Knockout or Knock-in? A Truncated D2 Receptor Protein Is Expressed in the Brain of Functional D2 Receptor Knockout Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Horizontal Locomotor Activity
2.3. Bioinformatics Research
2.4. RT-PCR and 3′RACE
2.5. Cloning of Truncated D2R and Immunodetection in the HEK293T Cell Line
2.6. In Vivo Brain Microdialysis
2.7. Analysis of Dialysate Samples
2.8. Immunofluorescence in Brain Tissue
2.9. Statistical Analysis
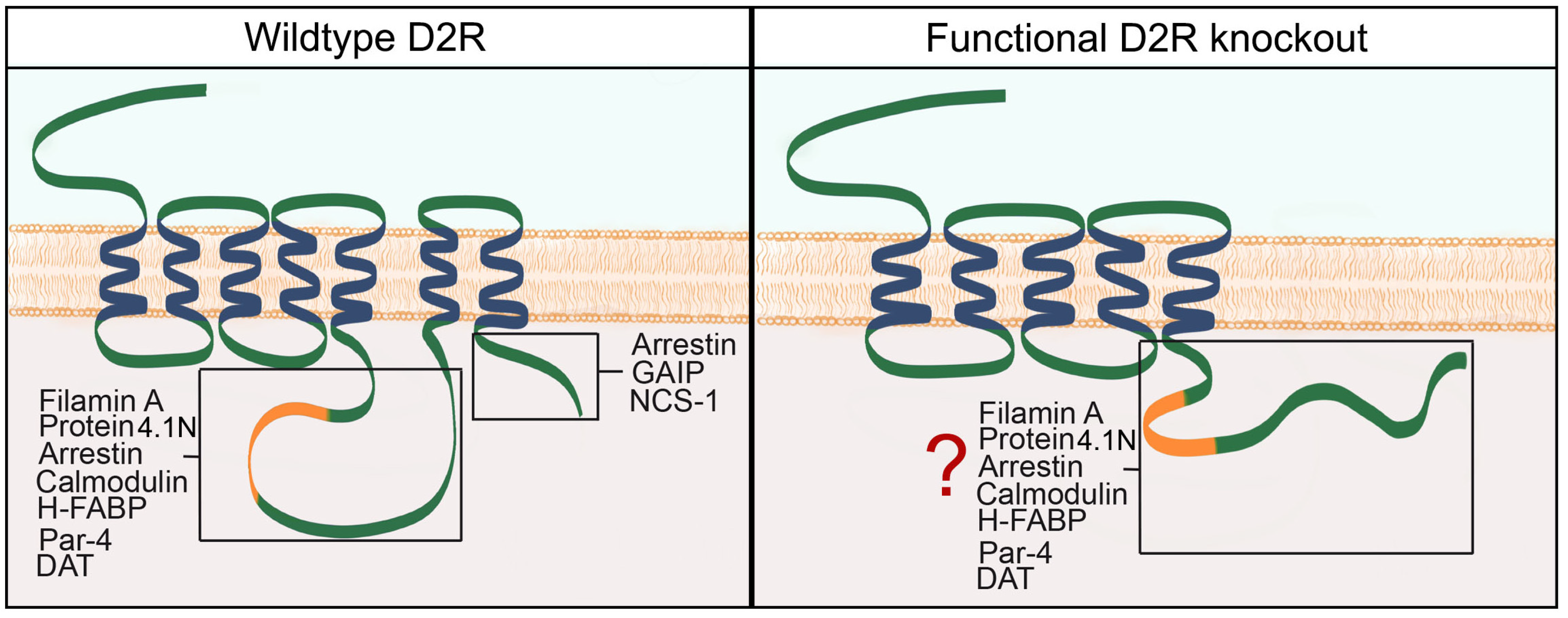
3. Results and Discussion
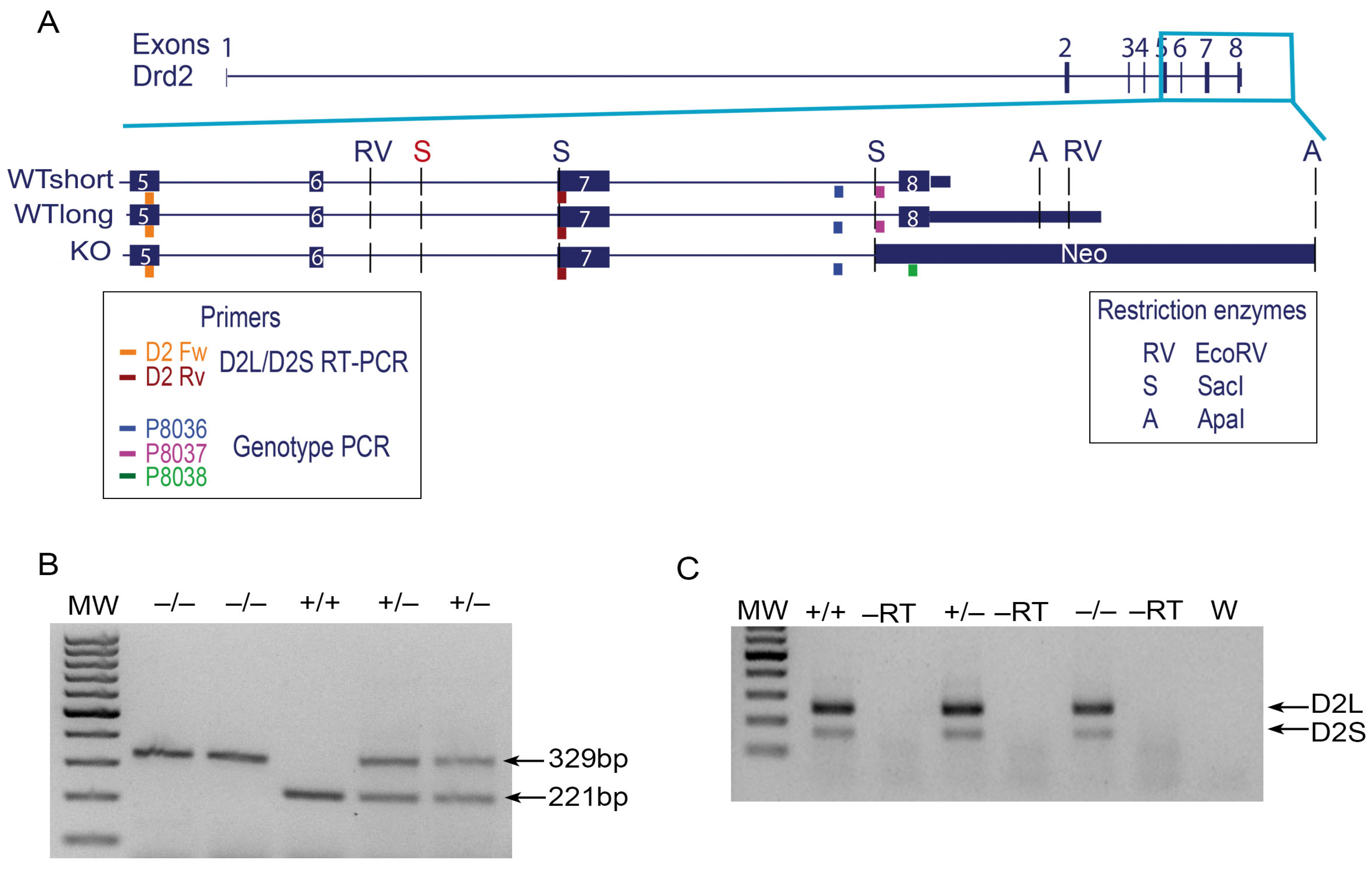
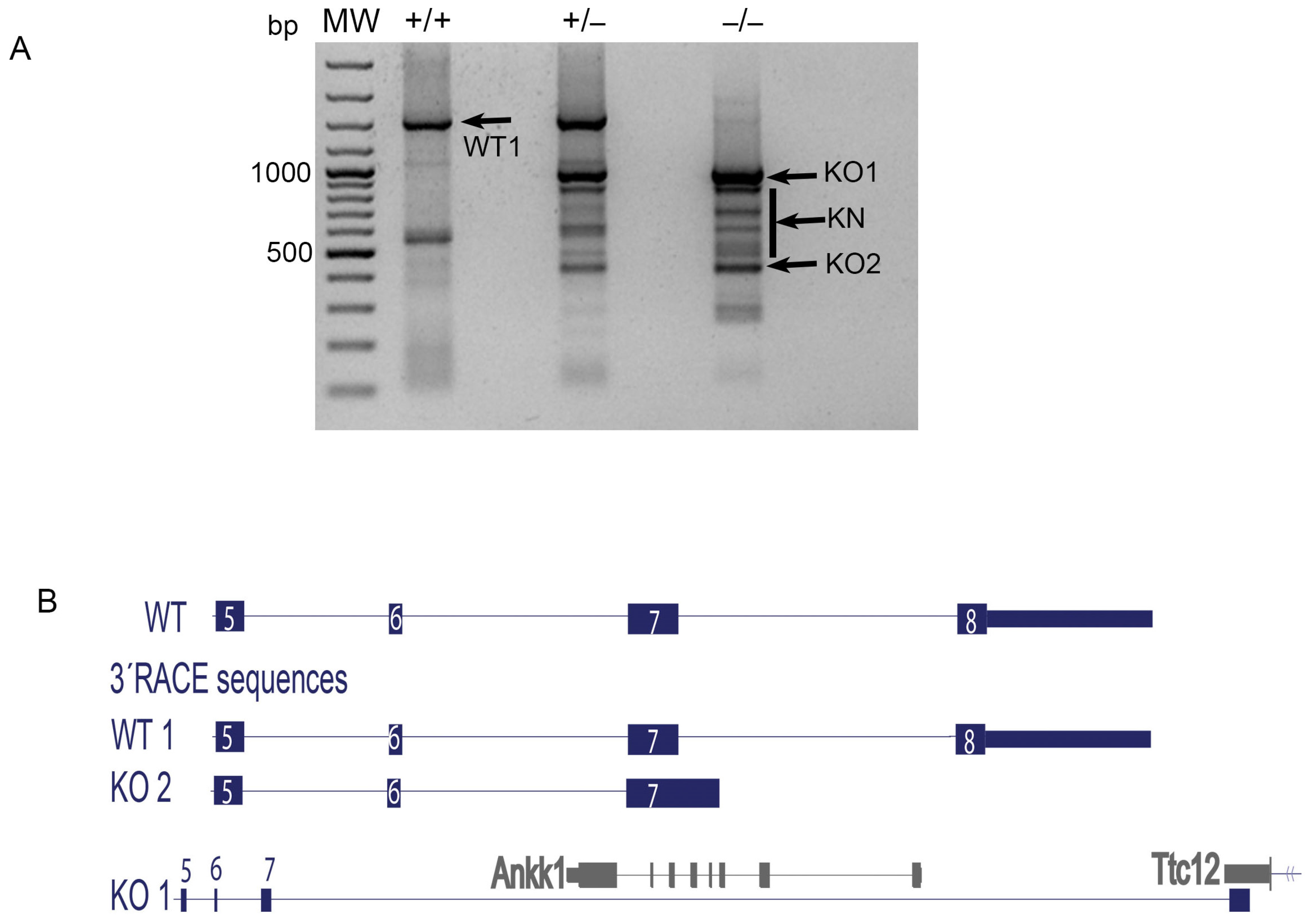
3.1. D2R Functional Knockout Mice Express Mature mRNA Transcripts That Lack Only Exon Eight
3.2. D2R Functional Knockout Mouse Brain Bears a Protein Recognizable by Anti-D2R Antibodies
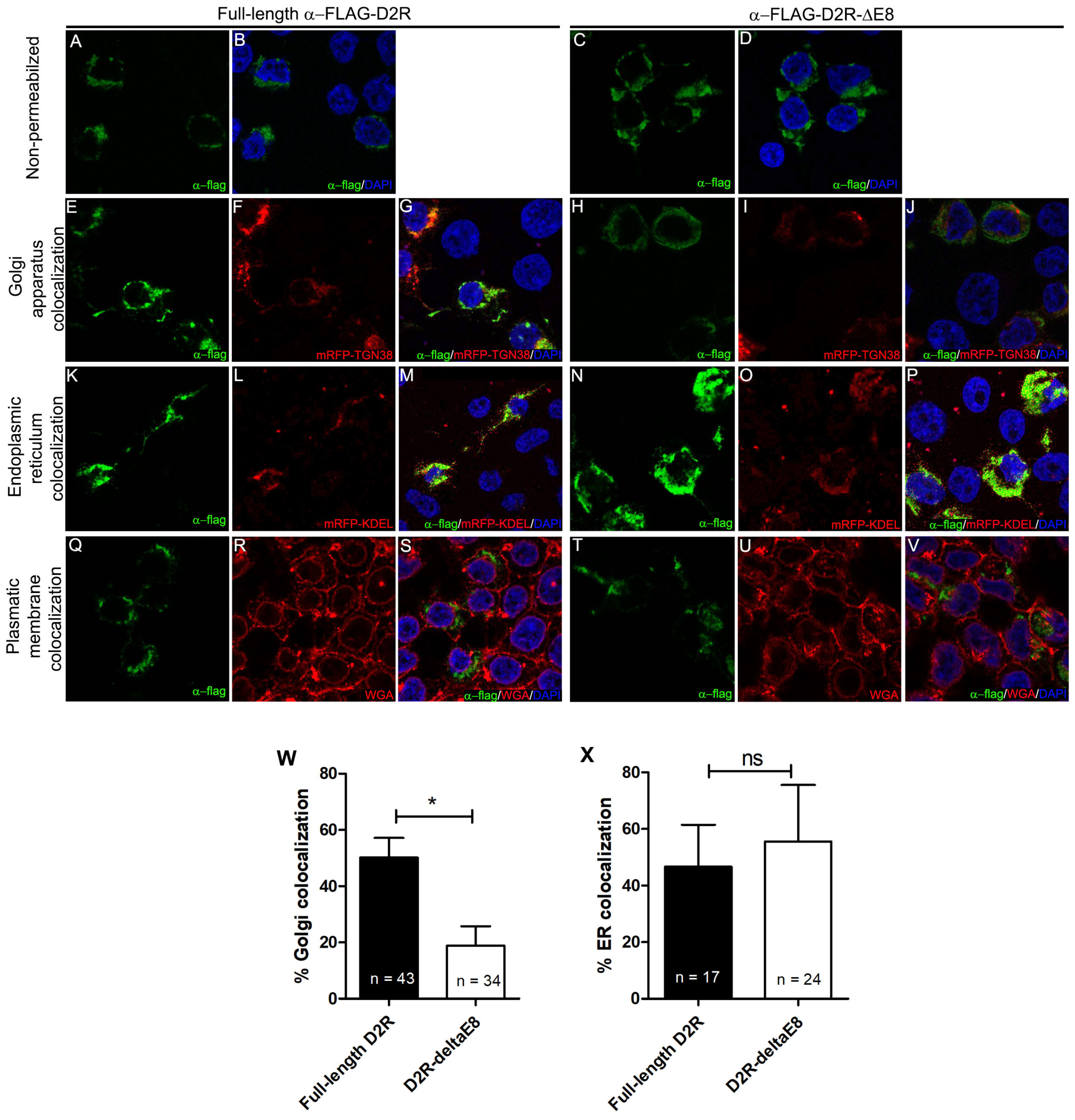
3.3. Recombinant Truncated D2R Shows Less Localization in the Golgi Apparatus Than Native D2R in HEK293T Cell Line
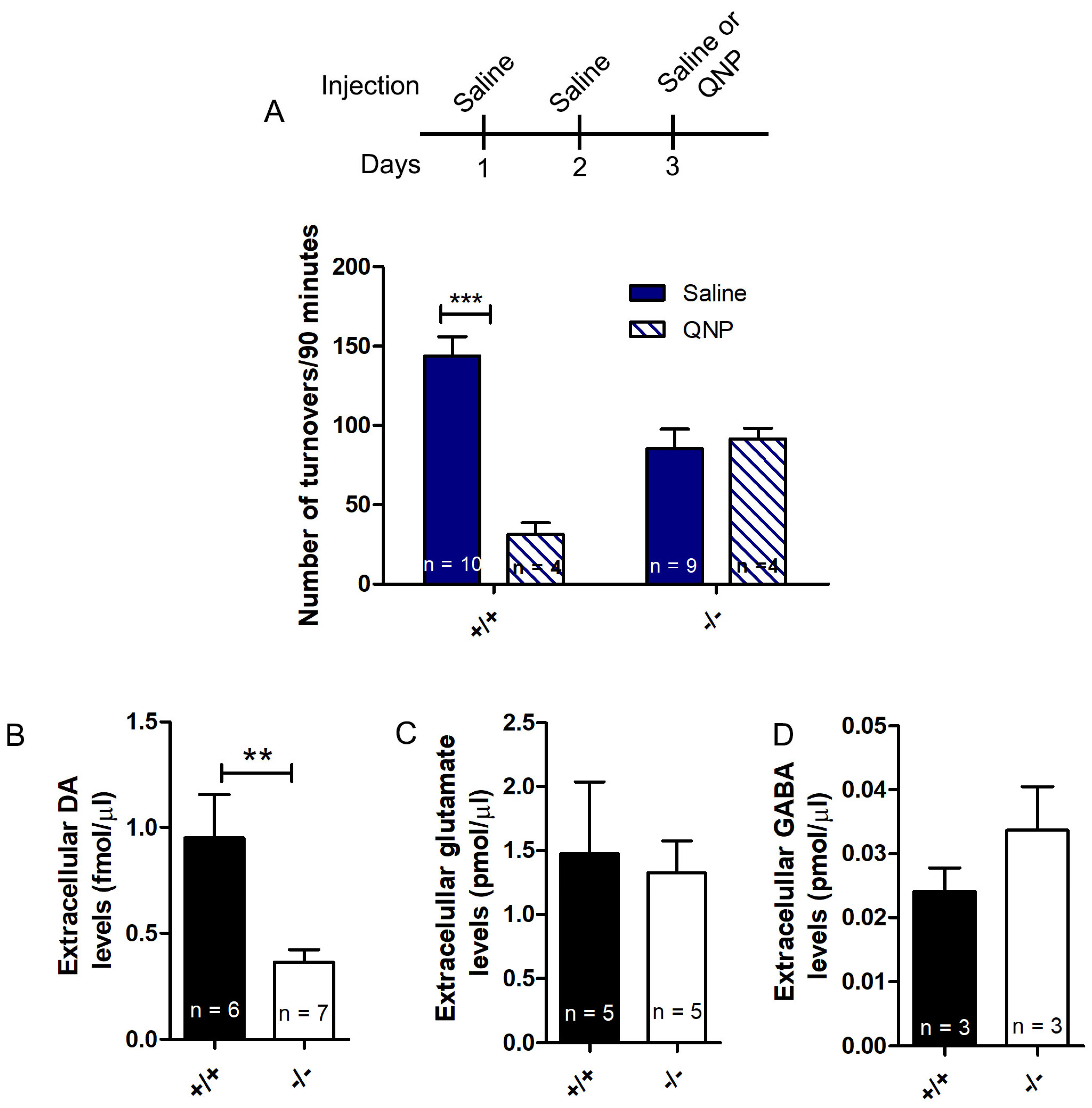
3.4. Diminished Locomotor Activity and Extracellular Dopamine Levels in D2R Functional Knockout Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giros, B.; Sokoloff, P.; Martres, M.P.; Riou, J.F.; Emorine, L.J.; Schwartz, J.C. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature 1989, 342, 923–926. [Google Scholar] [CrossRef]
