Salivary Transcriptome and Mitochondrial Analysis of Autism Spectrum Disorder Children Compared to Healthy Controls
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Microbiome Differences
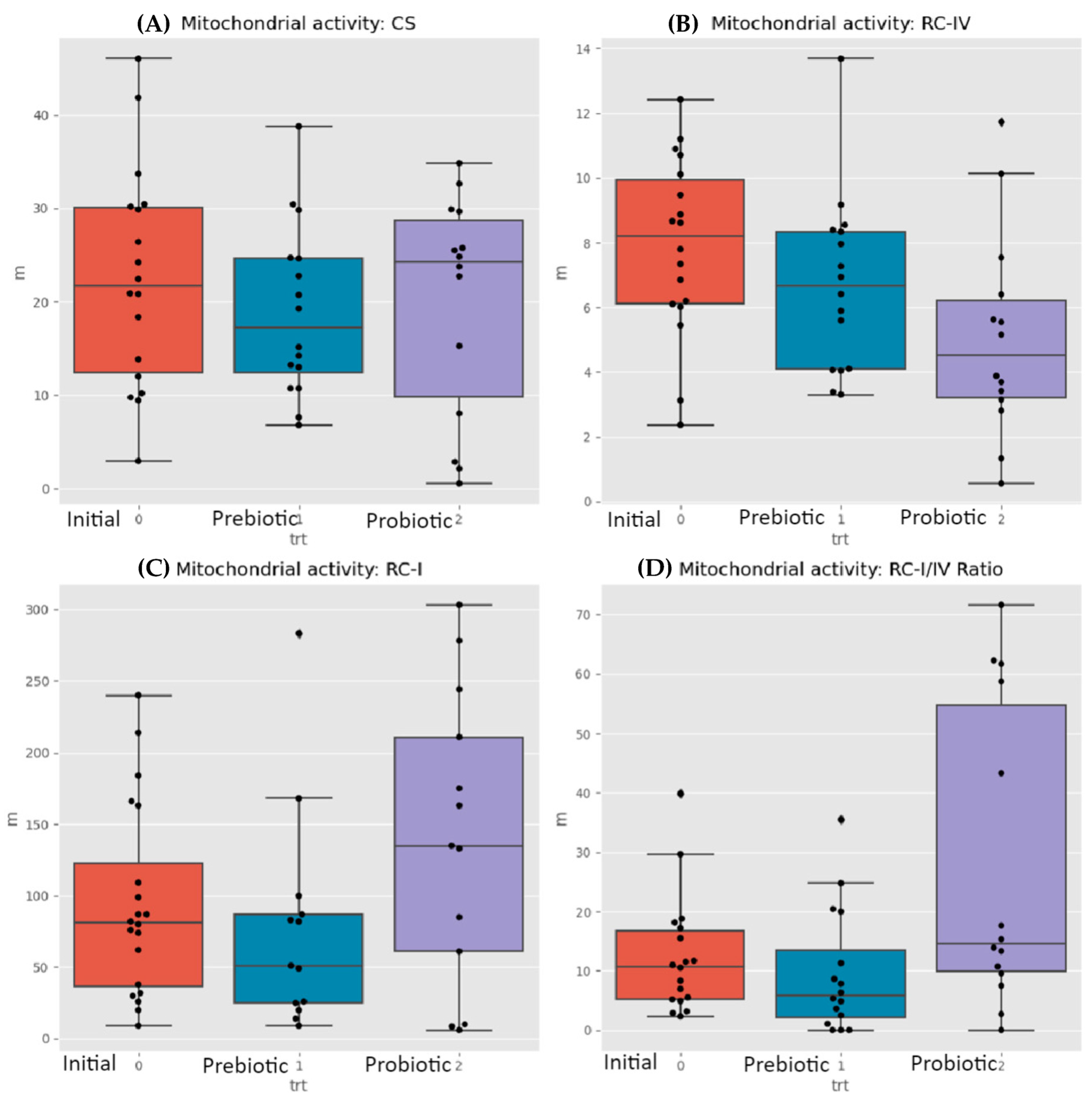
3.2. Mictochondrial Differences
3.3. KEGG Orthology
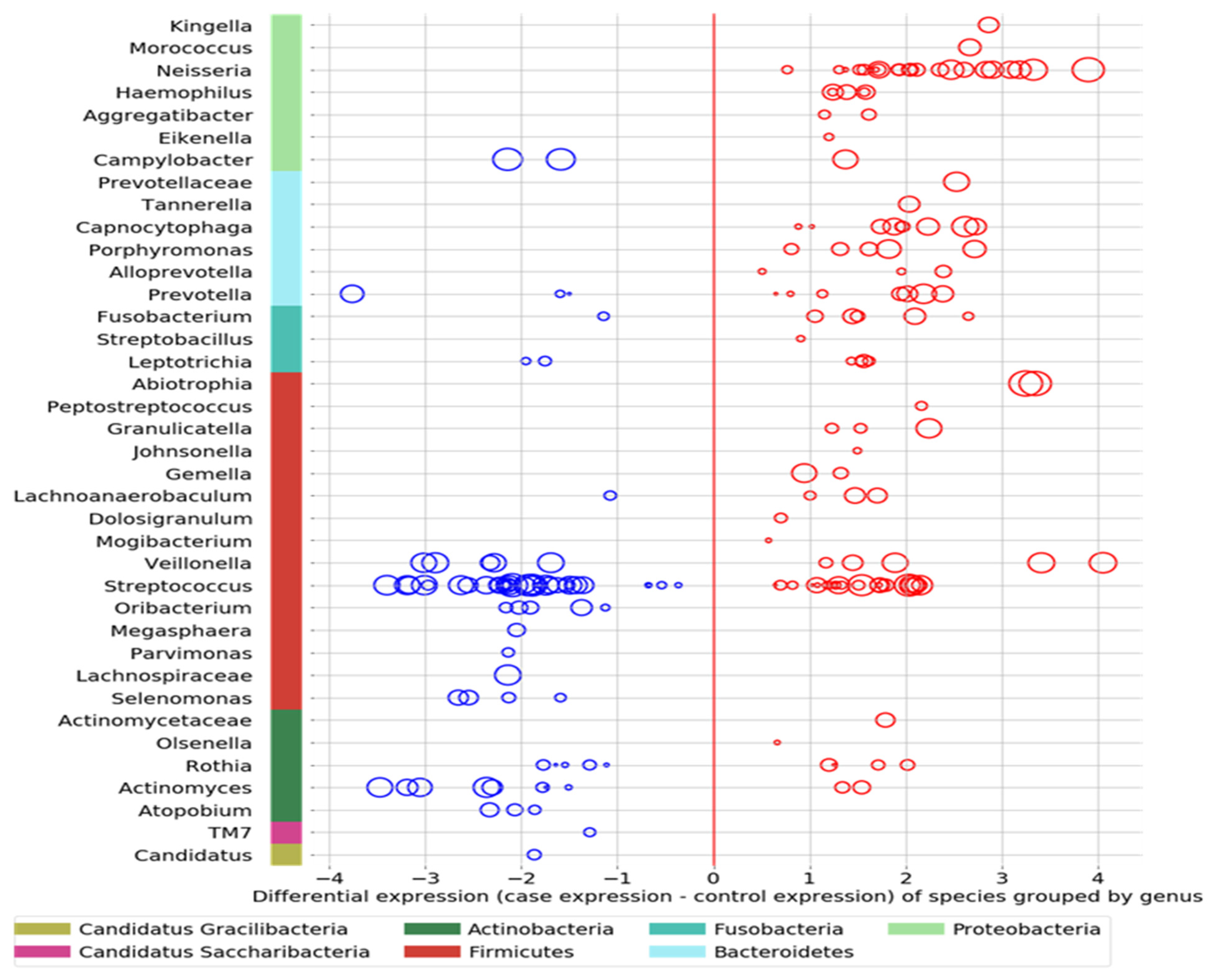
- Comparing the ASD subjects to the neurotypical, different species prevalence between the groups was demonstrated in 11 genera. Specific taxa, such as the Streptococcus genus, had up-regulated and down-regulated strains in the ASD group (see Figure 3). Neisseria, Leptotrichia, Prevotella, Capnoctyophaga, and Fusobacterium genera were up-regulated in the ASD group. The oral microbiome of the ASD subjects was significantly different from that of the neurotypical and “Blue Zone” controls. Species from the genera Treponema, Prevotella, Eubacterium, Selenomonas, Oribacterium, Lachnospiraceae, and Actinomyces were increased in the neurotypical (see Figure 4).
