Investigation of the Neuroprotective Action of Japanese Sake Yeast on Dementia Type of Alzheimer Disease in Rats: Behavioral and Neurobiochemical Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Surgery and Drug Administration
2.4. Behavioral Tests
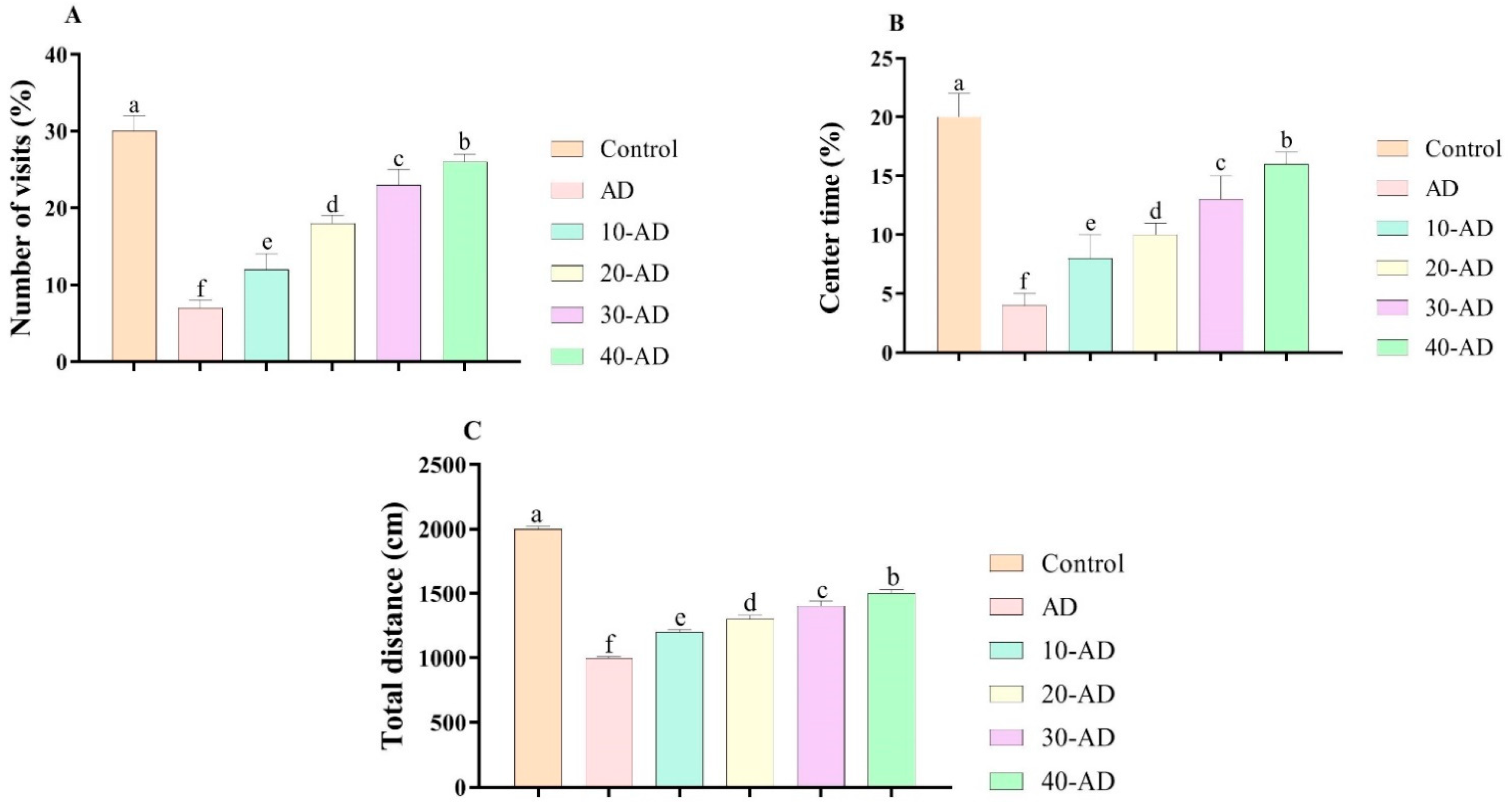
2.4.1. Open-Field Test (OFT)
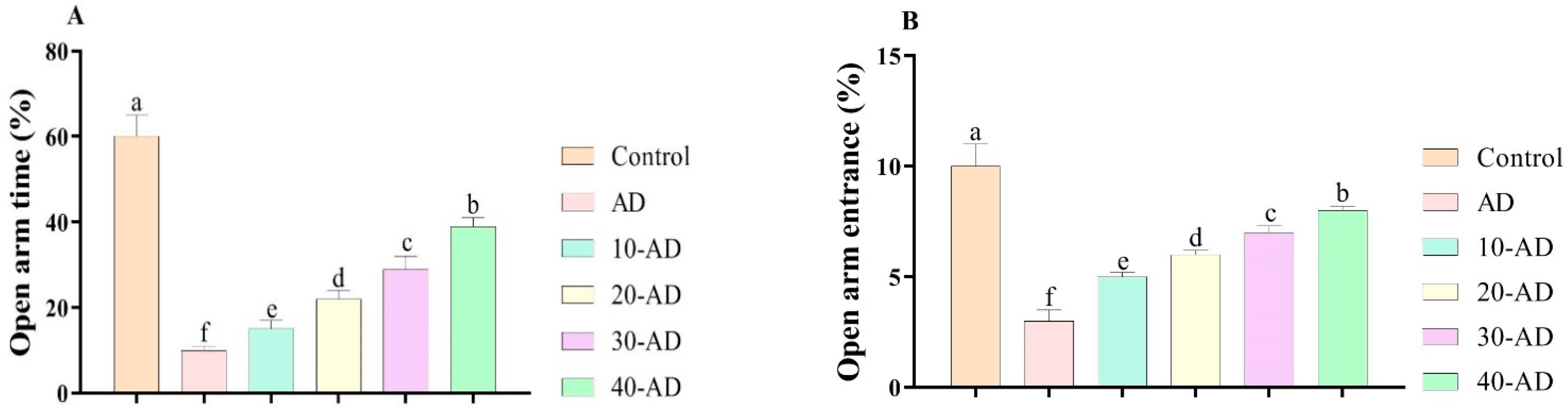
2.4.2. Elevated Plus Maze (EPM)
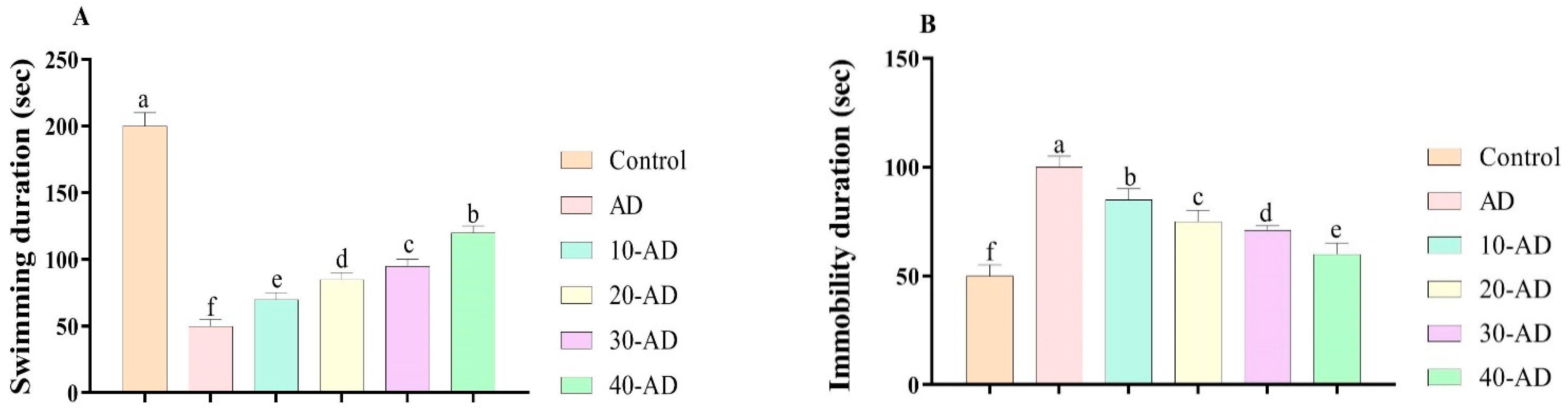
2.4.3. Force Swimming Test (FST)
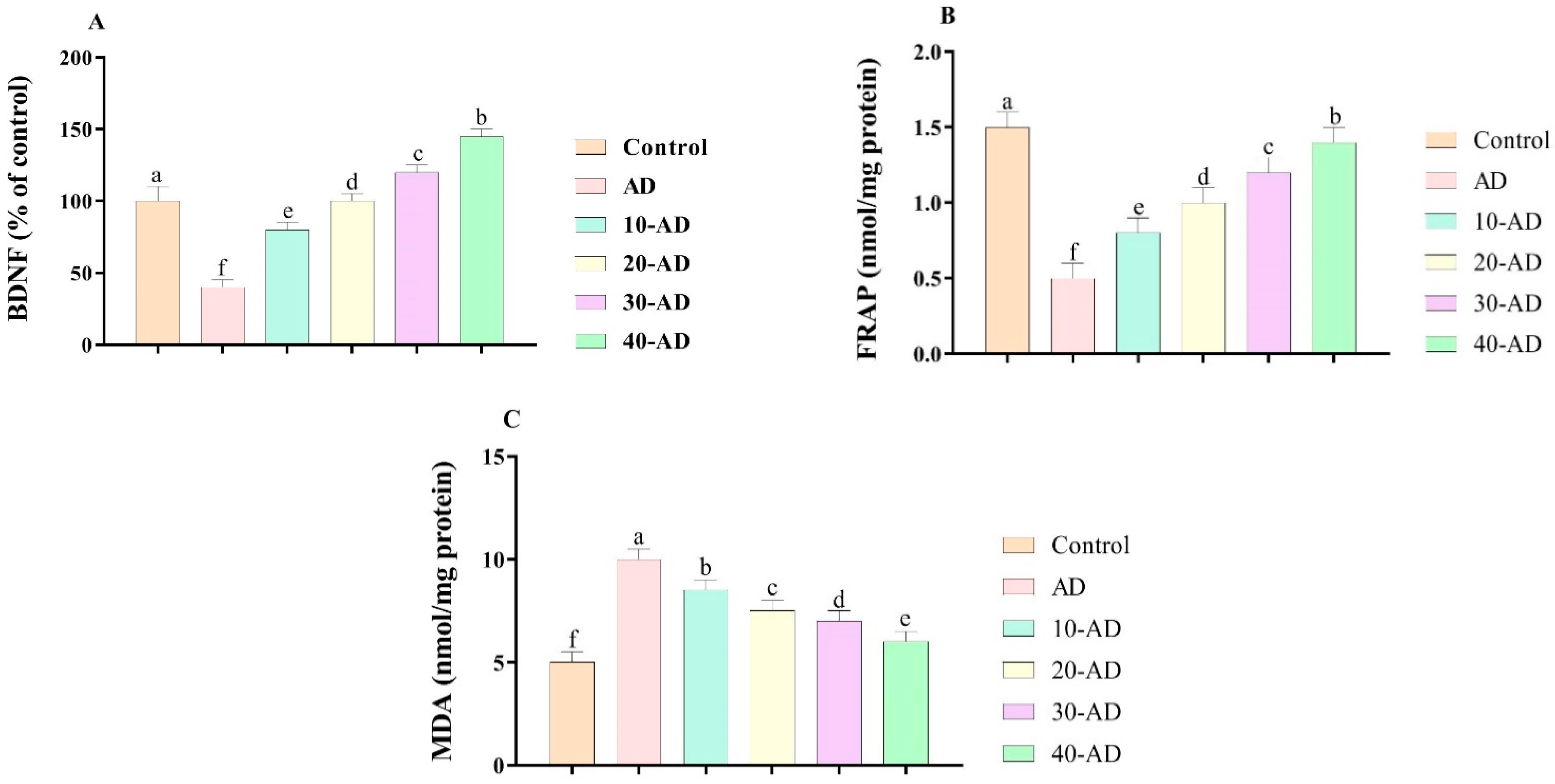
2.5. The Assessment of BDNF and Antioxidant-Associated Factors
2.6. The Expression of Inflammatory Factors
2.7. Data Analysis
3. Results
3.1. Anxiety-like Behaviors
3.2. Depression-like Behaviors
3.3. Biochemical Parameters
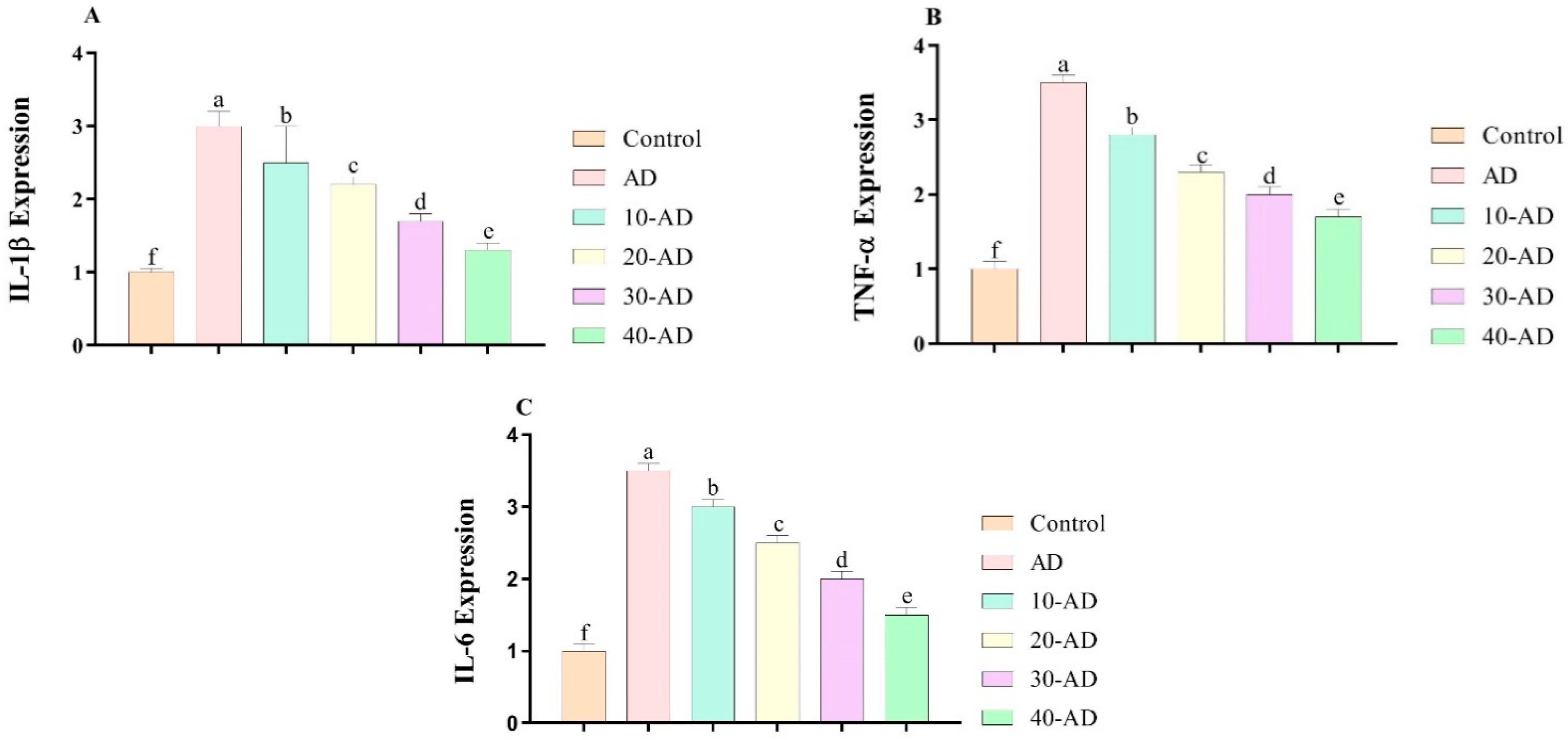
3.4. The Expression of Inflammatory Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2022, 10, 512–536. [Google Scholar] [CrossRef] [PubMed]
- Zucchella, C.; Sinforiani, E.; Tamburin, S.; Federico, A.; Mantovani, E.; Bernini, S.; Casale, R.; Bartolo, M. The multidisciplinary approach to Alzheimer’s disease and dementia. A narrative review of non-pharmacological treatment. Front. Neurol. 2018, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Rosness, T.A.; Engedal, K.; Chemali, Z. Frontotemporal dementia: An updated clinician’s guide. J. Geriatr. Psychiatry Neurol. 