- Monsma, F.J., Jr.; McVittie, L.D.; Gerfen, C.R.; Mahan, L.C.; Sibley, D.R. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature 1989, 342, 926–929. [Google Scholar] [CrossRef]
- De Mei, C.; Ramos, M.; Iitaka, C.; Borrelli, E. Getting specialized: Presynaptic and postsynaptic dopamine D2 receptors. Curr. Opin. Pharm. 2009, 9, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Radl, D.; Chiacchiaretta, M.; Lewis, R.G.; Brami-Cherrier, K.; Arcuri, L.; Borrelli, E. Differential regulation of striatal motor behavior and related cellular responses by dopamine D2L and D2S isoforms. Proc. Natl. Acad. Sci. USA 2018, 115, 198–203. [Google Scholar] [CrossRef]
- Deng, Y.P.; Lei, W.L.; Reiner, A. Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. J. Chem. Neuroanat. 2006, 32, 101–116. [Google Scholar] [CrossRef]
- Bertran-Gonzalez, J.; Bosch, C.; Maroteaux, M.; Matamales, M.; Herve, D.; Valjent, E.; Girault, J.A. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci. 2008, 28, 5671–5685. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.; Bevan, M.D.; Shink, E.; Bolam, J.P. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience 1998, 86, 353–387. [Google Scholar] [CrossRef] [PubMed]