3.4. Verbal Skills Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pulikkan, J.; Mazumder, A.; Grace, T. Role of the Gut Microbiome in Autism Spectrum Disorders. Adv. Exp. Med. Biol. 2019, 1118, 253–269. [Google Scholar] [CrossRef]
- Khalil, R.; Tindle, R.; Boraud, T.; Moustafa, A.A.; Karim, A.A. Social decision making in autism: On the impact of mirror neurons, motor control, and imitative behaviors. CNS Neurosci. Ther. 2018, 24, 669–676. [Google Scholar] [CrossRef]
- Waye, M.M.Y.; Cheng, H.Y. Genetics and epigenetics of autism: A Review. Psychiatry Clin. Neurosci. 2017, 72, 228–244. [Google Scholar] [CrossRef]
- Siu, M.T.; Weksberg, R. Epigenetics of Autism Spectrum Disorder. Adv. Exp. Med. Biol. 2017, 978, 63–90. [Google Scholar] [CrossRef]
- Duffney, L.J.; Valdez, P.; Tremblay, M.W.; Cao, X.; Montgomery, S.; McConkie-Rosell, A.; Jiang, Y. Epigenetics and autism spectrum disorder: A report of an autism case with mutation in H1 linker histone HIST1H1E and literature review. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2018, 177, 426–433. [Google Scholar] [CrossRef]
- Xu, G.; Strathearn, L.; Liu, B.; O’brien, M.; Kopelman, T.G.; Zhu, J.; Snetselaar, L.G.; Bao, W. Prevalence and Treatment Patterns of Autism Spectrum Disorder in the United States, 2016. JAMA Pediatr. 2019, 173, 153–159. [Google Scholar] [CrossRef]
- Karimi, P.; Kamali, E.; Mousavi, S.M.; Karahmadi, M. Environmental factors influencing the risk of autism. J. Res. Med. Sci. 2017, 22, 27. [Google Scholar] [CrossRef]
- Ronald, A.; Hoekstra, R.A. Autism spectrum disorders and autistic traits: A decade of new twin studies. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2011, 156, 255–274. [Google Scholar] [CrossRef]
- Hallmayer, J.; Cleveland, S.; Torres, A.; Phillips, J.; Cohen, B.; Torigoe, T.; Miller, J.; Fedele, A.; Collins, J.; Smith, K.; et al. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch. Gen. Psychiatry 2011, 68, 1095–1102. [Google Scholar] [CrossRef]
- Herbert, M.R. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr. Opin. Neurol. 2010, 23, 103–110. [Google Scholar] [CrossRef]
- Deth, R.; Muratore, C.; Benzecry, J.; Power-Charnitsky, V.-A.; Waly, M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. NeuroToxicology 2007, 29, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.; Herbstman, J. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 2011, 31, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Microbiome Disturbances and Autism Spectrum Disorders. Drug Metab. Dispos. 2015, 43, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Microbiota is essential for social development in the mouse. Mol. Psychiatry 2014, 19, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Golubeva, A.V.; Joyce, S.A.; Moloney, G.; Burokas, A.; Sherwin, E.; Arboleya, S.; Flynn, I.; Khochanskiy, D.; Moya-Pérez, A.; Peterson, V.; et al. Microbiota-related Changes in Bile Acid & Tryptophan Metabolism are Associated with Gastrointestinal Dysfunction in a Mouse Model of Autism. eBioMedicine 2017, 24, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef]
- Henry, L.P.; Bruijning, M.; Forsberg, S.K.; Ayroles, J.F. Can the microbiome influence host evolutionary trajectories? bioRxiv 2019, 700237. [Google Scholar] [CrossRef]
- Davenport, E.R.; Sanders, J.G.; Song, S.J.; Amato, K.R.; Clark, A.G.; Knight, R. The human microbiome in evolution. BMC Biol. 2017, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Montiel-Castro, A.J.; Gonzalez-Cervantes, R.M.; Bravo-Ruiseco, G.; Pacheco-Lopez, G. The microbiota-gut-brain axis: Neuro-behavioral correlates, health and sociality. Front. Integr. Neurosci. 2013, 7, 70. [Google Scholar] [CrossRef]