2016, 29, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Spina, S.; Miller, B.L. Frontotemporal dementia. Lancet 2015, 386, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.; Rios, C.L.O.; Lahmann, C.; Ruecker, G.; Bauer, J.; Boeker, M. Anxiety as a risk factor of Alzheimer’s disease and vascular dementia. Br. J. Psychiatry 2018, 213, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.-M. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s disease (AD): A systematic review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef]
- Wojsiat, J.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. Oxidant/antioxidant imbalance in Alzheimer’s disease: Therapeutic and diagnostic prospects. Oxidative Med. Cell. Longev. 2018, 2018, 6435861. [Google Scholar] [CrossRef]
- Darweesh, S.K.; Wolters, F.J.; Ikram, M.A.; de Wolf, F.; Bos, D.; Hofman, A. Inflammatory markers and the risk of dementia and Alzheimer’s disease: A meta-analysis. Alzheimer’s Dement. 2018, 14, 1450–1459. [Google Scholar] [CrossRef]
- Lott, I.T.; Head, E. Dementia in Down syndrome: Unique insights for Alzheimer disease research. Nat. Rev. Neurol. 2019, 15, 135–147. [Google Scholar] [CrossRef]
- Leblhuber, F.; Steiner, K.; Schuetz, B.; Fuchs, D.; Gostner, J.M. Probiotic supplementation in patients with Alzheimer’s dementia-an explorative intervention study. Curr. Alzheimer Res. 2018, 15, 1106–1113. [Google Scholar] [CrossRef]
- Davoodi, M.; Karimooy, F.N.; Budde, T.; Ortega-Martinez, S.; Moradi-Kor, N. Beneficial effects of Japanese sake yeast supplement on biochemical, antioxidant, and anti-inflammatory factors in streptozotocin-induced diabetic rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1667. [Google Scholar] [CrossRef] [PubMed]
