Intraoperative Fluorescein Sodium in Pediatric Neurosurgery: A Preliminary Case Series from a Singapore Children’s Hospital
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Outline of Operative Procedure and Perioperative Management
2.3. Patient Demographics, Radiological Features, and Variables of Interest
3. Results
3.1. Overview of Study Population and Its Characteristics
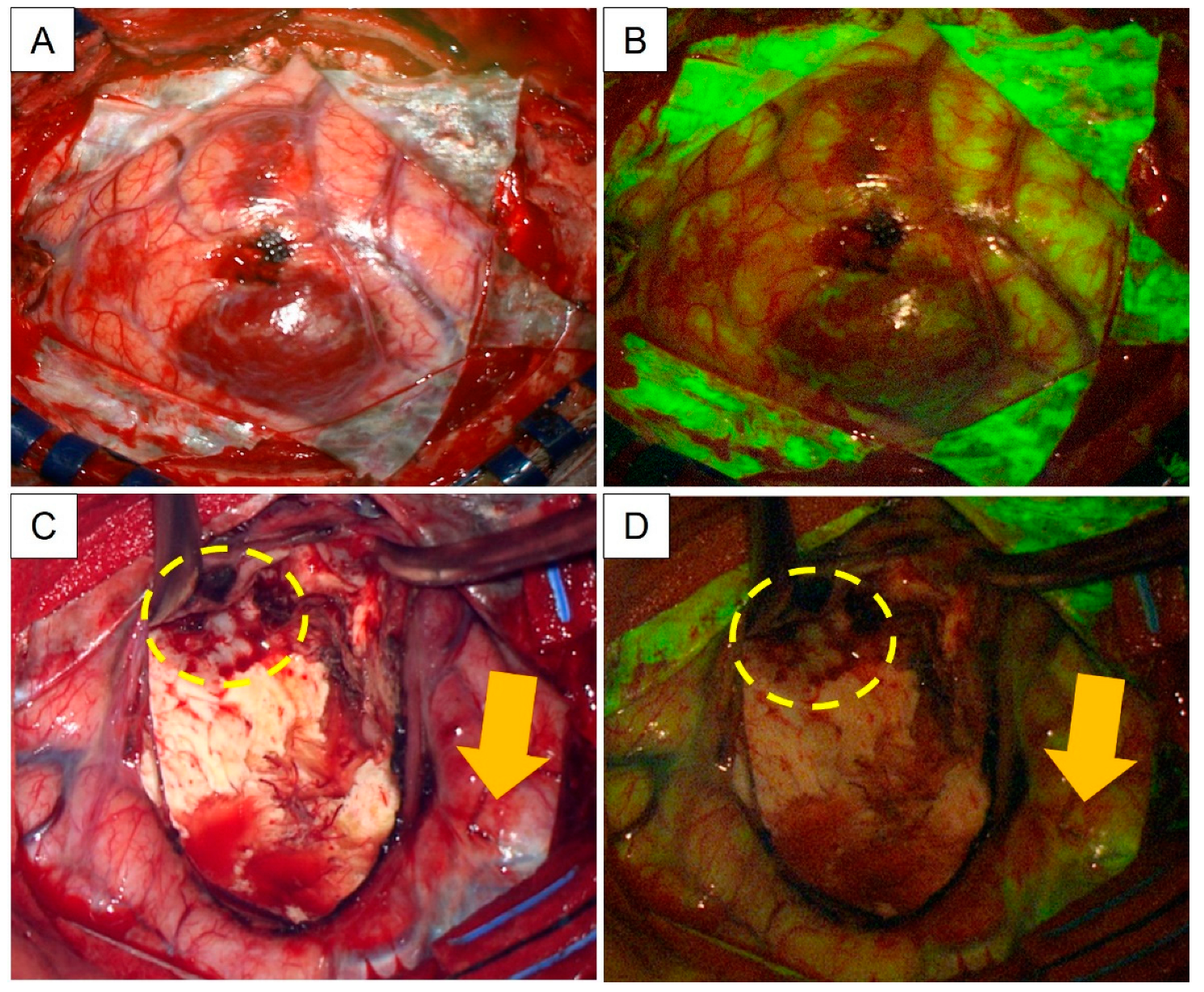
3.2. Evaluation of Na-Fl as an Intraoperative Adjunct
3.3. Illustrative Cases of Interest: Radiation-Induced Gliomas
4. Discussion
4.1. Surgery for Pediatric Brain Tumors: Technical Challenges
4.2. Fluorescent Dyes for Brain Tumor Surgery: An Overview
4.3. Use of Intraoperative Na-Fl: Institutional Reflections
4.4. Study Critique and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belykh, E.; Shaffer, K.V.; Lin, C.; Byvaltsev, V.A.; Preul, M.C.; Chen, L. Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 2020, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Widhalm, G.; Stummer, W. What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery 2015, 77, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Hohne, J.; Schebesch, K.M.; de Laurentis, C.; Akcakaya, M.O.; Pedersen, C.B.; Brawanski, A.; Poulsen, F.R.; Kiris, T.; Cavallo, C.; Broggi, M.; et al. Fluorescein Sodium in the Surgical Treatment of Recurrent Glioblastoma Multiforme. World Neurosurg. 2019, 125, e158–e164. [Google Scholar] [CrossRef] [PubMed]
- Falco, J.; Cavallo, C.; Vetrano, I.G.; de Laurentis, C.; Siozos, L.; Schiariti, M.; Broggi, M.; Ferroli, P.; Acerbi, F. Fluorescein Application in Cranial and Spinal Tumors Enhancing at Preoperative MRI and Operated With a Dedicated Filter on the Surgical Microscope: Preliminary Results in 279 Patients Enrolled in the FLUOCERTUM Prospective Study. Front. Surg. 2019, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.J.; Gohil, K.; Thompson, C.M.; Naik, A.; Hassaneen, W. Fluorescein-Guided Resection of High Grade Gliomas: A Meta-Analysis. World Neurosurg. 2021, 155, 181–188.e7. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Schebesch, K.M.; Hohne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef]
- Erdman, C.M.; Christie, C.; Iqbal, M.O.; Mazzola, C.A.; Tomycz, L. The utilization of sodium fluorescein in pediatric brain stem gliomas: A case report and review of the literature. Childs Nerv. Syst. 2021, 37, 1753–1758. [Google Scholar] [CrossRef]
- Goker, B.; Kiris, T. Sodium fluorescein-guided brain tumor surgery under the YELLOW-560-nm surgical microscope filter in pediatric age group: Feasibility and preliminary results. Childs Nerv. Syst. 2019, 35, 429–435. [Google Scholar] [CrossRef]
