Chanwuyi Lifestyle Medicine Program Alleviates Immunological Deviation and Improves Behaviors in Autism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Intervention
2.4. Immunological Measures
2.5. Behavioral Measures
2.5.1. Social Communication and Stereotypic/Repetitive Behaviors
2.5.2. Hyperactivity Behaviors
2.6. Data Analyses
3. Results
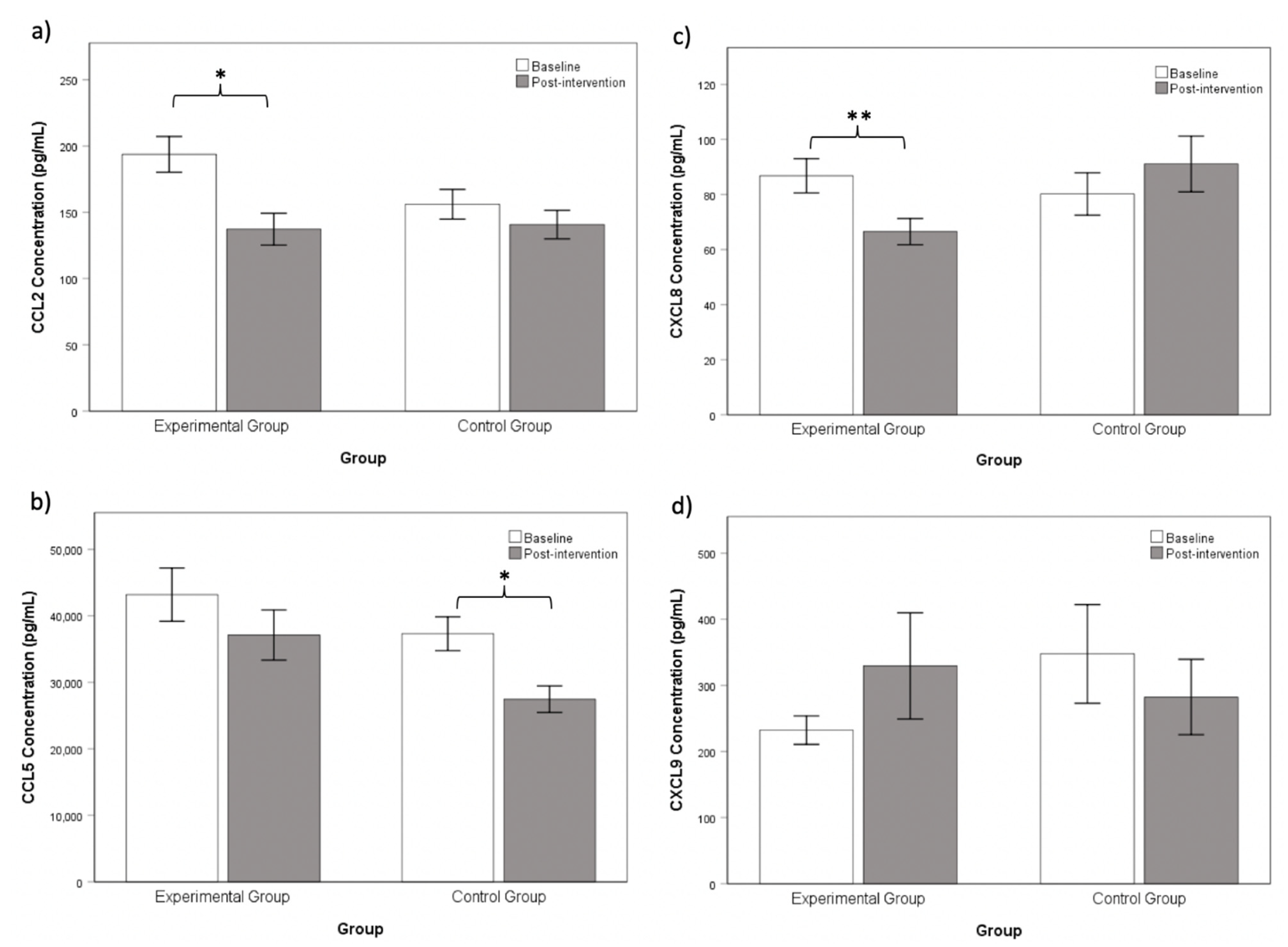
3.1. Chanwuyi Lifestyle Medicine Program Modulated Pro-Inflammatory Chemokines in ASD
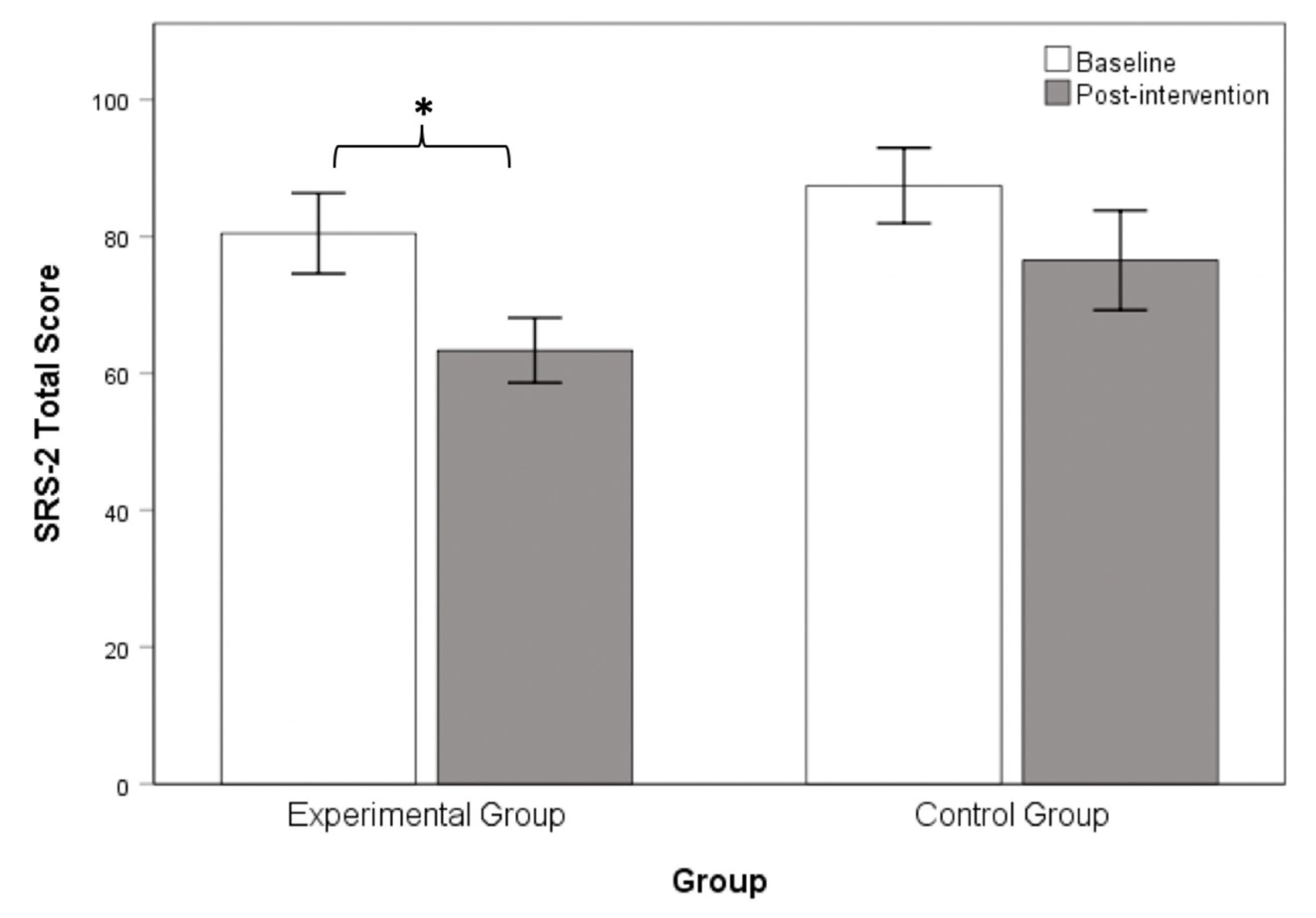
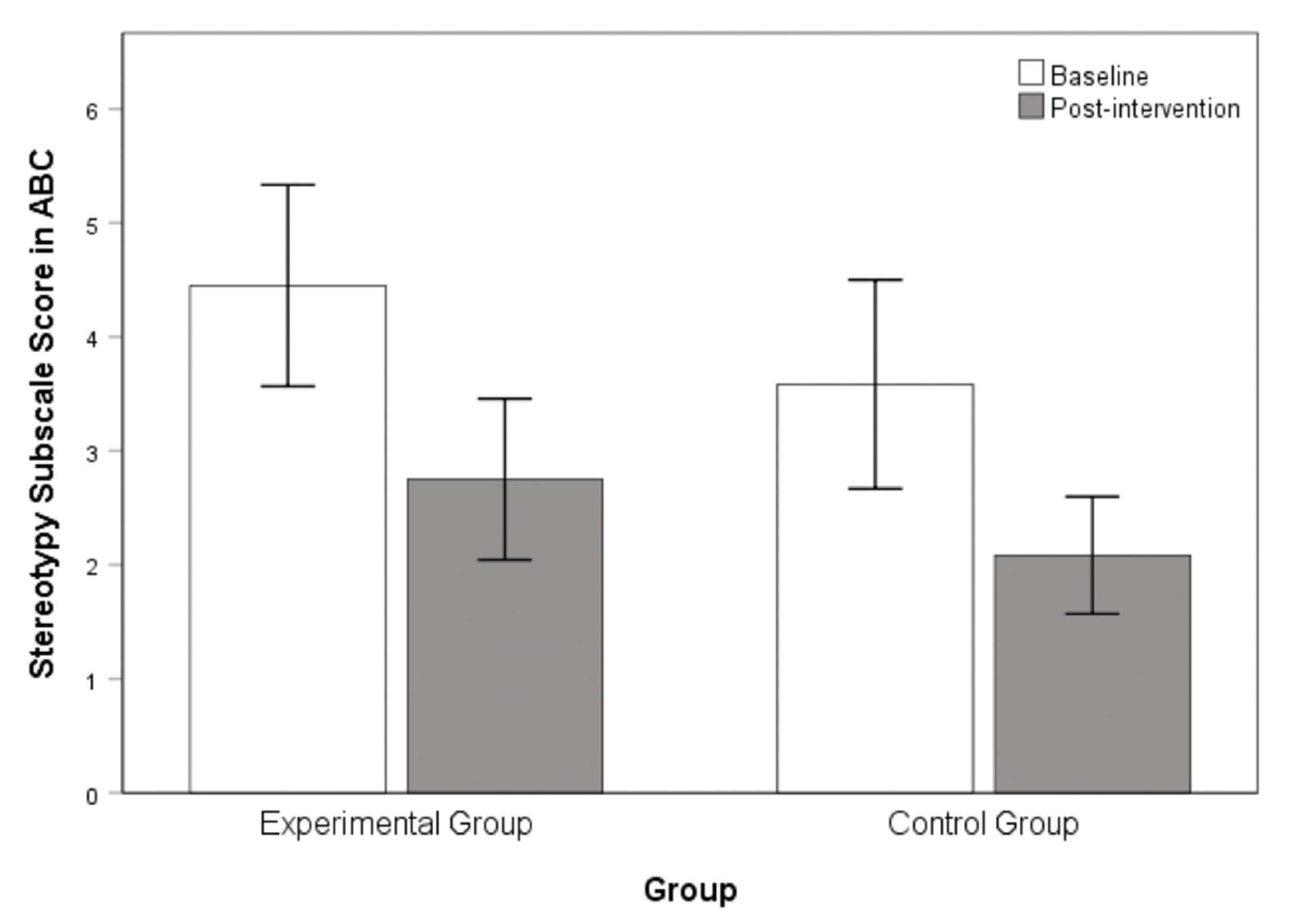
3.2. Chanwuyi Lifestyle Medicine Program Reduced Core Symptoms in ASD
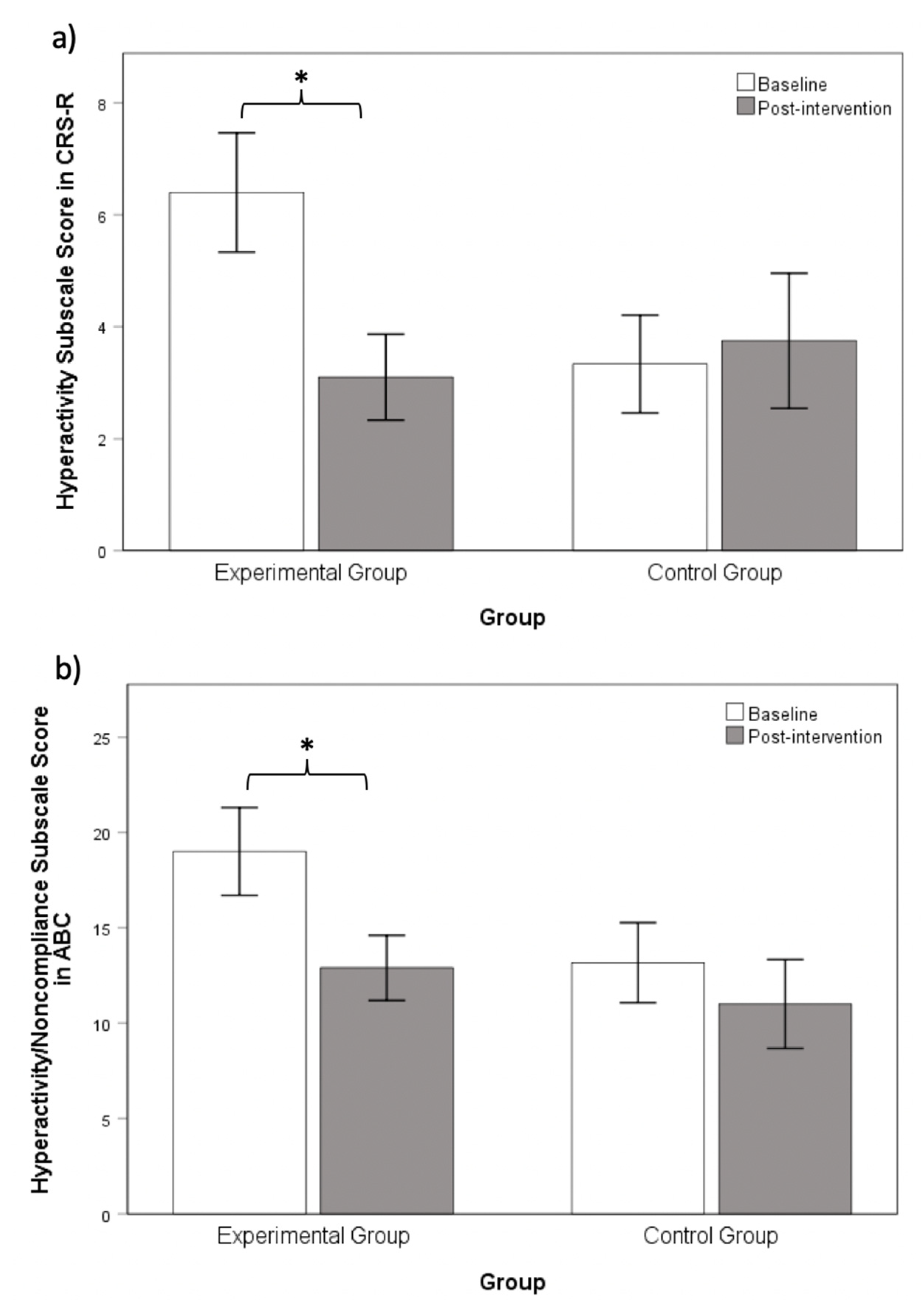
3.3. Chanwuyi Lifestyle Medicine Program Reduced Hyperactivity Behaviors in ASD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stubbs, E.G.; Crawford, M.L. Depressed lymphocyte responsiveness in autistic children. J. Autism Child. Schizophr. 1977, 7, 49–55. [Google Scholar] [CrossRef]