- Haghipanah, M.; Saadat, M.; Safarbalou, A.; Budde, T.; Mohamed, W.; Afraz, E.S.; Moradikor, N. The effects of oral supplementation of Japanese sake yeast on anxiety, depressive-like symptoms, oxidative stress, and BDNF changes in chronically stressed adolescent rats. bioRxiv 2022. [Google Scholar] [CrossRef]
- Yamashita, S.; Hata, M.; Kikuchi, N.; Kinoshita, M.; Miyazawa, T. Effects of dietary ethanol extracts from sake rice and sake lees on intestinal impairment in mice. J. Oleo Sci. 2020, 69, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, S.; Sarkaki, A.; Farbood, Y.; Eidi, A.; Mortazavi, P.; Valizadeh, Z. Effect of gallic acid on dementia type of Alzheimer disease in rats: Electrophysiological and histological studies. Basic Clin. Neurosci. 2016, 7, 97. [Google Scholar] [CrossRef]
- Moradi-Kor, N.; Dadkhah, M.; Ghanbari, A.; Rashidipour, H.; Bandegi, A.R.; Barati, M.; Kokhaei, P.; Rashidy-Pour, A. Protective effects of spirulina platensis, voluntary exercise and environmental interventions against adolescent stress-induced anxiety and depressive-like symptoms, oxidative stress and alterations of BDNF and 5HT-3 receptors of the prefrontal cortex in female rats. Neuropsychiatr. Dis. Treat. 2020, 16, 1777. [Google Scholar]
- Borsini, F.; Meli, A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology 1988, 94, 147–160. [Google Scholar] [CrossRef]
- Zheng, X.-Y.; Mao, C.-Y.; Qiao, H.; Zhang, X.; Yu, L.; Wang, T.-Y.; Lu, E.-Y. Plumbagin suppresses chronic periodontitis in rats via down-regulation of TNF-α, IL-1β and IL-6 expression. Acta Pharmacol. Sin. 2017, 38, 1150–1160. [Google Scholar] [CrossRef]