- Sesack, S.R.; Grace, A.A. Cortico-Basal Ganglia reward network: Microcircuitry. Neuropsychopharmacology 2010, 35, 27–47. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef]
- Baik, J.H.; Picetti, R.; Saiardi, A.; Thiriet, G.; Dierich, A.; Depaulis, A.; Le Meur, M.; Borrelli, E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 1995, 377, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.A.; Rubinstein, M.; Asa, S.L.; Zhang, G.; Saez, C.; Bunzow, J.R.; Allen, R.G.; Hnasko, R.; Ben-Jonathan, N.; Grandy, D.K.; et al. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 1997, 19, 103–113. [Google Scholar] [CrossRef]
- Jung, M.Y.; Skryabin, B.V.; Arai, M.; Abbondanzo, S.; Fu, D.; Brosius, J.; Robakis, N.K.; Polites, H.G.; Pintar, J.E.; Schmauss, C. Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience 1999, 91, 911–924. [Google Scholar] [CrossRef]
- Escobar, A.P.; Cornejo, F.A.; Olivares-Costa, M.; Gonzalez, M.; Fuentealba, J.A.; Gysling, K.; Espana, R.A.; Andres, M.E. Reduced dopamine and glutamate neurotransmission in the nucleus accumbens of quinpirole-sensitized rats hints at inhibitory D2 autoreceptor function. J. Neurochem. 2015, 134, 1081–1090. [Google Scholar] [CrossRef]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The human genome browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef]