- Young, A.M.H.; Chakrabarti, B.; Roberts, D.; Lai, M.C.; Suckling, J.; Baron-Cohen, S. From molecules to neural morphology: Understanding neuroinflammation in autism spectrum condition. Mol. Autism 2016, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.M.; Cheung, W.K.; Wong, C.K.; Sze, S.L.; Cheng, T.W.; Yeung, M.K.; Chan, A.S. Distinct cytokine and chemokine profiles in autism spectrum disorders. Front. Immunol. 2017, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.M.; Ji, L.; Brown, T.; Malik, M. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.N.; Van de Water, J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J. Neuroimmunol. 2011, 32, 196–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korzeniewski, S.J.; Allred, E.N.; O’Shea, T.M.; Leviton, A.; Kuban, K.C. Elevated protein concentrations in newborn blood and the risks of autism spectrum disorder, and of social impairment, at age 10 years among infants born before the 28th week of gestation. Transl. Psychiatry 2018, 8, 115. [Google Scholar] [CrossRef]
- Leviton, A.; Joseph, R.M.; Allred, E.N.; Fichorova, R.N.; O’Shea, M.; Kuban, K.K.C.; Dammann, O. The risk of neurodevelopmental disorders at age 10 years associated with blood concentrations of interleukins 4 and 10 during the first postnatal month of children born extremely preterm. Cytokine 2018, 110, 181–188. [Google Scholar] [CrossRef]
- Shen, Y.; Ou, J.; Liu, M.; Shi, L.; Li, Y.; Xiao, L.; Dong, H.; Zhang, F.; Xia, K.; Zhao, J. Altered plasma levels of chemokines in autism and their association with social behaviors. Psychiatry Res. 2016, 244, 300–305. [Google Scholar] [CrossRef]
- Gupta, S. Immunological treatments for autism. J. Autism Dev. Disord. 2000, 30, 475–479. [Google Scholar] [CrossRef]
- Gupta, S.; Samra, D.; Agrawal, S. Adaptive and innate immune responses in autism: Rationale for therapeutic use of intravenous immunoglobulin. J. Clin. Immunol. 2010, 30, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y. Effects of water exercise swimming program on aquatic skills and social behaviors in children with autism spectrum disorder. Autism 2010, 14, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.O.; Dobbin, A.R.; Rose, G.D.; Soper, H.V. Vigorous, aerobic exercise versus general motor training activities: Effects on maladaptive and stereotypic behaviors of adults with both autism and mental retardation. J. Autism Dev. Disord. 1994, 24, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, I.; Yanardağ, M.; Birkan, B.; Bumin, G. Effects of swimming training on physical fitness and water orientation in autism. Pediatr. Int. 2004, 46, 624–626. [Google Scholar] [CrossRef]
- Powers, S.; Thibadeau, S.; Rose, K. Antecedent exercise and its effects on self-stimulation. Behav. Intervent. 1992, 7, 15–22. [Google Scholar] [CrossRef]
- Ashwood, P.; Van de Water, J. A review of autism and the immune response. Clin. Dev. Immunol. 2004, 11, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucarelli, S.; Frediani, T.; Zingoni, A.M.; Ferruzzi, F.; Giardini, O.; Quintieri, F.; Barbato, M.; D’Eufemia, P.; Cardi, E. Food allergy and infantile autism. Panminerva Med. 1995, 37, 137–141. [Google Scholar] [PubMed]
- American College of Lifestyle Medicine. Available online: https://lifestylemedicine.org (accessed on 21 November 2018).