- Arora, T.; Bäckhed, F. The gut microbiota and metabolic disease: Current understanding and future perspectives. J. Intern. Med. 2016, 280, 339–349. [Google Scholar] [CrossRef]
- Shams, S.; Foley, K.A.; Kavaliers, M.; MacFabe, D.F.; Ossenkopp, K. Systemic treatment with the enteric bacterial metabolic product propionic acid results in reduction of social behavior in juvenile rats: Contribution to a rodent model of autism spectrum disorder. Dev. Psychobiol. 2019, 61, 688–699. [Google Scholar] [CrossRef] [PubMed]
- MacFabe, D.F.; Cain, D.P.; Rodriguezcapote, K.; Franklin, A.E.; Hoffman, J.E.; Boon, F.; Taylor, A.R.; Kavaliers, M.; Ossenkopp, K.-P. Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 2006, 176, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.R.; MacFabe, D.F.; Ossenkopp, K.-P.; Scratch, S.; Whelan, J.; Taylor, R.; Cain, D.P. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: Implications for an animal model of autism. Neuropharmacology 2008, 54, 901–911. [Google Scholar] [CrossRef]
- Shultz, S.R.; Macfabe, D.F.; Martin, S.; Jackson, J.; Taylor, R.; Boon, F.; Ossenkopp, K.P.; Cain, D.P. Intracerebroventricular injections of the enteric bacterial metabolic product pro-pionic acid impair cognition and sensorimotor ability in the Long-Evans rat: Further development of a rodent model of autism. Behav. Brain Res. 2009, 200, 33–41. [Google Scholar] [CrossRef]
- MacFabe, D.F.; Cain, N.E.; Boon, F.; Ossenkopp, K.P.; Cain, D.P. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behav. Brain Res. 2011, 217, 47–54. [Google Scholar] [CrossRef]
- Frye, R.E.; Nankova, B.; Bhattacharyya, S.; Rose, S.; Bennuri, S.C.; MacFabe, D.F. Modulation of Immunological Pathways in Autistic and Neurotypical Lymphoblastoid Cell Lines by the Enteric Microbiome Metabolite Propionic Acid. Front. Immunol. 2017, 8, 1670. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Bennuri, S.C.; Davis, J.E.; Wynne, R.; Slattery, J.C.; Tippett, M.; Delhey, L.; Melnyk, S.; Kahler, S.G.; MacFabe, D.F.; et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl. Psychiatry 2018, 8, 42. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Dengate, S.; Ruben, A. Controlled trial of cumulative behavioural effects of a common bread preservative*. J. Paediatr. Child Health 2002, 38, 373–376. [Google Scholar] [CrossRef]
- Akram, M.; Shah, S.M.A.; Munir, N.; Daniyal, M.; Tahir, I.M.; Mahmood, Z.; Irshad, M.; Akhlaq, M.; Sultana, S.; Zainab, R. Hexose monophosphate shunt, the role of its metabolites and associated disorders: A review. J. Cell. Physiol. 2019, 234, 14473–14482. [Google Scholar] [CrossRef] [PubMed]
- Bär, A. Sugar alcohols in the diabetic diet. In Sugars and Sweeteners; Kretchmer, N., Hollenbeck, C.B., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 131–150. [Google Scholar]
- Meyer-Gerspach, A.C.; Drewe, J.; Verbeure, W.; le Roux, C.W.; Dellatorre-Teixeira, L.; Rehfeld, J.F.; Holst, J.J.; Hartmann, B.; Tack, J.; Peterli, R.; et al. Effect of the Natural Sweetener Xylitol on Gut Hormone Secretion and Gastric Emptying in Humans: A Pilot Dose-Ranging Study. Nutrients 2021, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, C.; Kumar, C.D.; Joseph, J. Xylitol in preventing dental caries: A systematic review and meta-analyses. J. Nat. Sci. Biol. Med. 2017, 8, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Kõljalg, S.; Smidt, I.; Chakrabarti, A.; Bosscher, D.; Mändar, R. Exploration of singular and synergistic effect of xylitol and erythritol on causative agents of dental caries. