; Małecka, E.; Socha, T. Motor assessment in Parkinson’s disease. Ann. Agric. Environ. Med. 2017, 24, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R. Parkinson’s Disease: From Pathogenesis to Pharmacogenomics. Int. J. Mol. Sci. 2017, 18, 551. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System during Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Ho, M.S. Microglia in Parkinson’s Disease. Adv. Exp. Med. Biol. 2019, 1175, 335–353. [Google Scholar]
- Ho, D.H.; Nam, D.; Seo, M.; Park, S.W.; Seol, W.; Son, I. LRRK2 Inhibition Mitigates the Neuroinflammation Caused by TLR2-Specific α-Synuclein and Alleviates Neuroinflammation-Derived Dopaminergic Neuronal Loss. Cells 2022, 11, 861. [Google Scholar] [CrossRef]
- Usmani, A.; Shavarebi, F.; Hiniker, A. The Cell Biology of LRRK2 in Parkinson’s Disease. Mol. Cell. Biol. 2021, 41, e00660-20. [Google Scholar] [CrossRef]
- Ho, D.H.; Je, A.R.; Lee, H.; Son, I.; Kweon, H.S.; Kim, H.G.; Seol, W. LRRK2 Kinase Activity Induces Mitochondrial Fission in Microglia via Drp1 and Modulates Neuroinflammation. Exp. Neurobiol. 2018, 27, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Migheli, R.; Del Giudice, M.G.; Spissu, Y.; Sanna, G.; Xiong, Y.; Dawson, T.M.; Dawson, V.L.; Galioto, M.; Rocchitta, G.; Biosa, A.; et al. LRRK2 affects vesicle trafficking, neurotransmitter extracellular level and membrane receptor localization. PLoS ONE 2013, 8, e77198. [Google Scholar] [CrossRef]
- Pajarillo, E.; Kim, S.; Digman, A.; Dutton, M.; Son, D.S.; Aschner, M.; Lee, E. The role of microglial LRRK2 kinase in manganese-induced inflammatory neurotoxicity via NLRP3 inflammasome and RAB10-mediated autophagy dysfunction. J. Biol. Chem. 2023, 299, 104879. [Google Scholar] [CrossRef]
- Russo, I.; Bubacco, L.; Greggio, E. LRRK2 and neuroinflammation: Partners in crime in Parkinson’s disease? J. Neuroinflamm. 2014, 11, 52. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef] [PubMed]
- Desplats, P.; Lee, H.J.; Bae, E.J.; Patrick, C.; Rockenstein, E.; Crews, L.; Spencer, B.; Masliah, E.; Lee, S.J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. USA 2009, 106, 13010–13015. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ho, D.H.; Suk, J.E.; You, S.; Michael, S.; Kang, J.; Joong Lee, S.; Masliah, E.; Hwang, D.; Lee, H.J.; et al. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013, 4, 1562. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Suk, J.E.; Patrick, C.; Bae, E.J.; Cho, J.H.; Rho, S.; Hwang, D.; Masliah, E.; Lee, S.J. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010, 285, 9262–9272. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Khoshaghideh, F.; Patel, S.; Lee, S.J. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J. Neurosci. 2004, 24, 1888–1896. [Google Scholar] [CrossRef]
- Elbatrawy, A.A.; Ademoye, T.A.; Alnakhala, H.; Tripathi, A.; Zami, A.; Ostafe, R.; Dettmer, U.; Fortin, J.S. Discovery of small molecule benzothiazole and indole derivatives tackling tau 2N4R and α-synuclein fibrils. Bioorg. Med. Chem. 2024, 100, 117613. [Google Scholar] [CrossRef]
- Hong, B.; Ohtake, Y.; Itokazu, T.; Yamashita, T. Glial senescence enhances α-synuclein pathology owing to its insufficient clearance caused by autophagy dysfunction. Cell Death Discov. 2024, 10, 50. [Google Scholar] [CrossRef]
- Park, M.H.; Han, J.S. Protective Effect of Padina arborescens Extract against High Glucose-induced Oxidative Damage in Human Umbilical Vein Endothelial Cells. Prev. Nutr. Food Sci. 2013, 18, 11–17. [Google Scholar] [CrossRef][Green Version]
- Bae, E.J.; Ho, D.H.; Park, E.; Jung, J.W.; Cho, K.; Hong, J.H.; Lee, H.J.; Kim, K.P.; Lee, S.J. Lipid peroxidation product 4-hydroxy-2-nonenal promotes seeding-capable oligomer formation and cell-to-cell transfer of α-synuclein. Antioxid. Redox Signal. 2013, 18, 770–783. [Google Scholar] [CrossRef]