- Ornish, D.; Scherwitz, L.W.; Billings, J.H.; Gould, K.L.; Merritt, T.A.; Sparler, S.; Armstrong, W.T.; Ports, T.A.; Kirkeeide, R.L.; Hogeboom, C.; et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998, 280, 2001–2007. [Google Scholar] [CrossRef]
- Ornish, D.; Magbanua, M.J.M.; Weidner, G.; Weinberg, V.; Kemp, C.; Green, C.; Mattie, M.D.; Marlin, R.; Simko, J.; Shinohara, K.; et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc. Natl. Acad. Sci. USA 2008, 105, 8369–8374. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Giovannucci, E. Preventable incidence and mortality of carcinoma associated with lifestyle factors among white adults in the United States. JAMA Oncol. 2016, 2, 1154–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galimanis, A.; Mono, M.L.; Arnold, M.; Nedeltchev, K.; Mattle, H.P. Lifestyle and stroke risk: A review. Curr. Opin. Neurol. 2009, 22, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Gow, M.L.; Baur, L.A.; Johnson, N.A.; Cowell, C.T.; Garnett, S.P. Reversal of type 2 diabetes in youth who adhere to a very-low-energy diet: A pilot study. Diabetologia 2017, 60, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.U.; Collins, C.E.R. Lifestyle Redesign® for chronic pain management: A retrospective clinical efficacy study. Am. J. Occup. Ther. 2017, 71, 7104190040. [Google Scholar] [CrossRef]
- Gardner, C.D.; Kiazand, A.; Alhassan, S.; Kim, S.; Stafford, R.S.; Balise, R.R.; Kraemer, H.C.; King, A.C. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: The A TO Z Weight Loss Study: A randomized trial. JAMA 2007, 297, 969–977. [Google Scholar] [CrossRef]
- Ornish, D.; Weidner, G.; Fair, W.R.; Marlin, R.; Pettengill, E.B.; Raisin, C.J.; Dunn-Emke, S.; Crutchfield, L.; Jacobs, F.N.; Barnard, R.J.; et al. Intensive lifestyle changes may affect the progression of prostate cancer. J. Urol. 2005, 174, 1065–1070. [Google Scholar] [CrossRef]
- Chan, A.S. The Shaolin Chanwuyi: A Chinese Chan Buddhism; Chanwuyi Publishing: Hong Kong, China, 2010. [Google Scholar]
- Chan, A.S. Contemporary Application of Shaolin Medicine: Dejian Mind- Body Intervention, 5th ed.; Chanwuyi Publishing: Hong Kong, China, 2013. [Google Scholar]
- Chan, A.S.; Cheung, M.C.; Tsui, W.J.; Sze, S.L.; Shi, D. Dejian mind-body intervention on depressive mood of community-dwelling adults: A randomized controlled trial. Evid.-Based Complement. Altern. Med. 2011, 2011, 473961. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.S.; Cheung, M.C.; Sze, S.L.; Leung, W.W.M.; Shi, D. Shaolin Dan Tian breathing fosters relaxed and attentive mind: A randomized controlled neuro-electrophysiological study. Evid.-Based Complement. Altern. Med. 2011, 2011, 180704. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Woo, J.; Chan, A.S.; Sze, S.L. A Chinese Chan-based mind–body intervention improves psychological well-being and physical health of community-dwelling elderly: A pilot study. Clin. Interv. Aging 2014, 9, 727–736. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.S.; Sze, S.L.; Woo, J.; Yu, R.H. A Chinese Chan-based lifestyle intervention improves memory of older adults. Front. Aging Neurosci. 2014, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.S.; Cheung, W.K.; Yeung, M.K.; Woo, J.; Kwok, T.; Shum, D.H.K.; Yu, R.; Cheung, M.C. A Chinese Chan-based Mind-Body Intervention Improves Memory of Older Adults. Front. Aging Neurosci. 2017, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.S.; Cheung, K.Y.; Yeung, M.K.; Lee, T.L. Sustained Effects of Memory and Lifestyle Interventions on Memory Functioning of Older Adults: An 18-Month Follow-Up Study. Front. Aging Neurosci. 