Prevalence, Symptom Profiles, and Correlates of Mixed Anxiety–Depression in Male and Female Autistic Youth
Abstract
1. Introduction
1.1. Autism Symptomatology and Comorbidities
1.2. Measuring Anxiety and Depression in Autism
1.3. Sex Differences
1.4. Correlates of Mixed Anxiety and Depression
1.5. Aims of This Study
2. Materials and Methods
2.1. Participants
2.2. Age
2.3. Instruments
2.3.1. Autism
2.3.2. IQ
2.3.3. Anxiety and Depression
2.3.4. Source of GAD-MDD Data
2.3.5. The Autism Spectrum Disorder Behaviour Checklist (ASDBC)
2.4. Saliva Assays
2.5. Procedure
2.6. Statistical Analysis
3. Results
3.1. Data
3.2. Sex Differences in GAD-MDD
3.2.1. Total GAD-MDD Scores
3.2.2. Factor Structure of GAD-MDD
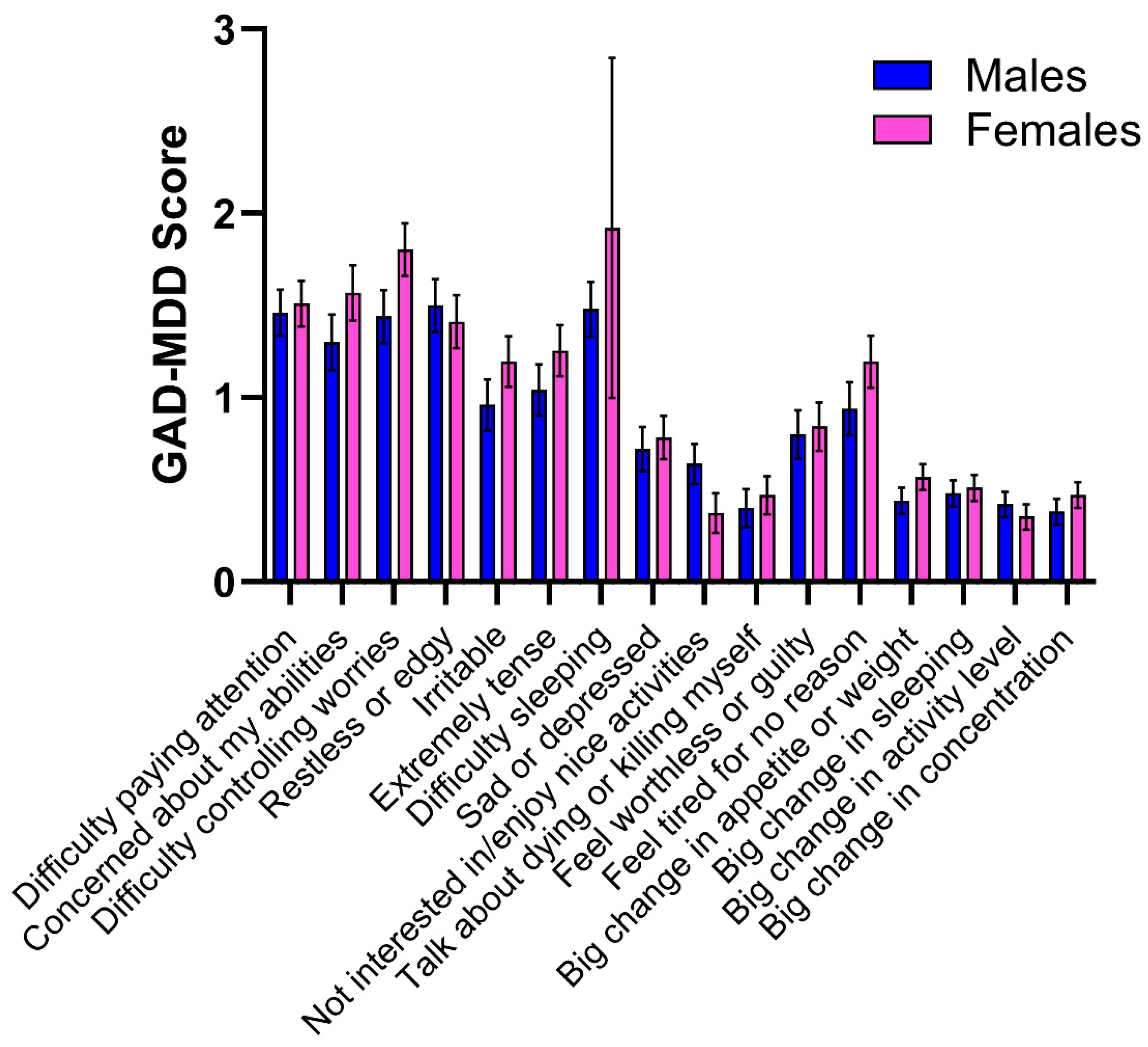
3.2.3. Individual Item Comparisons
3.3. Correlates of GAD-MDD
4. Discussion
4.1. Hypothesis 1: Sex Differences
4.2. Hypothesis 2: Correlates of GAD-MDD
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Khachadourian, V.; Mahjani, B.; Sandin, S.; Kolevzon, A.; Buxbaum, J.D.; Reichenberg, A.; Janecka, M. Comorbidities in autism spectrum disorder and their etiologies. Transl. Psychiatry 2023, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Mutluer, T.; Aslan Genç, H.; Özcan Morey, A.; Yapici Eser, H.; Ertinmaz, B.; Can, M.; Munir, K. Population-Based Psychiatric Comorbidity in Children and Adolescents With Autism Spectrum Disorder: A Meta-Analysis. Front. Psychiatry 2022, 13, 856208. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, K.; Adams, D.; Simpson, K.; Keen, D. Exploring profiles of anxiety symptoms in male and female children on the autism spectrum. Res. Autism Spectr. Disord. 2020, 76, 101601. [Google Scholar] [CrossRef]
- Bougeard, C.; Picarel-Blanchot, F.; Schmid, R.; Campbell, R.; Buitelaar, J. Prevalence of autism spectrum disorder and co-morbidities in children and adolescents: A systematic literature review. Front. Psychiatry 2021, 12, 744709. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.M.; Martin, K.; Fazi, M.; Oldridge, J.; Piper, A.; Rhodes, S.M. A systematic review of the rates of depression in autistic children and adolescents without intellectual disability. Psychol. Psychother. Theory Res. Pract. 2022, 95, 313–344. [Google Scholar] [CrossRef]
- Zinbarg, R.E.; Barlow, D.H.; Liebowitz, M.; Street, L.; Broadhead, E.; Katon, W.; Roy-Byrne, P.; Lepine, J.-P.; Teherani, M.; Richards, J.; et al. The DSM-IV Field Trial for Mixed Anxiety-Depression. Am. J. Psychiatry 1994, 151, 1153–1162. [Google Scholar] [PubMed]
- Barlow, D.; Campbell, L. Mixed anxiety-depression and its implications for models of mood and anxiety disorders. Compr. Psychiatry 2000, 41, 55–60. [Google Scholar] [CrossRef] [PubMed]