- Almojuela, A.; Honey, C.M.; Gomez, A.; Hasen, M.; MacDonald, C.; Kazina, C.; Serletis, D. Using Fluorescein in the Resection of a Pediatric Posterior Fossa Tumor. Can. J. Neurol. Sci. 2020, 47, 578–580. [Google Scholar] [CrossRef]
- Xue, Z.; Kong, L.; Pan, C.C.; Wu, Z.; Zhang, J.T.; Zhang, L.W. Fluorescein-Guided Surgery for Pediatric Brainstem Gliomas: Preliminary Study and Technical Notes. J. Neurol. Surg. B Skull Base 2018, 79, S340–S346. [Google Scholar] [CrossRef]
- Singh, D.K.; Khan, K.A.; Singh, A.K.; Kaif, M.; Yadav, K.; Kumar Singh, R.; Ahmad, F. Fluorescein sodium fluorescence: Role in stereotactic brain biopsy. Br. J. Neurosurg. 2021, 1–4. [Google Scholar] [CrossRef]
- Catapano, G.; Sgulo, F.G.; Seneca, V.; Iorio, G.; de Notaris, M.; di Nuzzo, G. Fluorescein-assisted stereotactic needle biopsy of brain tumors: A single-center experience and systematic review. Neurosurg. Rev. 2019, 42, 309–318. [Google Scholar] [CrossRef]
- Nevzati, E.; Chatain, G.P.; Hoffman, J.; Kleinschmidt-DeMasters, B.K.; Lillehei, K.O.; Ormond, D.R. Reliability of fluorescein-assisted stereotactic brain biopsies in predicting conclusive tissue diagnosis. Acta Neurochir. 2020, 162, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Ellison, D.W.; Figarella-Branger, D.; Perry, A.; Reifenberger, G.; von Deimling, A. WHO Classification of Tumours of the Central Nervous System, 4th ed.; IARC: Lyon, France, 2016. [Google Scholar]
- WHO Classification of Tumours Editorial Board (Ed.) WHO Classification of Tumours, Central Nervous System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; p. 568. [Google Scholar]
- Barry, R.E.; Behrendt, W.A. Studies on the pharmacokinetics of fluorescein and its dilaurate ester under the conditions of the fluorescein dilaurate test. Arzneimittelforschung 1985, 35, 644–648. [Google Scholar] [PubMed]
- Hohne, J.; Acerbi, F.; Falco, J.; Akcakaya, M.O.; Schmidt, N.O.; Kiris, T.; de Laurentis, C.; Ferroli, P.; Broggi, M.; Schebesch, K.M. Lighting Up the Tumor-Fluorescein-Guided Resection of Gangliogliomas. J. Clin. Med. 2020, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Markosian, C.; Mazzola, C.A.; Tomycz, L.D. Sodium Fluorescein-Guided Gross Total Resection of Pediatric Exophytic Brainstem Glioma: 2-Dimensional Operative Video. Oper. Neurosurg. 2021, 20, E146–E147. [Google Scholar] [CrossRef]
- Packer, R.J. Brain tumors in children. Arch Neurol. 1999, 56, 421–425. [Google Scholar] [CrossRef]