2018, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Sze, S.L.; Dejian, S. Traditional Chinese Mind-Body Exercises Improve Self-Control Ability of an Adolescent with Asperger’s Disorder. J. Psychol. Chin. Soc. 2008, 9, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.S.; Sze, S.L.; Cheung, M.C.; Han, Y.M.; Leung, W.W.; Shi, D. Dejian mind-body intervention improves the cognitive functions of a child with autism. Evid.-Based Complement. Altern. Med. 2011, 2011, 549254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, A.S.; Sze, S.L.; Han, Y.M.; Cheung, M.C. A Chan dietary intervention enhances executive functions and anterior cingulate activity in autism spectrum disorders: A randomized controlled trial. Evid.-Based Complement. Altern. Med. 2012, 2012, 262136. [Google Scholar] [CrossRef]
- Chan, A.S.; Sze, S.L.; Siu, N.Y.; Lau, E.M.; Cheung, M.C. A Chinese mind-body exercise improves self-control of children with autism: A randomized controlled trial. PLoS ONE 2013, 8, e68184. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.S.Y.; Han, Y.M.Y.; Cheung, M.C. Chinese Chan-based prospective neuropsychological intervention for autistic children. In Comprehensive Guide to Autism; Patel, V.B., Preedy, V.R., Martin, C.R., Eds.; Springer: New York, NY, USA, 2014; pp. 2333–2355. ISBN 978-1-4614-4787-0. [Google Scholar]
- Chan, A.S.; Han, Y.M.; Sze, S.L.; Lau, E.M. Neuroenhancement of memory for children with autism by a mind-body exercise. Front. Psychol. 2015, 6, 1893. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Wong, Q.Y.; Sze, S.L.; Kwong, P.P.; Han, Y.M.; Cheung, M.C. A Chinese Chan-based mind-body intervention for patients with depression. J. Affect. Disord. 2012, 142, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Wong, Q.Y.; Sze, S.L.; Kwong, P.P.; Han, Y.M.; Cheung, M.C. A Chinese Chan-based mind-body intervention improves sleep on patients with depression: A randomized controlled trial. Sci. World J. 2012, 2012, 235206. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.S.; Han, Y.M.; Sze, S.L.; Wong, Q.Y.; Cheung, M.C. A randomized controlled neurophysiological study of a Chinese Chan-based mind-body intervention in patients with major depressive disorder. Evid.-Based Complement. Altern. Med. 2013, 2013, 812096. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.S.; Sze, S.L.; Cheung, M.C.; Lam, J.M.K.; Shi, D. Dejian mind-body intervention improves the functioning of a patient with chronic epilepsy: A case report. Cases J. 2009, 2, 9080. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; ISBN 978-089-042-555-8. [Google Scholar]
- Lord, C.; Rutter, M.; LeCouteur, A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; King-May Psychological Assessment: Hong Kong, China, 2010. [Google Scholar]
- Constantino, J.N.; Gruber, C.P. The Social Responsiveness Scale Manual, 2nd ed.; Western Psychological Services: Los Angeles, CA, USA, 2012; ISBN 978-074-916-743-1. [Google Scholar]
- Conners, C.K. Conners’ Rating Scales-Revised: Short Form; Multi-Heath Systems: North Tonawanda, NY, USA, 1997. [Google Scholar]
- Aman, M.G.; Singh, N.N. Aberrant Behavior Checklist: Manual; Slosson: East Aurora, NY, USA, 1986. [Google Scholar]
- Xu, J.; Dong, H.; Qian, Q.; Zhang, X.; Wang, Y.; Jin, W.; Qian, Y. Astrocyte-derived CCL2 participates in surgery-induced cognitive dysfunction and neuroinflammation via evoking microglia activation. Behav. Brain Res. 2017, 332, 145–153. [Google Scholar] [CrossRef]
- Bartosik-Psujek, H.; Stelmasiak, Z. The levels of chemokines CXCL8, CCL2 and CCL5 in multiple sclerosis patients are linked to the activity of the disease. Eur. J. Neurol. 2005, 12, 49–54. [Google Scholar] [CrossRef]