- van Heijst, B.F.; Deserno, M.K.; Rhebergen, D.; Geurts, H.M. Autism and depression are connected: A report of two complimentary network studies. Autism 2020, 24, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Das-Munshi, J.; Goldberg, D.; Bebbington, P.E.; Bhugra, D.K.; Brugha, T.S.; Dewey, M.E.; Jenkins, R.; Stewart, R.; Prince, M. Public health significance of mixed anxiety and depression: Beyond current classification. Br. J. Psychiatry 2008, 192, 171–177. [Google Scholar] [CrossRef]
- Moeller, H.-J.; Bandelow, B.; Volz, H.-P.; Barnikol, U.B.; Seifritz, E.; Kasper, S. The relevance of ‘mixed anxiety and depression’as a diagnostic category in clinical practice. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 725–736. [Google Scholar] [CrossRef]
- Fombonne, E.; Quirke, S.; Hagen, A. Epidemiology of pervasive developmental disorders. In Autism Spectrum Disorders; Amaral, D.G., Dawson, G., Geschwind, D.H., Eds.; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Tubío-Fungueiriño, M.; Cruz, S.; Sampaio, A.; Carracedo, A.; Fernández-Prieto, M. Social Camouflaging in Females with Autism Spectrum Disorder: A Systematic Review. J. Autism Dev. Disord. 2021, 51, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, A.M.; Frosch, I.R.; Li, C.E.; Cardinaux, A.L.; Gabrieli, J.D. Exclusion of females in autism research: Empirical evidence for a “leaky” recruitment-to-research pipeline. Autism Res. 2022, 15, 1929–1940. [Google Scholar] [CrossRef]
- Napolitano, A.; Schiavi, S.; La Rosa, P.; Rossi-Espagnet, M.C.; Petrillo, S.; Tagliente, E.; Longo, D.; Valeri, G.; Piemonte, F.; Trezza, V. Sex differences in autism spectrum disorder: Diagnostic, neurobiological, and behavioral features. Front. Psychiatry 2022, 13, 889636. [Google Scholar] [CrossRef] [PubMed]
- de Giambattista, C.; Ventura, P.; Trerotoli, P.; Margari, F.; Margari, L. Sex Differences in Autism Spectrum Disorder: Focus on High Functioning Children and Adolescents. Front. Psychiatry 2021, 12, 539835. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lecavalier, L. Depression in young autistic people: A scoping review. Res. Autism Spectr. Disord. 2021, 88, 101841. [Google Scholar] [CrossRef]
- Solomon, M.; Miller, M.; Taylor, S.; Hinshaw, S.; Carter, C. Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. J. Autism Dev. Disabil. 2012, 42, 48–59. [Google Scholar] [CrossRef]
- Schwartzman, J.M.; Williams, Z.J.; Corbett, B.A. Diagnostic-and sex-based differences in depression symptoms in autistic and neurotypical early adolescents. Autism 2022, 26, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Sukhodolsky, D.; Scahill, L.; Gadow, K.; Arnold, L.; Aman, M.; McDougle, C.; McCracken, J.; Tierney, E.; White, S.; Lecavalier, L.; et al. Parent-rated anxiety symptoms in children with Pervasive Developmental Disorders: Frequency and association with core Autism symtpoms and cognitive functioning. J. Abnorm. Child Psychol. 2008, 36, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Vasa, R.; Keefer, A.; McDonald, R.; Hunsche, M.; Kerns, C. A Scoping Review of Anxiety in Young Children with Autism Spectrum Disorder. Autism Res. 2020, 13, 2038–2057. [Google Scholar] [CrossRef]