- García-Alberca, J.M.; Lara, J.P.; Berthier, M.L. Anxiety and depression in caregivers are associated with patient and caregiver characteristics in Alzheimer’s disease. Int. J. Psychiatry Med. 2011, 41, 57–69. [Google Scholar] [CrossRef]
- Pietrzak, R.H.; Lim, Y.Y.; Neumeister, A.; Ames, D.; Ellis, K.A.; Harrington, K.; Lautenschlager, N.T.; Restrepo, C.; Martins, R.N.; Masters, C.L. Amyloid-β, anxiety, and cognitive decline in preclinical Alzheimer disease: A multicenter, prospective cohort study. JAMA Psychiatry 2015, 72, 284–291. [Google Scholar] [CrossRef]
- Salim, S.; Chugh, G.; Asghar, M. Inflammation in anxiety. Adv. Protein Chem. Struct. Biol. 2012, 88, 1–25. [Google Scholar]
- Lopresti, A.L.; Maker, G.L.; Hood, S.D.; Drummond, P.D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Antioxidant and anti-inflammatory mechanisms of neuroprotection by ursolic acid: Addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxidative Med. Cell. Longev. 2019, 2019, 8512048. [Google Scholar] [CrossRef] [PubMed]
- Manosso, L.M.; Camargo, A.; Dafre, A.L.; Rodrigues, A.L.S. Vitamin E for the management of major depressive disorder: Possible role of the anti-inflammatory and antioxidant systems. Nutr. Neurosci. 2022, 25, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Tanila, H. The role of BDNF in Alzheimer’s disease. Neurobiol. Dis. 2017, 97, 114–118. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.