- Yates, A.; Akanni, W.; Amode, M.R.; Barrell, D.; Billis, K.; Carvalho-Silva, D.; Cummins, C.; Clapham, P.; Fitzgerald, S.; Gil, L.; et al. Ensembl 2016. Nucleic Acids Res. 2016, 44, D710–D716. [Google Scholar] [CrossRef] [PubMed]
- Scotto-Lavino, E.; Du, G.; Frohman, M.A. 3′ end cDNA amplification using classic RACE. Nat. Protoc. 2006, 1, 2742–2745. [Google Scholar] [CrossRef]
- Escobar, A.P.; Gonzalez, M.P.; Meza, R.C.; Noches, V.; Henny, P.; Gysling, K.; Espana, R.A.; Fuentealba, J.A.; Andres, M.E. Mechanisms of Kappa Opioid Receptor Potentiation of Dopamine D2 Receptor Function in Quinpirole-Induced Locomotor Sensitization in Rats. Int. J. Neuropsychopharmacol. 2017, 20, 660–669. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Sotomayor-Zarate, R.; Araya, K.A.; Pereira, P.; Blanco, E.; Quiroz, G.; Pozo, S.; Carreno, P.; Andres, M.E.; Forray, M.I.; Gysling, K. Activation of GABA-B receptors induced by systemic amphetamine abolishes dopamine release in the rat lateral septum. J. Neurochem. 2010, 114, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Xie, Z.; Varghese, G.; Nguyen, T.; O’Dowd, B.F.; George, S.R. Oligomerization of dopamine and serotonin receptors. Neuropsychopharmacology 2000, 23 (Suppl. 4), S32–S40. [Google Scholar] [CrossRef]