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, K.K. Sugar alcohols and prevention of oral diseases―comments and rectifications. Oral Health Care 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Salli, K.; Lehtinen, M.J.; Tiihonen, K.; Ouwehand, A.C. Xylitol’s Health Benefits beyond Dental Health: A Com-prehensive Review. Nutrients 2019, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, C.I.; Islam, M.S. Xylitol: One name, numerous benefits. In Sweeteners: Pharmacology, Biotechnology, and Applications; Merillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–27. [Google Scholar]
- Wölnerhanssen, B.K.; Meyer-Gerspach, A.C.; Beglinger, C.; Islam, S. Metabolic effects of the natural sweeteners xylitol and erythritol: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1986–1998. [Google Scholar] [CrossRef]
- Benahmed, A.G.; Gasmi, A.; Arshad, M.; Shanaida, M.; Lysiuk, R.; Peana, M.; Pshyk-Titko, I.; Adamiv, S.; Shanaida, Y.; Bjørklund, G. Health benefits of xylitol. Appl. Microbiol. Biotechnol. 2020, 104, 7225–7237. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.L.; Merchant, M.; Kabat, W.; Catherine, L.; White, K.; Unruh, B.; Ramones, A. In Vitro Studies of Xylitol and Erythritol Inhibition of Streptococcus Mutans and Streptococcus Sobrinus Growth and Biofilm Production. J. Clin. Pediatr. Dent. 2020, 44, 307–314. [Google Scholar] [CrossRef]
- Park, E.; Park, M.H.; Na, H.S.; Chung, J. Xylitol induces cell death in lung cancer A549 cells by autophagy. Biotechnol. Lett. 2015, 37, 983–990. [Google Scholar] [CrossRef]
- Tomonobu, N.; Komalasari, N.L.G.Y.; Sumardika, I.W.; Jiang, F.; Chen, Y.; Yamamoto, K.-I.; Kinoshita, R.; Murata, H.; Inoue, Y.; Sakaguchi, M. Xylitol acts as an anticancer monosaccharide to induce selective cancer death via regulation of the glutathione level. Chem. Interact. 2020, 324, 109085. [Google Scholar] [CrossRef] [PubMed]
- Sahasakul, Y.; Angkhasirisap, W.; Lam-Ubol, A.; Aursalung, A.; Sano, D.; Takada, K.; Trachootham, D. Partial Substitution of Glucose with Xylitol Prolongs Survival and Suppresses Cell Proliferation and Glycolysis of Mice Bearing Orthotopic Xenograft of Oral Cancer. Nutrients 2022, 14, 2023. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.A.; Saad, K.; El-Asheer, O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr. Neurosci. 2018, 21, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fu, X.; Liao, X.; Li, Y. Effects of gut microbial-based treatments on gut microbiota, behavioral symptoms, and gastrointestinal symptoms in children with autism spectrum disorder: A systematic review. Psychiatry Res. 2020, 293, 113471. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, W.; Tang, F.; Chen, X.; Song, G. Effects of Probiotics on Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrients 2023, 15, 1415. [Google Scholar] [CrossRef] [PubMed]
- Abujamel, T.S.; Al-Otaibi, N.M.; Abuaish, S.; AlHarbi, R.H.; Assas, M.B.; Alzahrani, S.A.; Alotaibi, S.M.; El-Ansary, A.; Aabed, K. Different Alterations in Gut Microbiota between Bifidobacterium longum and Fecal Microbiota Transplantation Treatments in Propionic Acid Rat Model of Autism. Nutrients 2022, 14, 608. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yan, J.; Yang, T.; Zhu, J.; Li, T.; Wei, H.; Chen, J. Fecal Microbiome Transplantation from Children with Autism Spectrum Disorder Modulates Tryptophan and Serotonergic Synapse Metabolism and Induces Altered Behaviors in Germ-Free Mice. mSystems 2021, 6, 10-1128. [Google Scholar] [CrossRef]