-K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Fanaei, H.; Karimian, S.M.; Sadeghipour, H.R.; Hassanzade, G.; Kasaeian, A.; Attari, F.; Khayat, S.; Ramezani, V.; Javadimehr, M. Testosterone enhances functional recovery after stroke through promotion of antioxidant defenses, BDNF levels and neurogenesis in male rats. Brain Res. 2014, 1558, 74–83. [Google Scholar] [CrossRef]
- Fahnestock, M.; Marchese, M.; Head, E.; Pop, V.; Michalski, B.; Milgram, W.N.; Cotman, C.W. BDNF increases with behavioral enrichment and an antioxidant diet in the aged dog. Neurobiol. Aging 2012, 33, 546–554. [Google Scholar] [CrossRef]
- Zhang, J.-C.; Yao, W.; Hashimoto, K. Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef]
- Goldstein, B.I.; Young, L.T. Toward clinically applicable biomarkers in bipolar disorder: Focus on BDNF, inflammatory markers, and endothelial function. Curr. Psychiatry Rep. 2013, 15, 425. [Google Scholar] [CrossRef]
- Giera, M.; Lingeman, H.; Niessen, W. Recent advancements in the LC-and GC-based analysis of malondialdehyde (MDA): A brief overview. Chromatographia 2012, 75, 433–440. [Google Scholar] [CrossRef]
- da Silva, S.L.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma nutrient status of patients with Alzheimer’s disease: Systematic review and meta-analysis. Alzheimer’s Dement. 2014, 10, 485–502. [Google Scholar] [CrossRef] [PubMed]
- A Kosenko, E.; Aliev, G.; A Tikhonova, L.; Li, Y.; C Poghosyan, A.; G Kaminsky, Y. Antioxidant status and energy state of erythrocytes in Alzheimer dementia: Probing for markers. CNS Neurol. Disord. Drug Targets 2012, 11, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A mini review. Oxidative Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef] [PubMed]