- Fuxe, K.; OBorroto-Escuela, D.; Marcellino, D.; Romero-Fernandez, W.; Frankowska, M.; Guidolin, D.; Filip, M.; Ferraro, L.; Woods, A.S.; Tarakanov, A.; et al. GPCR heteromers and their allosteric receptor-receptor interactions. Curr. Med. Chem. 2012, 19, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Prou, D.; Gu, W.J.; Le Crom, S.; Vincent, J.D.; Salamero, J.; Vernier, P. Intracellular retention of the two isoforms of the D2 dopamine receptor promotes endoplasmic reticulum disruption. J. Cell Sci. 2001, 114, 3517–3527. [Google Scholar] [CrossRef]
- Voulalas, P.J.; Schetz, J.; Undieh, A.S. Differential subcellular distribution of rat brain dopamine receptors and subtype-specific redistribution induced by cocaine. Mol. Cell Neurosci. 2011, 46, 645–654. [Google Scholar] [CrossRef]
- Tirotta, E.; Fontaine, V.; Picetti, R.; Lombardi, M.; Samad, T.A.; Oulad-Abdelghani, M.; Edwards, R.; Borrelli, E. Signaling by dopamine regulates D2 receptors trafficking at the membrane. Cell Cycle 2008, 7, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Sotnikova, T.D.; Marion, S.; Lefkowitz, R.J.; Gainetdinov, R.R.; Caron, M.G. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 2005, 122, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Shioda, N. Novel dopamine D2 receptor signaling through proteins interacting with the third cytoplasmic loop. Mol. Neurobiol. 