- Guidetti, C.; Salvini, E.; Viri, M.; Deidda, F.; Amoruso, A.; Visciglia, A.; Drago, L.; Calgaro, M.; Vitulo, N.; Pane, M.; et al. Randomized Double-Blind Crossover Study for Evaluating a Probiotic Mixture on Gastrointestinal and Behavioral Symptoms of Autistic Children. J. Clin. Med. 2022, 11, 5263. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.H.; Oliver, B. Microarrays, deep sequencing and the true measure of the transcriptome. BMC Biol. 2011, 9, 34. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Wakayama, M.; Katsuno, Y.; Hayashi, S.-I.; Miyamoto, Y.; Sakai, K.; Moriguchi, M. Cloning and Sequencing of a Gene Encoding D-Aminoacylase from Alcaligenes xylosoxydans subsp. xylosoxydans A-6 and Expression of the Gene in Escherichia coli. Biosci. Biotechnol. Biochem. 1995, 59, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.; Vaughan, C.W.; Vandenberg, R.J. N-Acyl amino acids and N-acyl neurotransmitter conjugates: Neuromodulators and probes for new drug targets. Br. J. Pharmacol. 2010, 160, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.C. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J. Neural Transm. 2014, 121, 891–905. [Google Scholar] [CrossRef]
- Tirouvanziam, R.; Obukhanych, T.V.; Laval, J.; Aronov, P.A.; Libove, R.; Banerjee, A.G.; Parker, K.J.; O’hara, R.; Herzenberg, L.A.; Hardan, A.Y. Distinct Plasma Profile of Polar Neutral Amino Acids, Leucine, and Glutamate in Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2011, 42, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef] [PubMed]
- Walker, H.B.; Midvedt, T. Microbiota Transplant Intervention Shows Promising Results in Some Children with Autism Spectrum Disorder (ASD); Program No. 458.03. 2019 Neuroscience Meeting Planner; Society for Neuroscience: Chicago, IL, USA, 2019. [Google Scholar]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Kobayashi, R.; Ogawa, Y.; Hashizume-Takizawa, T.; Kurita-Ochiai, T. Oral bacteria affect the gut microbiome and intestinal immunity. Pathog. Dis. 2020, 78, ftaa024. [Google Scholar] [CrossRef]
- Shoemark, D.K.; Allen, S.J. The Microbiome and Disease: Reviewing the Links Between the Oral Microbiome, Aging, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 43, 725–738. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed]
- Cha, G.S.; Kim, H.-J.; Kwon, E.-Y.; Lee, J.-Y.; Choi, J.; Joo, J.-Y. Porphyromonas gingivalis accelerates atherosclerosis through oxidation of high-density lipoprotein. J. Periodontal Implant. Sci. 2018, 48, 60–68. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Aburaya, S.; Sugiyama, N.; Narukawa, Y.; Sakamoto, Y.; Takahashi, M.; Uemura, H.; Yamashita, R.; Tominaga, S.; Hayashi, S.; et al. Porphyromonas gingivalis induces entero-hepatic metabolic derangements with alteration of gut microbiota in a type 2 diabetes mouse model. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Hanel, A.N.; Herzog, H.M.; James, M.G.; Cuadra, G.A. Effects of Oral Commensal Streptococci on Porphyromonas gingivalis Invasion into Oral Epithelial Cells. Dent. J. 2020, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Uebanso, T.; Kano, S.; Yoshimoto, A.; Naito, C.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of Consuming Xylitol on Gut Microbiota and Lipid Metabolism in Mice. Nutrients 2017, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Kikuko, A.; Arai, H.; Takashi, U.; Fukaya, M.; Koganei, M.; Sasaki, H.; Yamamoto, H.; Taketani, Y.; Takeda, E. Effects of xylitol on metabolic parameters and visceral fat accumulation. J. Clin. Biochem. Nutr. 2011, 49, 1–7. [Google Scholar] [CrossRef]