- Dobrovoljac, M.; Hengartner, H.; Boltshauser, E.; Grotzer, M.A. Delay in the diagnosis of paediatric brain tumours. Eur. J. Pediatr. 2002, 161, 663–667. [Google Scholar] [CrossRef]
- Akshulakov, S.K.; Kerimbayev, T.T.; Biryuchkov, M.Y.; Urunbayev, Y.A.; Farhadi, D.S.; Byvaltsev, V.A. Current Trends for Improving Safety of Stereotactic Brain Biopsies: Advanced Optical Methods for Vessel Avoidance and Tumor Detection. Front. Oncol. 2019, 9, 947. [Google Scholar] [CrossRef]
- Gerard, I.J.; Kersten-Oertel, M.; Petrecca, K.; Sirhan, D.; Hall, J.A.; Collins, D.L. Brain shift in neuronavigation of brain tumors: A review. Med. Image Anal. 2017, 35, 403–420. [Google Scholar] [CrossRef]
- Giussani, C.; Trezza, A.; Ricciuti, V.; Di Cristofori, A.; Held, A.; Isella, V.; Massimino, M. Intraoperative MRI versus intraoperative ultrasound in pediatric brain tumor surgery: Is expensive better than cheap? A review of the literature. Childs Nerv. Syst. 2022, 38, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Boop, F.A.; Ruge, J. The use of 5-aminolevulinic acid in resection of pediatric brain tumors: A critical review. J. Neurooncol. 2019, 141, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.S. The fluorescein-guided technique. Neurosurg. Focus 2014, 36, E6. [Google Scholar] [CrossRef] [PubMed]
- Schebesch, K.M.; Brawanski, A.; Hohenberger, C.; Hohne, J. Fluorescein Sodium-Guided Surgery of Malignant Brain Tumors: History, Current Concepts, and Future Project. Turk Neurosurg. 2016, 26, 185–194. [Google Scholar] [CrossRef]
- Hollon, T.; Stummer, W.; Orringer, D.; Suero Molina, E. Surgical Adjuncts to Increase the Extent of Resection: Intraoperative MRI, Fluorescence, and Raman Histology. Neurosurg Clin. N. Am. 2019, 30, 65–74. [Google Scholar] [CrossRef]
- Bander, E.D.; Jones, S.H.; Pisapia, D.; Magge, R.; Fine, H.; Schwartz, T.H.; Ramakrishna, R. Tubular brain tumor biopsy improves diagnostic yield for subcortical lesions. J. Neurooncol. 2019, 141, 121–129. [Google Scholar] [CrossRef]
- Teng, C.W.; Huang, V.; Arguelles, G.R.; Zhou, C.; Cho, S.S.; Harmsen, S.; Lee, J.Y.K. Applications of indocyanine green in brain tumor surgery: Review of clinical evidence and emerging technologies. Neurosurg. Focus 2021, 50, E4. [Google Scholar] [CrossRef]
- Labuschagne, J.J. The Use of 5-Aminolevulinic Acid to Assist Gross Total Resection of Paediatric Posterior Fossa Tumours. Pediatr. Neurosurg. 2020, 55, 268–279. [Google Scholar] [CrossRef]
- Beez, T.; Sarikaya-Seiwert, S.; Steiger, H.J.; Hanggi, D. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of brain tumors in children--a technical report. Acta Neurochir. 2014, 156, 597–604. [Google Scholar] [CrossRef]