- Semple, B.D.; Kossmann, T.; Morganti-Kossmann, M.C. Role of chemokines in CNS health and pathology: A focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cereb. Blood Flow Metab. 2010, 30, 459–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrêa, J.D.; Starling, D.; Teixeira, A.L.; Caramelli, P.; Silva, T.A. Chemokines in CSF of Alzheimer’s disease patients. Arq. Neuropsiquiatr. 2011, 69, 455–459. [Google Scholar] [CrossRef]
- Kimura, A.; Yoshikura, N.; Hayashi, Y.; Inuzuka, T. Cerebrospinal Fluid C-C Motif Chemokine Ligand 2 Correlates with Brain Atrophy and Cognitive Impairment in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 61, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Grozdanov, V.; Bliederhaeuser, C.; Ruf, W.P.; Roth, V.; Fundel-Clemens, K.; Zondler, L.; Brenner, D.; Martin-Villalba, A.; Hengerer, B.; Kassubek, J.; et al. Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol. 2014, 128, 651–663. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, P.; Khan, S.A. Significance of CCL2, CCL5 and CCR2 polymorphisms for adverse prognosis of Japanese encephalitis from an endemic population of India. Sci. Rep. 2017, 7, 13716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magge, S.N.; Malik, S.Z.; Royo, N.C.; Chen, H.I.; Yu, L.; Snyder, E.Y. Role of monocyte chemoattractant protein-1 (MCP-1/CCL2) in migration of neural progenitor cells toward glial tumors. J. Neurosci. 2009, 87, 1547–1555. [Google Scholar] [CrossRef]
- Mazdeh, M.; Omrani, M.D.; Sayad, A.; Komaki, A.; Arsang-Jang, S.; Taheri, M.; Ghafouri-Fard, S. Expression analysis of cytokine coding genes in epileptic patients. Cytokine 2018, 110, 284–287. [Google Scholar] [CrossRef]
- Zheng, J.C.; Huang, Y.; Tang, K.; Cui, M.; Niemann, D.; Lopez, A.; Morgello, S.; Chen, S. HIV-1-infected and/or immune-activated macrophages regulate astrocyte CXCL8 production through IL-1β and TNF-α: Involvement of mitogen-activated protein kinases and protein kinase R. J. Neuroimmunol. 2008, 200, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wu, K.; Yu, Q.; Zhang, S.; Gao, Z.; Liu, Y.; Ni, L.; Cheng, Y.; Guan, Z.; Shi, M.; et al. CXCL13, CXCL10 and CXCL8 as potential biomarkers for the diagnosis of neurosyphilis patients. Sci. Rep. 2016, 6, 33569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittaluga, A. CCL5-Glutamate Cross-Talk in Astrocyte-Neuron Communication in Multiple Sclerosis. Front. Immunol. 2017, 8, 1079. [Google Scholar] [CrossRef] [Green Version]
- Sankar, S.; Wood, L. Differences in inflammatory signaling between males and females with Alzheimer’s disease. Alzheimers Dement. 2017, 13, 984. [Google Scholar] [CrossRef]
- Aldinucci, D.; Colombatti, A. The inflammatory chemokine CCL5 and cancer progression. Mediat. Inflamm. 2014, 2014, 292376. [Google Scholar] [CrossRef] [Green Version]
- Kelder, W.; McArthur, J.C.; Nance-Sproson, T.; McClernon, D.; Griffin, D.E. β-Chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann. Neurol. 1998, 44, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Zerbo, O.; Yoshida, C.; Grether, J.K.; Van de Water, J.; Ashwood, P.; Delorenze, G.N.; Hansen, R.L.; Kharrazi, M.; Croen, L.A. Neonatal cytokines and chemokines and risk of autism spectrum disorder: The Early Markers for Autism (EMA) study: A case-control study. J. Neuroinflamm. 2014, 11, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.; Vakilian, A.; Mahmoodi, M.H.; Hassanshahi, G.; Falahati-pour, S.K.; Dolatabadi, M.R.; Nadimi, A.E. Circulatory Levels of C-X-C Motif Chemokine Ligands 1, 9, and 10 Are Elevated in Patients with Ischemic Stroke. Eurasian J. Med. 2017, 49, 92–96. [Google Scholar] [CrossRef]