- Varela, R.E.; DuPont, R.; Kamps, J.L.; Weems, C.F.; Niditch, L.; Beaton, E.A.; Pucci, G. Age differences in expression of generalized and social anxiety among youth with autism spectrum disorder. J. Autism Dev. Disord. 2020, 50, 730–740. [Google Scholar] [CrossRef]
- DeFilippis, M. Depression in Children and Adolescents with Autism Spectrum Disorder. Children 2018, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Matthey, S.; Petrovski, P. The Children’s Depression Inventory: Error in cutoff scores for screening purposes. Psychol. Assess. 2002, 14, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Szatmari, P.; Bryson, S.; Streiner, D.; Wilson, F. The prevalence of anxiety and mood problems among children with autism and Asperger syndrome. Autism 2000, 4, 117–132. [Google Scholar] [CrossRef]
- Kerns, C.M.; Rast, J.E.; Shattuck, P.T. Prevalence and correlates of caregiver-reported mental health conditions in youth with autism spectrum disorder in the United States. J. Clin. Psychiatry 2020, 82, 11637. [Google Scholar] [CrossRef]
- Taylor, E.C.; Livingston, L.A.; Callan, M.J.; Ashwin, C.; Shah, P. Autonomic dysfunction in autism: The roles of anxiety, depression, and stress. Autism 2021, 25, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, Y.; Hunter, R.; Wei, Y.; Blumenthal, R.; Falke, C.; Khairova, R.; Zhou, R.; Yuan, P.; Machado-Viera, R.; et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc. Natl. Acad. Sci. USA 2009, 106, 3543–3548. [Google Scholar] [CrossRef]
- Gillespie, C.F.; Nemeroff, C.B. Hypercortisolemia and Depression. Psychosom. Med. 2005, 67, S26–S28. [Google Scholar] [CrossRef]
- Sharpley, C.; Bitsika, V.; McMillan, M.; Agnew, L. Incidence, profiles and correlates of the Cortisol Awakening Response in high-functioning young males with ASD. Res. Autism Spectr. Disord. 2019, 57, 145–153. [Google Scholar] [CrossRef]
- Oakley, B.; Loth, E.; Murphy, D.G. Autism and mood disorders. Int. Rev. Psychiatry 2021, 33, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Bitsika, V.; Sharpley, C. Brain-Behaviour Research Group Autism Study; University of New England: Biddeford, ME, USA, 2016. [Google Scholar]
- Lord, C.; Rutter, M.; DiLavore, P.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule, 2nd ed.; (ADOS-2); Western Psychological Services: Los Angeles, CA, USA, 2012. [Google Scholar]
- Cohen, J. The cost of dichotomization. Appl. Psychol. Meas. 1983, 7, 249–253. [Google Scholar] [CrossRef]
- Chen, H.; Cohen, P.; Chen, S. Biased odds ratios from dichotomization of age. Stat. Med. 2007, 26, 3487–3497. [Google Scholar] [CrossRef] [PubMed]