2012, 45, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, N.; Levenson, R. A proteomic approach to receptor signaling: Molecular mechanisms and therapeutic implications derived from discovery of the dopamine D2 receptor signalplex. Eur. J. Pharmacol. 2007, 572, 83–93. [Google Scholar] [CrossRef]
- Sidhu, A.; Niznik, H.B. Coupling of dopamine receptor subtypes to multiple and diverse G proteins. Int. J. Dev. Neurosci. 2000, 18, 669–677. [Google Scholar] [CrossRef]
- Lee, F.J.; Pei, L.; Moszczynska, A.; Vukusic, B.; Fletcher, P.J.; Liu, F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 2007, 26, 2127–2136. [Google Scholar] [CrossRef]
- Kelly, M.A.; Rubinstein, M.; Phillips, T.J.; Lessov, C.N.; Burkhart-Kasch, S.; Zhang, G.; Bunzow, J.R.; Fang, Y.; Gerhardt, G.A.; Grandy, D.K.; et al. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J. Neurosci. 1998, 18, 3470–3479. [Google Scholar] [CrossRef]
- Zapata, A.; Shippenberg, T.S. Lack of functional D2 receptors prevents the effects of the D3-preferring agonist (+)-PD 128907 on dialysate dopamine levels. Neuropharmacology 2005, 48, 43–50. [Google Scholar] [CrossRef]