- Lind, L.; Studahl, M.; Berg, L.P.; Eriksson, K. CXCL11 production in cerebrospinal fluid distinguishes herpes simplex meningitis from herpes simplex encephalitis. J. Immunol. 2017, 14, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farias-Moeller, R.; LaFrance-Corey, R.; Bartolini, L.; Wells, E.M.; Baker, M.; Doslea, A.; Suslovic, W.; Greenberg, J.; Carpenter, J.L.; Howe, C.L. Fueling the FIRES: Hemophagocytic lymphohistiocytosis in febrile infection-related epilepsy syndrome. Epilepsia 2018, 59, 1753–1763. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, T. A plant-based diet and stroke. J. Geriatr. Cardiol. 2017, 14, 321–326. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Gheewala, N.M.; O’Keefe, J.O. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J. Am. Coll. Cardiol. 2018, 51, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Jyonouchi, H.; Geng, L.; Ruby, A.; Zimmerman-Bier, B. Dysregulated innate immune responses in young children with autism spectrum disorders: Their relationship to gastrointestinal symptoms and dietary intervention. Neuropsychobiology 2005, 51, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Spielman, L.J.; Little, J.P.; Klegeris, A. Physical activity and exercise attenuate neuroinflammation in neurological diseases. Brain Res. Bull. 2016, 125, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Emmons, R.A.; Stern, R. Gratitude as a psychotherapeutic intervention. J. Clin. Psychol. 2013, 69, 846–855. [Google Scholar] [CrossRef]
- Pan, C.Y. Objectively measured physical activity between children with autism spectrum disorders and children without disabilities during inclusive recess settings in Taiwan. J. Autism Dev. Disord. 2008, 38, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Sowa, M.; Meulenbroek, R. Effects of physical exercise on autism spectrum disorders: A meta-analysis. Res. Autism Spectr. Disord. 2012, 6, 46–57. [Google Scholar] [CrossRef]

| Experimental Group (n = 20) | Control Group (n = 12) | t | p | |
|---|---|---|---|---|
| Age, years | 11.08 (3.54) | 13.39 (3.97) | −1.71 | 0.10 |
| IQ | 86.65 (16.55) | 80.00 (9.14) | 1.46 | 0.15 |
| ADI-R Social Interaction | 19.70 (5.54) | 20.17 (5.80) | −0.23 | 0.82 |
| ADI-R Communication | 14.35 (5.64) | 16.67 (3.68) | −1.27 | 0.22 |
| ADI-R Stereotyped Behavior | 5.25 (2.29) | 5.17 (1.70) | 0.11 | 0.91 |
| SRS-2 Total Score | 80.45 (26.29) | 87.42 (19.09) | −0.80 | 0.43 |
| CRS-R Hyperactivity | 6.40 (4.76) | 3.33 (3.03) | 2.00 | 0.06 |
| ABC Stereotypic Behavior | 4.45 (3.95) | 3.58 (3.18) | 0.64 | 0.53 |
| ABC Hyperactivity/Noncompliance | 19.00 (10.31) | 13.17 (7.25) | 1.72 | 0.10 |
| Experimental Group (n = 20) | Control Group (n = 12) | t | p | |
|---|---|---|---|---|
| CCL2 | 193.73 (60.25) | 156.03 (38.83) | 2.15 | 0.04 |
| CCL5 | 43,176.60 (18,019.80) | 37,264.78 (8839.55) | 1.24 | 0.23 |
| CXCL8 | 86.81 (27.87) | 80.20 (26.61) | 0.66 | 0.51 |
| CXCL9 | 232.33 (96.57) | 347.52 (257.99) | 1.49 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, A.S.; Han, Y.M.Y.; Sze, S.L.; Wong, C.-k.; Chu, I.M.T.; Cheung, M.-c. Chanwuyi Lifestyle Medicine Program Alleviates Immunological Deviation and Improves Behaviors in Autism. NeuroSci 2021, 2, 207-223. https://doi.org/10.3390/neurosci2020015
Chan AS, Han YMY, Sze SL, Wong C-k, Chu IMT, Cheung M-c. Chanwuyi Lifestyle Medicine Program Alleviates Immunological Deviation and Improves Behaviors in Autism. NeuroSci. 2021; 2(2):207-223. https://doi.org/10.3390/neurosci2020015
Chicago/Turabian StyleChan, Agnes S., Yvonne M. Y. Han, Sophia L. Sze, Chun-kwok Wong, Ida M. T. Chu, and Mei-chun Cheung. 2021. "Chanwuyi Lifestyle Medicine Program Alleviates Immunological Deviation and Improves Behaviors in Autism" NeuroSci 2, no. 2: 207-223. https://doi.org/10.3390/neurosci2020015
APA StyleChan, A. S., Han, Y. M. Y., Sze, S. L., Wong, C.-k., Chu, I. M. T., & Cheung, M.-c. (2021). Chanwuyi Lifestyle Medicine Program Alleviates Immunological Deviation and Improves Behaviors in Autism. NeuroSci, 2(2), 207-223. https://doi.org/10.3390/neurosci2020015