- Han, S.-J.; Jeong, S.-Y.; Nam, Y.-J.; Yang, K.-H.; Lim, H.-S.; Chung, J. Xylitol Inhibits Inflammatory Cytokine Expression Induced by Lipopolysaccharide from Porphyromonas gingivalis. Clin. Vaccine Immunol. 2005, 12, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.L.; Westover, J.; Ferrar, G.; Bleher, R. Anti-viral Potential of nasal spray constituents against SARS-CoV-2. bioRxiv 2020. bioRxiv: 2020.12.02.408575. [Google Scholar]
- Xu, M.L.; Wi, G.R.; Kim, H.J. Ameliorating Effect of Dietary Xylitol on Human Respiratory Syncytial Virus (hRSV) Infection. Biol. Pharm. Bull. 2016, 39, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.Y.; Kim, H.J. Protective Effect of Dietary Xylitol on Influenza A Virus Infection. PLoS ONE 2014, 9, e84633. [Google Scholar] [CrossRef][Green Version]
- Jones, A.H. Xylitol Compositions for Treating Upper Respiratory Conditions. WO 99/48361, 30 September 1999. [Google Scholar]
- Kaur, K.; Chauhan, V.; Gu, F.; Chauhan, A. Bisphenol A induces oxidative stress and mitochondrial dysfunction in lymphoblasts from children with autism and unaffected siblings. Free. Radic. Biol. Med. 2014, 76, 25–33. [Google Scholar] [CrossRef]
- Morimoto, M.; Hashimoto, T.; Tsuda, Y.; Nakatsu, T.; Kitaoka, T.; Kyotani, S. Assessment of oxidative stress in autism spectrum disorder using reactive oxygen metabolites and biological antioxidant potential. PLoS ONE 2020, 15, e0233550. [Google Scholar] [CrossRef]
- Olsen, I.; Hicks, S.D. Oral microbiota and autism spectrum disorder (ASD). J. Oral Microbiol. 2020, 12, 1702806. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.X.; Henders, A.K.; Alvares, G.A.; Wood, D.L.; Krause, L.; Tyson, G.W.; Restuadi, R.; Wallace, L.; McLaren, T.; Hansell, N.K.; et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 2021, 184, 5916–5931.e17. [Google Scholar] [CrossRef]
- Feng, P.; Zhao, S.; Zhang, Y.; Li, E. A review of probiotics in the treatment of autism spectrum disorders: Perspectives from the gut–brain axis. Front. Microbiol. 2023, 14, 1123462. [Google Scholar] [CrossRef]
- Fernandez-Feo, M.; Wei, G.; Blumenkranz, G.; Dewhirst, F.; Schuppan, D.; Oppenheim, F.; Helmerhorst, E. The cultivable human oral gluten-degrading microbiome and its potential implications in coeliac disease and gluten sensitivity. Clin. Microbiol. Infect. 2013, 19, E386–E394. [Google Scholar] [CrossRef] [PubMed]
- Zamakhchari, M.; Wei, G.; Dewhirst, F.; Lee, J.; Schuppan, D.; Oppenheim, F.G.; Helmerhorst, E.J. Identification of Rothia Bacteria as Gluten-Degrading Natural Colonizers of the Upper Gastro-Intestinal Tract. PLoS ONE 2011, 6, e24455. [Google Scholar] [CrossRef] [PubMed]
- Caminero, A.; Nistal, E.; Herrán, A.R.; Pérez-Andrés, J.; Ferrero, M.A.; Ayala, L.V.; Vivas, S.; de Morales, J.M.G.R.; Albillos, S.M.; Casqueiro, F.J. Differences in gluten metabolism among healthy volunteers, coeliac disease patients and first-degree relatives. Br. J. Nutr. 2015, 114, 1157–1167. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; German, J.B.; Slupsky, C.M.; et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLOS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Yang, J.-J.; Zhao, D.-M.; Chen, B.; Zhang, G.-Q.; Chen, S.; Cao, R.-F.; Yu, H.; Zhao, C.-Y.; et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol. Res. 2020, 157, 104784. [Google Scholar] [CrossRef]
- Duque, A.L.R.F.; Demarqui, F.M.; Santoni, M.M.; Zanelli, C.F.; Adorno, M.A.T.; Milenkovic, D.; Mesa, V.; Sivieri, K. Effect of probiotic, prebiotic, and synbiotic on the gut microbiota of autistic children using an in vitro gut microbiome model. Food Res. Int. 2021, 149, 110657. [Google Scholar] [CrossRef]