- Filipek, P.; Accardo, P.; Baranek, G.; Cook, E.; Dawson, G.; Gordon, B.; Gravel, J.; Johnson, C.; Kallen, R.; Levy, S.; et al. The screening and diagnosis of autistic spectrum disorders. J. Autism Dev. Disabil. 1999, 29, 439–484. [Google Scholar] [CrossRef]
- National Research Council. Educating Children with Autism; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Lebersfeld, J.B.; Swanson, M.; Clesi, C.D.; O’Kelley, S.E. Systematic review and meta-analysis of the clinical utility of the ADOS-2 and the ADI-R in diagnosing autism spectrum disorders in children. J. Autism Dev. Disord. 2021, 51, 4101–4114. [Google Scholar] [CrossRef] [PubMed]
- Janvier, D.; Choi, Y.B.; Klein, C.; Lord, C.; Kim, S.H. Brief report: Examining test-retest reliability of the autism diagnostic observation schedule (ADOS-2) calibrated severity scores (CSS). J. Autism Dev. Disord. 2022, 52, 1388–1394. [Google Scholar] [CrossRef]
- Wechsler, D. The Wechsler Abbreviated Scale of Intelligence, 2nd ed.; Pearson: Bloomington, MN, USA, 2011. [Google Scholar]
- Minshew, N.; Turner, C.; Goldstein, G. The Application of Short Forms of the Wechsler Intelligence Scales in Adults and Children with High Functioning Autism. J. Autism Dev. Disord. 2005, 35, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gadow, K.; Sprafkin, J. Child and Adolescent Symptom Inventory 4R: Screening and Norms Manual; Checkmate Plus: Stony Brook, NY, USA, 2010. [Google Scholar]
- Gadow, K.; Devincent, C.; Pomeroy, J.; Azizian, A. Comparison of DSM-IV symptoms in elementary school-age children with PDD versus clinic and community samples. Autism 2005, 9, 392–415. [Google Scholar] [CrossRef]
- Lecavalier, L.; Wood, J.; Halliday, A.; Jones, N.; Aman, M.; Cook, E. Measuring anxiety as a treatment endpoint in youth with Autism Spectrum Disorder. J. Autism Dev. Disord. 2014, 44, 1128–1143. [Google Scholar] [CrossRef]
- Bitsika, V.; Sharpley, C.; Andronicos, N.; Agnew, L. A test of the ‘Parent distortion’ hypothesis when assessing Generalised Anxiety Disorder in boys with an Autism Spectrum Disorder. Res. Autism Spectr. Disord. 2015, 15–16, 42–52. [Google Scholar] [CrossRef]
- Bitsika, V.; Sharpley, C. Mothers’ Depressive State ‘Distorts’ the Ratings of Depression they give for their Sons with an Autism Spectrum Disorder. Int. J. Disabil. Dev. Educ. 2016, 63, 491–499. [Google Scholar] [CrossRef]
- Bitsika, V.; Sharpley, C.; Sweeney, J.; McFarlane, J. HPA and SAM axis responses as correlates of self- vs parental ratings of anxiety in boys with an Autistic Disorder. Physiol. Behav. 2014, 127, 1–7. [Google Scholar] [CrossRef]
- Schwartzman, J.; Corbett, B. Higher depressive symptoms in early adolescents with Autism Spectrum Disorder by self- and parent-report compared to typically-developing peers. Res. Autism Spectr. Disord. 2020, 77, 101613. [Google Scholar] [CrossRef]