- Nam, D.; Lee, J.Y.; Lee, M.; Kim, J.; Seol, W.; Son, I.; Ho, D.H. Detection and Assessment of α-Synuclein Oligomers in the Urine of Parkinson’s Disease Patients. J. Parkinsons Dis. 2020, 10, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lim, H.S.; Kawasaki, I.; Shim, Y.H.; Vaikath, N.N.; El-Agnaf, O.M.; Lee, H.J.; Lee, S.J. Anti-aging treatments slow propagation of synucleinopathy by restoring lysosomal function. Autophagy 2016, 12, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Lee, D.; Lee, H.; Kim, D.; Son, H.G.; Yang, J.S.; Lee, S.V.; Kim, S. OASIS 2: Online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 2016, 7, 56147–56152. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Mazzulli, J.R.; Zunke, F.; Tsunemi, T.; Toker, N.J.; Jeon, S.; Burbulla, L.F.; Patnaik, S.; Sidransky, E.; Marugan, J.J.; Sue, C.M.; et al. Activation of β-Glucocerebrosidase Reduces Pathological α-Synuclein and Restores Lysosomal Function in Parkinson’s Patient Midbrain Neurons. J. Neurosci. 2016, 36, 7693–7706. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Mader, B.J.; Pivtoraiko, V.N.; Flippo, H.M.; Klocke, B.J.; Roth, K.A.; Mangieri, L.R.; Shacka, J.J. Rotenone inhibits autophagic flux prior to inducing cell death. ACS Chem. Neurosci. 2012, 3, 1063–1072. [Google Scholar] [CrossRef]
- Cook, C.; Stetler, C.; Petrucelli, L. Disruption of protein quality control in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009423. [Google Scholar] [CrossRef]
- Filippini, A.; Salvi, V.; Dattilo, V.; Magri, C.; Castrezzati, S.; Veerhuis, R.; Bosisio, D.; Gennarelli, M.; Russo, I. LRRK2 Kinase Inhibition Attenuates Astrocytic Activation in Response to Amyloid β(1-42) Fibrils. Biomolecules 2023, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, M.; Ali, M.H.; Jyoti, M.M.S.; Rezanujjaman, M.; Hassan, M.M.; Rana, M.R.; Hossain, M.F.; Kodani, S.; Tokumoto, T. The antagonistic activity of Padina arborescens extracts on mPRα. Nat. Prod. Res. 2023, 37, 1872–1876. [Google Scholar] [CrossRef]
- Castelnovo, L.F.; Thomas, P. Membrane progesterone receptor α (mPRα/PAQR7) promotes migration, proliferation and BDNF release in human Schwann cell-like differentiated adipose stem cells. Mol. Cell. Endocrinol. 2021, 531, 111298. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.H.; Nam, D.; Seo, M.K.; Park, S.W.; Seol, W.; Son, I. LRRK2 Kinase Inhibitor Rejuvenates Oxidative Stress-Induced Cellular Senescence in Neuronal Cells. Oxid. Med. Cell. Longev. 2021, 2021, 9969842. [Google Scholar] [CrossRef] [PubMed]

| Antibody | Manufacturer and Catalog Number |
|---|---|
| Anti-pS1292 LRRK2 | ab203181; Abcam, Cambridge, UK |
| Anti-pS935 LRRK2 | ab133450; Abcam |
| Anti-LRRK2 (N241A/34) | 75-253; NeuroMab, Davis, CA, USA |
| Anti-β-actin | sc-47778; Santa Cruz, Dallas, TX, USA |
| Anti-α-synuclein (Clone 42) | 610787; BD Bioscience, San Jose, CA, USA |
| Peroxidase-conjugated AffiniPure goat anti-mouse IgG (H + L) | 115-035-003; Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA |
| Peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H + L) | 115-035-144; Jackson Immunoresearch Laboratories, Inc. |
| Genes | Sequence (5′-3′) | ||
|---|---|---|---|
| TNF-α | Rat | Forward | ACTGAACTTCGGGGTGATTG |
| Reverse | GCTTGGTGGTTTGCTACGAC | ||
| iNOS (NOS2) | Forward | CACCTTGGAGTTCACCCAGT | |
| Reverse | ACCACTCGTACTTGGGATGC | ||
| β-actin | Forward | CAGGGTGTGATGGTGGGTATGG | |
| Reverse | AGTTGGTGACAATGCCGTGTTC | ||
| TNF-α | Mouse | Forward | CCGATGGGTTGTACCTTGTC |
| Reverse | GCTGGGTAGAGAATGGATGAACA | ||
| β-actin | Forward | TGTTACCAACTGGGACGACA | |
| Reverse | TCTCAGCTGTGGTGGTGAAG | ||
| Group | Mean | S.E.M | N |
|---|---|---|---|
| Vehicle | 16.05 | 0.28 | 100 |
| RTN | 9.38 | 0.47 | 100 |
| RTN + PA_EXT | 9.7 | 0.4433 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, D.H.; Kim, H.; Nam, D.; Seo, M.K.; Park, S.W.; Kim, D.-K.; Son, I. Therapeutic Effect of Padina arborescens Extract on a Cell System Model for Parkinson’s Disease. NeuroSci 2024, 5, 301-314. https://doi.org/10.3390/neurosci5030024
Ho DH, Kim H, Nam D, Seo MK, Park SW, Kim D-K, Son I. Therapeutic Effect of Padina arborescens Extract on a Cell System Model for Parkinson’s Disease. NeuroSci. 2024; 5(3):301-314. https://doi.org/10.3390/neurosci5030024
Chicago/Turabian StyleHo, Dong Hwan, Hyejung Kim, Daleum Nam, Mi Kyoung Seo, Sung Woo Park, Dong-Kyu Kim, and Ilhong Son. 2024. "Therapeutic Effect of Padina arborescens Extract on a Cell System Model for Parkinson’s Disease" NeuroSci 5, no. 3: 301-314. https://doi.org/10.3390/neurosci5030024
APA StyleHo, D. H., Kim, H., Nam, D., Seo, M. K., Park, S. W., Kim, D.-K., & Son, I. (2024). Therapeutic Effect of Padina arborescens Extract on a Cell System Model for Parkinson’s Disease. NeuroSci, 5(3), 301-314. https://doi.org/10.3390/neurosci5030024