- Schmitt, L.M.; Smith, E.G.; Pedapati, E.V.; Horn, P.S.; Will, M.; Lamy, M.; Barber, L.; Trebley, J.; Meyer, K.; Heiman, M.; et al. Results of a phase Ib study of SB-121, an investigational probiotic formulation, a randomized controlled trial in participants with autism spectrum disorder. Sci. Rep. 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Vargason, T.; Santiago, M.; Hahn, J.; Krajmalnik-Brown, R. Distinct Fecal and Plasma Metabolites in Children with Autism Spectrum Disorders and Their Modulation after Microbiota Transfer Therapy. mSphere 2020, 5, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, B.; Kokhaei, P.; Mehranfar, F.; Bahar, A.; Abdolshahi, A.; Emadi, A.; Eslami, M. The role of the host microbiome in autism and neurodegenerative disorders and effect of epigenetic procedures in the brain functions. Neurosci. Biobehav. Rev. 2021, 132, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Marzal, L.N.; Rojas-Velazquez, D.; Rigters, D.; Prince, N.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P.; Lopez-Rincon, A. A robust microbiome signature for autism spectrum disorder across different studies using machine learning. Sci. Rep. 2024, 14, 814. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Uhlig, R.; Afshari, P.; Williams, J.; Chroneos, M.; Tierney-Aves, C.; Wagner, K.; Middleton, F.A. Oral microbiome activity in children with autism spectrum disorder. Autism Res. 2018, 11, 1286–1299. [Google Scholar] [CrossRef]
- Ling, Z.; Cheng, Y.; Liu, X.; Yan, X.; Wu, L.; Shao, L.; Gao, J.; Lei, W.; Song, Q.; Zhao, L.; et al. Altered oral microbiota and immune dysfunction in Chinese elderly patients with schizophrenia: A cross-sectional study. Transl. Psychiatry 2023, 13, 383. [Google Scholar] [CrossRef]
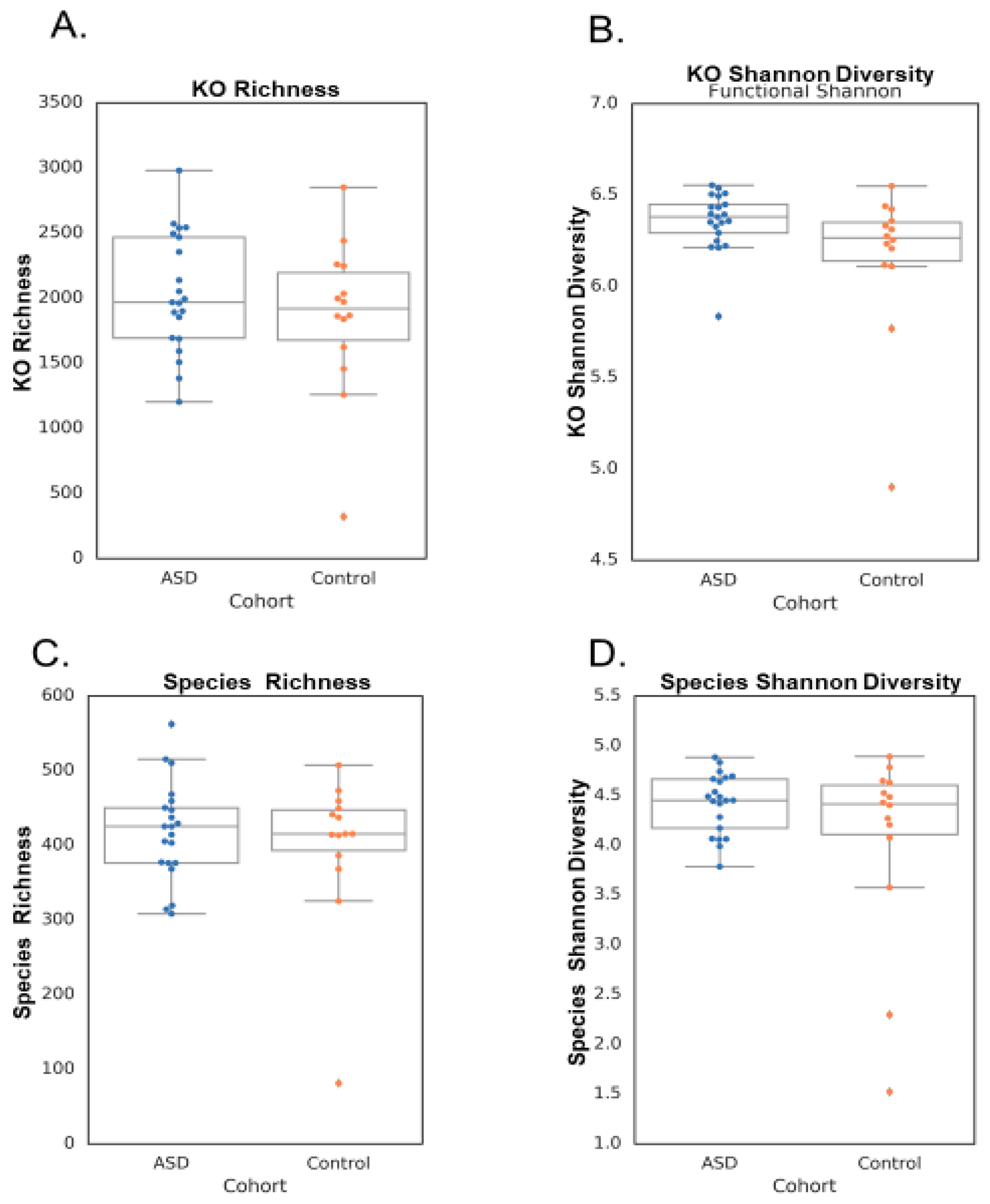


| Metric | Test p Value | ASD Mean +/− Std | Control Mean +/− Std |
|---|---|---|---|
| Microbial Richness | 0.491677 | 418.43 +/− 65.60 | 398.79 +/− 101.95 |
| Functional Richness | 0.32413 | 2035.14 +/− 453.53 | 1857.29 +/− 597.31 |
| Microbial Shannon | 0.113801 | 4.42 +/− 0.30 | 4.05 +/− 0.98 |
| Functional Shannon | 0.056327 | 6.36 +/− 0.16 | 6.16 +/− 0.41 |
| Subjects | ETC Complex I Defect | ETC Complex IV Defect |
|---|---|---|
| ASD children | 7 | 3 |
| Neurotypical controls | 4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannon, M.; Toma, R.; Ganeshan, S.; de Jesus Alvarez Varela, E.; Vuyisich, M.; Banavar, G. Salivary Transcriptome and Mitochondrial Analysis of Autism Spectrum Disorder Children Compared to Healthy Controls. NeuroSci 2024, 5, 276-290. https://doi.org/10.3390/neurosci5030022
Cannon M, Toma R, Ganeshan S, de Jesus Alvarez Varela E, Vuyisich M, Banavar G. Salivary Transcriptome and Mitochondrial Analysis of Autism Spectrum Disorder Children Compared to Healthy Controls. NeuroSci. 2024; 5(3):276-290. https://doi.org/10.3390/neurosci5030022
Chicago/Turabian StyleCannon, Mark, Ryan Toma, Sri Ganeshan, Emmery de Jesus Alvarez Varela, Momchilo Vuyisich, and Guruduth Banavar. 2024. "Salivary Transcriptome and Mitochondrial Analysis of Autism Spectrum Disorder Children Compared to Healthy Controls" NeuroSci 5, no. 3: 276-290. https://doi.org/10.3390/neurosci5030022
APA StyleCannon, M., Toma, R., Ganeshan, S., de Jesus Alvarez Varela, E., Vuyisich, M., & Banavar, G. (2024). Salivary Transcriptome and Mitochondrial Analysis of Autism Spectrum Disorder Children Compared to Healthy Controls. NeuroSci, 5(3), 276-290. https://doi.org/10.3390/neurosci5030022