- Bitsika, V.; Sharpley, C.F. “They don’t understand how bad I feel”: Inconsistencies between mother-rated and self-rated symptoms of depression in autistic girls. Res. Autism Spectr. Disord. 2023, 104, 102145. [Google Scholar] [CrossRef]
- Bitsika, V. The Autism Spectrum Disorder Behaviour Checklist (ASDBC); Behaviour Analysis and Consulting Services: Coolangatta, Australia, 2000. [Google Scholar]
- Schopler, E.; Reichler, R.; Renner, B. The Childhood Autism Rating Scale (CARS) for Diagnostic Screening and Classification of Autism; Western Psychological Services: Los Angeles, CA, USA, 1999. [Google Scholar]
- Sparrow, S.; Balla, D.; Cicchetti, D. Vineland Adaptive Behaviour Scales: Interview Edition; American Guidance: Circle Pines, MN, USA, 1984. [Google Scholar]
- Bitsika, V.; Sharpley, C.; Orapeleng, S. Using cognitive, adaptive and behavioral indices for Cluster Analysis of ASD subgroups. J. Intellect. Disabil. Res. 2008, 52, 973–985. [Google Scholar] [CrossRef]
- Stevens, J. Applied Multivariate Statistics for the Social Sciences; Lawrence Erlbaum: Mahwah, NJ, USA, 1996. [Google Scholar]
- Rothman, K. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef]
- Rothman, K. Curbing type I and type II errors. Eur. J. Epidemiol. 2010, 25, 223–224. [Google Scholar] [CrossRef]
- Team, A. JASP; University of Amsterdam: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Tabachnik, B.; Fidell, L. Using Multivariate Statistics, 6th ed.; Pearson Education: Boston, MA, USA, 2013. [Google Scholar]
- Nájera, P.; Abad, F.J.; Sorrel, M.A. Is exploratory factor analysis always to be preferred? A systematic comparison of factor analytic techniques throughout the confirmatory—Exploratory continuum. Psychol. Methods 2023. Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Sapnas, K.G.; Zeller, R.A. Minimizing sample size when using exploratory factor analysis for measurement. J. Nurs. Meas. 2002, 10, 135–154. [Google Scholar] [CrossRef]
- Thurstone, L.L. Multiple Factor Analysis; Chicago University Press: Chicago, IL, USA, 1947. [Google Scholar]
- Nutt, D. Anxiety and depression: Individual entities or two sides of the same coin? Int. J. Psychiatry Clin. Pract. 2004, 8, 19–24. [Google Scholar] [CrossRef]
- Hyde, J.S.; Mezulis, A.H. Gender differences in depression: Biological, affective, cognitive, and sociocultural factors. Harv. Rev. Psychiatry 2020, 28, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Steinsbekk, S.; Ranum, B.; Wichstrøm, L. Prevalence and course of anxiety disorders and symptoms from preschool to adolescence: A 6-wave community study. J. Child Psychol. Psychiatry 2022, 63, 527–534. [Google Scholar] [CrossRef]
- Mylett, M.L.; Boucher, T.Q.; Scheerer, N.E.; Iarocci, G. Examining the Relations between Social Competence, Autistic Traits, Anxiety and Depression in Autistic and Non-Autistic Children. J. Autism Dev. Disord. 2023, 54, 3094–3106. [Google Scholar] [CrossRef]