- Abdelhafeez, A.; Talbot, L.; Murphy, A.J.; Davidoff, A.M. Indocyanine Green-Guided Pediatric Tumor Resection: Approach, Utility, and Challenges. Front. Pediatr. 2021, 9, 689612. [Google Scholar] [CrossRef]
- Jiang, J.X.; Keating, J.J.; Jesus, E.M.; Judy, R.P.; Madajewski, B.; Venegas, O.; Okusanya, O.T.; Singhal, S. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 390–400. [Google Scholar] [PubMed]
- Kwiterovich, K.A.; Maguire, M.G.; Murphy, R.P.; Schachat, A.P.; Bressler, N.M.; Bressler, S.B.; Fine, S.L. Frequency of adverse systemic reactions after fluorescein angiography. Results of a prospective study. Ophthalmology 1991, 98, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.H.; Muqit, M.M.; Mordant, D.J.; Smith, L.M.; Patel, C.K. Noncontact high-resolution ultra-wide-field oral fluorescein angiography in premature infants with retinopathy of prematurity. JAMA Ophthalmol. 2014, 132, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-Gadol, A.A. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar] [CrossRef]
- Shinoda, J.; Yano, H.; Yoshimura, S.; Okumura, A.; Kaku, Y.; Iwama, T.; Sakai, N. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Technical note. J. Neurosurg. 2003, 99, 597–603. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Eoli, M.; Anghileri, E.; Cavallo, C.; Boffano, C.; Cordella, R.; Cuppini, L.; Pollo, B.; Schiariti, M.; et al. Is fluorescein-guided technique able to help in resection of high-grade gliomas? Neurosurg. Focus 2014, 36, E5. [Google Scholar] [CrossRef]
- Acerbi, F.; Cavallo, C.; Broggi, M.; Cordella, R.; Anghileri, E.; Eoli, M.; Schiariti, M.; Broggi, G.; Ferroli, P. Fluorescein-guided surgery for malignant gliomas: A review. Neurosurg. Rev. 2014, 37, 547–557. [Google Scholar] [CrossRef]
- Catapano, G.; Sgulo, F.G.; Seneca, V.; Lepore, G.; Columbano, L.; di Nuzzo, G. Fluorescein-Guided Surgery for High-Grade Glioma Resection: An Intraoperative “Contrast-Enhancer”. World Neurosurg. 2017, 104, 239–247. [Google Scholar] [CrossRef]
- Craig, S.E.L.; Wright, J.; Sloan, A.E.; Brady-Kalnay, S.M. Fluorescent-Guided Surgical Resection of Glioma with Targeted Molecular Imaging Agents: A Literature Review. World Neurosurg. 2016, 90, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rey-Dios, R.; Roberts, D.W.; Valdes, P.A.; Cohen-Gadol, A.A. Intraoperative fluorescence-guided resection of high-grade gliomas: A comparison of the present techniques and evolution of future strategies. World Neurosurg. 