- Farahpour, M. Evaluation of the combined effect of St John’s wort hydroethanolic flower extract and flaxseed oil on skin wound healing-in rats. Vet. Clin. Pathol. Q. Sci. J. 2014, 8, 417–426. [Google Scholar]
- Wyss-Coray, T.; Rogers, J. Inflammation in Alzheimer disease—A brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012, 2, a006346. [Google Scholar] [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Farahpour, M.R.; Pirkhezr, E.; Ashrafian, A.; Sonboli, A. Accelerated healing by topical administration of Salvia officinalis essential oil on Pseudomonas aeruginosa and Staphylococcus aureus infected wound model. Biomed. Pharmacother. 2020, 128, 110120. [Google Scholar] [CrossRef]
- Pourkarim, R.; Farahpour, M.R.; Rezaei, S.A. Comparison effects of platelet-rich plasma on healing of infected and non-infected excision wounds by the modulation of the expression of inflammatory mediators: Experimental research. Eur. J. Trauma Emerg. Surg. 2022, 48, 3339–3347. [Google Scholar] [CrossRef]
- Osman, N.N.; Alsharari, M.A.; Alsufiani, H.M. Peppermint (Mentha piperita L.) and Thyme (Thymus vulgaris) attenuate the Immune and Inflammatory Disorders in Rats Consumed Repeatedly heated Palm oil. Int. J. Pharm. Phytopharm. Res. 2020, 10, 59–66. [Google Scholar]