- Bauminger, N.; Kasari, C. Loneliness and friendship in high-functioning children with autism. Child Dev. 2000, 71, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Kuusikko, S.; Pollock-Wurman, R.; Jussila, K.; Carter, A.; Mattila, M.-L.; Ebeling, H.; Pauls, D.; Moilanen, I. Social anxiety in high-functioning children and adolescents with Autism and Asperger Syndrome. J. Autism Dev. Disord. 2008, 38, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Vickerstaff, S.; Heriot, S.; Wong, M.L.; Lopes, A.; Dossetor, D. Intellectual ability, self-perceived social competence, and depressive symptomatology in children with high-functioning autistic spectrum disorders. J. Autism Dev. Disord. 2007, 37, 1647–1664. [Google Scholar] [CrossRef]
| Males | Females | F | p | |
|---|---|---|---|---|
| ASDB communication | 13.92 (5.57) | 13.90 (5.32) | 0.000 | 0.987 |
| ASDBC social interaction | 17.46 (6.950 | 18.14 (5.98) | 0.276 | 0.601 |
| ASDBC adaptive behaviour | 15.64 (5.90) | 14.92 (5.16) | 0.425 | 0.516 |
| Morning cortisol | 10.56 (6.26) | 20.32 (13.16) | 22.095 | <0.001 |
| GAD-MDD Symptom | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| Feel worthless or guilty | 0.828 | ||
| Difficulty paying attention | 0.736 | ||
| Extremely tense | 0.731 | ||
| Talk about dying or killing myself | 0.71 | ||
| Difficulty controlling my worries | 0.684 | ||
| Restless or edgy | 0.684 | ||
| Irritable | 0.682 | ||
| Concerned about my abilities | 0.642 | ||
| Sad or depressed | 0.631 | ||
| Big change in sleeping | 0.793 | ||
| Big change in concentration | 0.739 | ||
| Big change in appetite or weight | 0.645 | ||
| Big change in activity level | 0.562 | ||
| Difficulty sleeping | 0.482 | ||
| Not interested/enjoy nice activities | 0.841 | ||
| Feel tired for no reason | 0.703 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitsika, V.; Sharpley, C.F.; Vessey, K.A.; Evans, I.D. Prevalence, Symptom Profiles, and Correlates of Mixed Anxiety–Depression in Male and Female Autistic Youth. NeuroSci 2024, 5, 315-327. https://doi.org/10.3390/neurosci5030025
Bitsika V, Sharpley CF, Vessey KA, Evans ID. Prevalence, Symptom Profiles, and Correlates of Mixed Anxiety–Depression in Male and Female Autistic Youth. NeuroSci. 2024; 5(3):315-327. https://doi.org/10.3390/neurosci5030025
Chicago/Turabian StyleBitsika, Vicki, Christopher F. Sharpley, Kirstan A. Vessey, and Ian D. Evans. 2024. "Prevalence, Symptom Profiles, and Correlates of Mixed Anxiety–Depression in Male and Female Autistic Youth" NeuroSci 5, no. 3: 315-327. https://doi.org/10.3390/neurosci5030025
APA StyleBitsika, V., Sharpley, C. F., Vessey, K. A., & Evans, I. D. (2024). Prevalence, Symptom Profiles, and Correlates of Mixed Anxiety–Depression in Male and Female Autistic Youth. NeuroSci, 5(3), 315-327. https://doi.org/10.3390/neurosci5030025