2014, 82, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Meza, D.; Sanai, N. Trends in fluorescence image-guided surgery for gliomas. Neurosurgery 2014, 75, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, X.; Zhu, X.; Wu, L.; Ma, S.; Yan, J.; Yan, D. Diffusion Tensor Imaging with Fluorescein Sodium Staining in the Resection of High-Grade Gliomas in Functional Brain Areas. World Neurosurg. 2019, 124, e595–e603. [Google Scholar] [CrossRef] [PubMed]
- Restelli, F.; Bonomo, G.; Monti, E.; Broggi, G.; Acerbi, F.; Broggi, M. Safeness of sodium fluorescein administration in neurosurgery: Case-report of an erroneous very high-dose administration and review of the literature. Brain Spine 2022, 2, 101703. [Google Scholar] [CrossRef]
- Dilek, O.; Ihsan, A.; Tulay, H. Anaphylactic reaction after fluorescein sodium administration during intracranial surgery. J. Clin. Neurosci. 2011, 18, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Moosbrugger, K.A.; Sheidow, T.G. Evaluation of the side effects and image quality during fluorescein angiography comparing 2 mL and 5 mL sodium fluorescein. Can J Ophthalmol 2008, 43, 571–575. [Google Scholar] [CrossRef]
- Tan, V.A.; Gerez, I.F.; Van Bever, H.P. Prevalence of drug allergy in Singaporean children. Singap. Med. J. 2009, 50, 1158–1161. [Google Scholar]
- Goh, D.Y.; Chew, F.T.; Quek, S.C.; Lee, B.W. Prevalence and severity of asthma, rhinitis, and eczema in Singapore schoolchildren. Arch. Dis. Child. 1996, 74, 131–135. [Google Scholar] [CrossRef]
- O’Goshi, K.; Serup, J. Safety of sodium fluorescein for in vivo study of skin. Skin Res. Technol. 2006, 12, 155–161. [Google Scholar] [CrossRef]
- Kwan, A.S.; Barry, C.; McAllister, I.L.; Constable, I. Fluorescein angiography and adverse drug reactions revisited: The Lions Eye experience. Clin. Exp. Ophthalmol. 2006, 34, 33–38. [Google Scholar] [CrossRef]
- De Laurentis, C.; Bteich, F.; Beuriat, P.A.; Almeida, L.C.A.; Combet, S.; Mottolese, C.; Vinchon, M.; Szathmari, A.; Di Rocco, F. Sodium fluorescein in pediatric oncological neurosurgery: A pilot study on 50 children. Childs Nerv. Syst. 2022. [Google Scholar] [CrossRef]
- Xue, Z.; Kong, L.; Hao, S.; Wang, Y.; Jia, G.; Wu, Z.; Jia, W.; Zhang, J.; Zhang, L. Combined Application of Sodium Fluorescein and Neuronavigation Techniques in the Resection of Brain Gliomas. Front. Neurol. 2021, 12, 747072. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value (%) |
|---|---|
| Total number of patients | 21 (100) |
| Age (years) | 12.1 ± 5.3 (mean ± SD) |
| Gender | |