- Modarresi, M.; Farahpour, M.-R.; Baradaran, B. Topical application of Mentha piperita essential oil accelerates wound healing in infected mice model. Inflammopharmacology 2019, 27, 531–537. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghipanah, M.; Ghalami, F.; Saadat, M.; Abbasi-Maleki, S.; Gholizadeh Salmani, R.H.; Budde, T.; Moradikor, N. Investigation of the Neuroprotective Action of Japanese Sake Yeast on Dementia Type of Alzheimer Disease in Rats: Behavioral and Neurobiochemical Assessment. NeuroSci 2023, 4, 45-53. https://doi.org/10.3390/neurosci4010006
Haghipanah M, Ghalami F, Saadat M, Abbasi-Maleki S, Gholizadeh Salmani RH, Budde T, Moradikor N. Investigation of the Neuroprotective Action of Japanese Sake Yeast on Dementia Type of Alzheimer Disease in Rats: Behavioral and Neurobiochemical Assessment. NeuroSci. 2023; 4(1):45-53. https://doi.org/10.3390/neurosci4010006
Chicago/Turabian StyleHaghipanah, Motahareh, Fatemeh Ghalami, Maryam Saadat, Saeid Abbasi-Maleki, Reza Hossein Gholizadeh Salmani, Thomas Budde, and Nasrollah Moradikor. 2023. "Investigation of the Neuroprotective Action of Japanese Sake Yeast on Dementia Type of Alzheimer Disease in Rats: Behavioral and Neurobiochemical Assessment" NeuroSci 4, no. 1: 45-53. https://doi.org/10.3390/neurosci4010006
APA StyleHaghipanah, M., Ghalami, F., Saadat, M., Abbasi-Maleki, S., Gholizadeh Salmani, R. H., Budde, T., & Moradikor, N. (2023). Investigation of the Neuroprotective Action of Japanese Sake Yeast on Dementia Type of Alzheimer Disease in Rats: Behavioral and Neurobiochemical Assessment. NeuroSci, 4(1), 45-53. https://doi.org/10.3390/neurosci4010006