- Fan, X.; Xu, M.; Hess, E.J. D2 dopamine receptor subtype-mediated hyperactivity and amphetamine responses in a model of ADHD. Neurobiol. Dis. 2010, 37, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, Y.; Schmauss, C.; Sulzer, D. Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J. Neurosci. 2002, 22, 8002–8009. [Google Scholar] [CrossRef] [PubMed]

| Method | Primer | Sequence (5′–3′) |
|---|---|---|
| Genotyping | P8036 | TGATGACTGGGAATGTTGGTGTGC |
| P8037 | CTCCCCAGACTTGTGGCAAAAGG | |
| P8038 | AGGATTGGGAAGACAATAGCAG | |
| RT-PCR | D2 Fw | CCCTTCATCGTCACCCTGCTGG |
| D2 Rv | CTCCATTTCCAGCTCCTGAG | |
| RT-PCR polyA transcripts | D2—1(Fw) | CTCAGGAGCTGGAAATGGAG |
| Transcription (poly-T) | GCTCAAGCTTTTTTTTTTTTTTTTT | |
| 3′RACE | QT (Rv) | CCAGTGAGCAGAGTGACGAGGACTCGA |
| D2—1(Fw) nested PCR1 | CTCAGGAGCTGGAAATGGAG | |
| D2—2(Fw) nested PCR2 | CCACGGTCTACATAGCAACC | |
| Q0 (Rv) nested PCR1 | CCAGTGAGCAGAGTGACG | |
| Q1 (Rv) nested PCR2 | GAGGACTCGAGCTCAAGC | |
| D2R subcloning | EcoRI-ATG-Flag-D2R Fw | GCATGAATTCATGGACTACAAGGACGATGATGACGCC |
| Flag-D2R-XhoI Rv | GCCTTCCTGAAGATCCTCCACTGCTGACTCGAGGCAT | |
| FLAG-D2R-ΔE8 Rv | ATGCCTCGAGTCAGAGAACAATGGCGAGCATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, N.; Olivares-Costa, M.; González, M.P.; Munita, R.; Escobar, A.P.; Meza, R.; Herrera-Rojas, M.; Albornoz, J.; Merello, G.; Andrés, M.E. Knockout