| Male | 13 (61.9) |
| Female | 8 (38.1) |
| Type of surgery | |
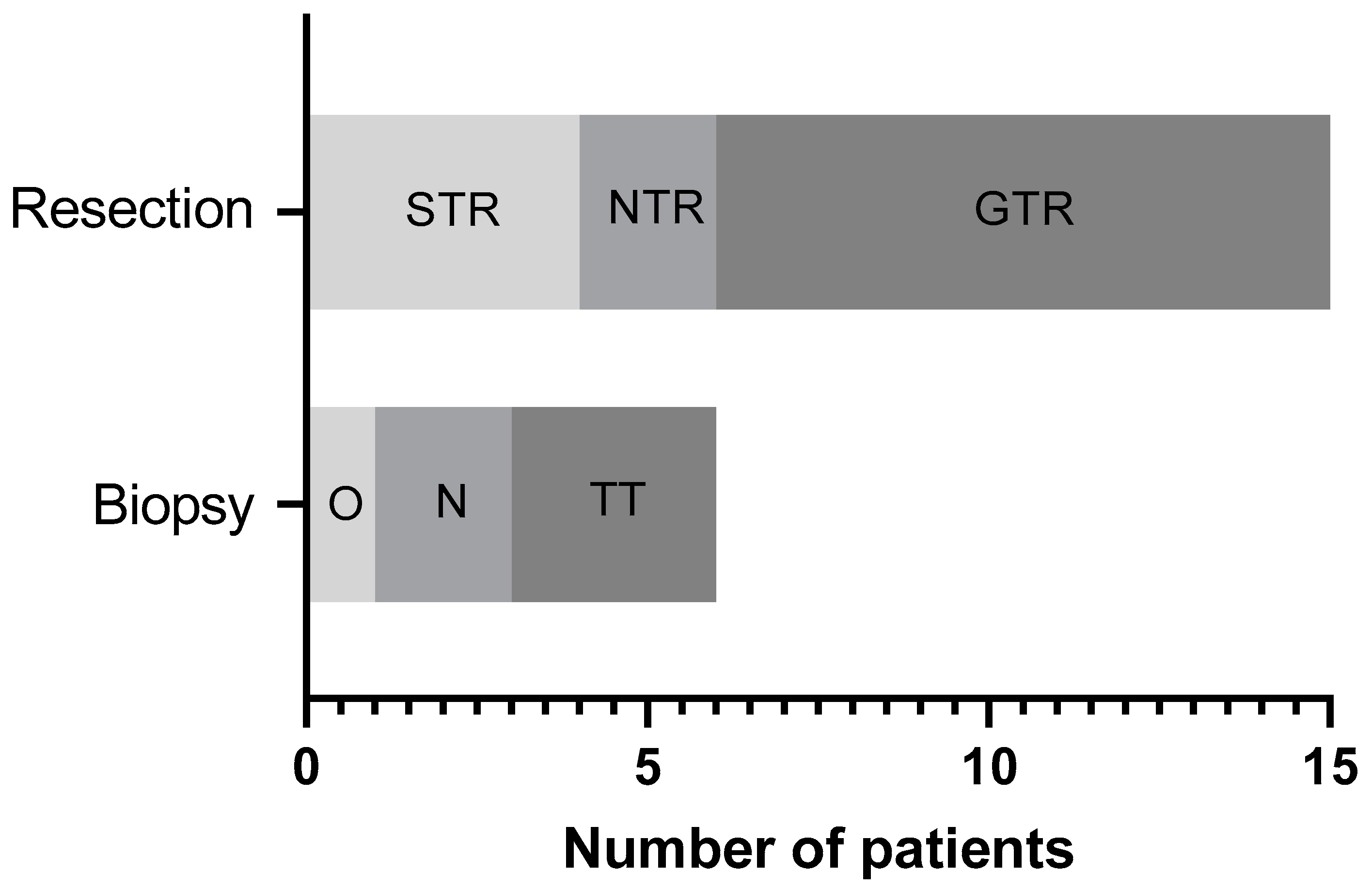
| Biopsy | 6 (28.6) |
| Resection | 15 (71.4) |
| Histopathological diagnosis 1 | |
| Non-neoplastic lesion (cavernoma) | 1 (4.8) |
| Neoplastic lesions | 20 (95.2) |
| Low grade glioma | 6 (28.6) |
| Hemispheric high grade glioma | 6 (28.6) |
| Medulloblastoma | 2 (9.5) |
| Diffuse midline glioma (H3K27M-altered)—1 thalamus and 2 brainstem | 3 (14.3) |
| Craniopharyngioma | 1 (4.8) |
| Choroid plexus carcinoma | 1 (4.8) |
| Primary intracranial malignant melanoma | 1 (4.8) |
| Adverse side effects from Na-Fl | |
| Yes | 0 (0) |
| No | 21 (100) |
| Score for Na-Fl fluorescence | |
| 0 | 3 (14.3) |
| 1 | 0 (0) |
| 2 | 5 (23.8) |
| 3 | 13 (61.9) |
| Concurrent operative adjuncts | |
| DTI imaging | 13 (61.9) |
| iMRI operating theatre | 1 (4.8) |
| IONM | 3 (14.3) |
| Transtubular system | 3 (14.3) |
| NIL | 6 (28.6) |
| Location of lesion | |
| Suprasellar | 2 (9.5) |
| Frontal | 2 (9.5) |
| Temporal | 1 (4.8) |
| Parietal | 4 (19.1) |
| Occipital | 1 (4.8) |
| Thalamic | 4 (19.1) |
| Intraventricular (frontal horn) | 1 (4.8) |
| Posterior fossa | 4 (19.1) |
| Brainstem | 2 (9.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, A.J.L.; Tey, M.L.; Seow, W.T.; Low, D.C.Y.; Chang, K.T.E.; Ng, L.P.; Looi, W.S.; Wong, R.X.; Tan, E.E.K.; Low, S.Y.Y. Intraoperative Fluorescein Sodium in Pediatric Neurosurgery: A Preliminary Case Series from a Singapore Children’s Hospital. NeuroSci 2023, 4, 54-64. https://doi.org/10.3390/neurosci4010007
Tan AJL, Tey ML, Seow WT, Low DCY, Chang KTE, Ng LP, Looi WS, Wong RX, Tan EEK, Low SYY. Intraoperative Fluorescein Sodium in Pediatric Neurosurgery: A Preliminary Case Series from a Singapore Children’s Hospital. NeuroSci. 2023; 4(1):54-64. https://doi.org/10.3390/neurosci4010007
Chicago/Turabian StyleTan, Audrey J. L., Min Li Tey, Wan Tew Seow, David C. Y. Low, Kenneth T. E. Chang, Lee Ping Ng, Wen Shen Looi, Ru Xin Wong, Enrica E. K. Tan, and Sharon Y. Y. Low. 2023. "Intraoperative Fluorescein Sodium in Pediatric Neurosurgery: A Preliminary Case Series from a Singapore Children’s Hospital" NeuroSci 4, no. 1: 54-64. https://doi.org/10.3390/neurosci4010007
APA StyleTan, A. J. L., Tey, M. L., Seow, W. T., Low, D. C. Y., Chang, K. T. E., Ng, L. P., Looi, W. S., Wong, R. X., Tan, E. E. K., & Low, S. Y. Y. (2023). Intraoperative Fluorescein Sodium in Pediatric Neurosurgery: A Preliminary Case Series from a Singapore Children’s Hospital. NeuroSci, 4(1), 54-64. https://doi.org/10.3390/neurosci4010007