or Knock-in? A Truncated D2 Receptor Protein Is Expressed in the Brain of Functional D2 Receptor Knockout Mice. NeuroSci 2021, 2, 193-206. https://doi.org/10.3390/neurosci2020014
Sánchez N, Olivares-Costa M, González MP, Munita R, Escobar AP, Meza R, Herrera-Rojas M, Albornoz J, Merello G, Andrés ME. Knockout or Knock-in? A Truncated D2 Receptor Protein Is Expressed in the Brain of Functional D2 Receptor Knockout Mice. NeuroSci. 2021; 2(2):193-206. https://doi.org/10.3390/neurosci2020014
Chicago/Turabian StyleSánchez, Natalia, Montserrat Olivares-Costa, Marcela P González, Roberto Munita, Angélica P Escobar, Rodrigo Meza, Mauricio Herrera-Rojas, Jessica Albornoz, Gianluca Merello, and María E Andrés. 2021. "Knockout or Knock-in? A Truncated D2 Receptor Protein Is Expressed in the Brain of Functional D2 Receptor Knockout Mice" NeuroSci 2, no. 2: 193-206. https://doi.org/10.3390/neurosci2020014
APA StyleSánchez, N., Olivares-Costa, M., González, M. P., Munita, R., Escobar, A. P., Meza, R., Herrera-Rojas, M., Albornoz, J., Merello, G., & Andrés, M. E. (2021). Knockout or Knock-in? A Truncated D2 Receptor Protein Is Expressed in the Brain of Functional D2 Receptor Knockout Mice. NeuroSci, 2(2), 193-206. https://doi.org/10.3390/neurosci2020014






