Alcohol Consumption during Adulthood Does Not Impair Later Go/No-Go Reversal Learning in Male Rats
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Behavioral Apparatus
2.3. Behavioral Procedure
2.4. Statistical Analysis
3. Results
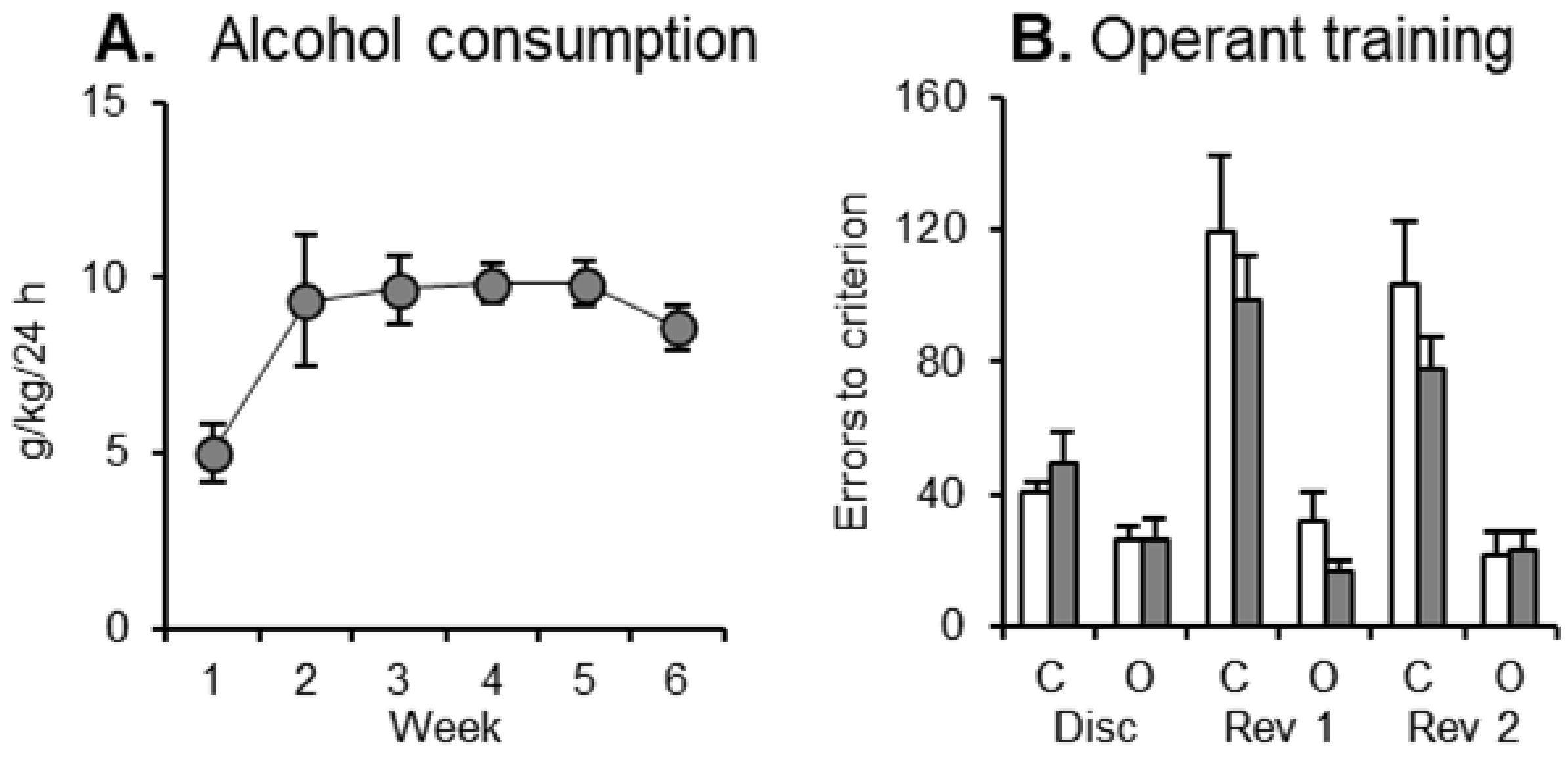
3.1. Alcohol Consumption
3.2. Discrimination Learning
3.3. Reversal Learning
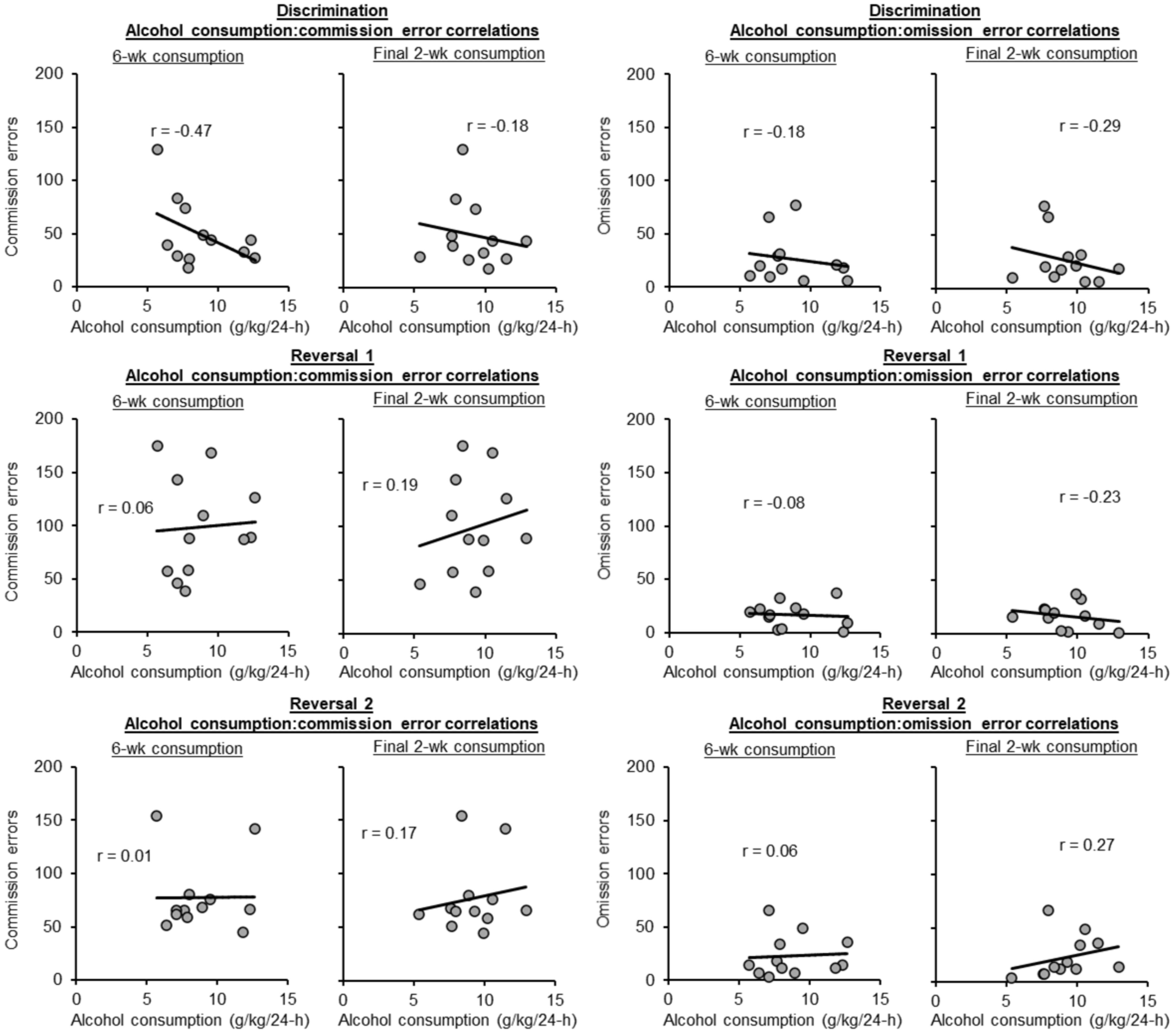
3.4. Alcohol-Operant Behavior Correlations
3.5. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fein, G.; Klein, L.; Finn, P. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol. Clin. Exp. Res. 2004, 28, 1487–1491. [Google Scholar] [CrossRef]
- Tomassini, A.; Struglia, F.; Spaziani, D.; Pacifico, R.; Stratta, P.; Rossi, A. Decision making, impulsivity, and personality traits in alcohol-dependent subjects. Am. J. Addict. 2012, 21, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.M. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology 2001, 154, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Coskunpinar, A.; Dir, A.L.; Cyders, M.A. Multidimensionality in impulsivity and alcohol use: A meta-analysis using the UPPS model of impulsivity. Alcohol. Clin. Exp. Res. 2013, 37, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-Garcia, A.; Lawrence, A.J.; Clark, L. Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 2008, 32, 777–810. [Google Scholar] [CrossRef] [PubMed]
- Malone, S.M.; Luciana, M.; Wilson, S.; Sparks, J.C.; Hunt, R.H.; Thomas, K.M.; Iacono, W.G. Adolescent drinking and motivated decision-making: A cotwin-control investigation with monozygotic twins. Behav. Genet. 2014, 44, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Khemiri, L.; Kuja-Halkola, R.; Larsson, H.; Jayaram-Lindstrom, N. Genetic overlap between impulsivity and alcohol dependence: A large-scale national twin study. Psychol. Med. 2016, 46, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Heitzeg, M.M.; Nigg, J.T.; Yau, W.Y.; Zucker, R.A.; Zubieta, J.K. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol. Psychiatry 2010, 68, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, A.; Liljequist, S.; Meis, J.; Chefer, V.; Shippenberg, T.; Bakalkin, G. Repeated moderate-dose ethanol bouts impair cognitive function in Wistar rats. Addict. Biol. 2012, 17, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, G.M.; Stewart, W.N.; Savage, L.M. Chronic Drinking During Adolescence Predisposes the Adult Rat for Continued Heavy Drinking: Neurotrophin and Behavioral Adaptation after Long-Term, Continuous Ethanol Exposure. PLoS ONE 2016, 11, e0149987. [Google Scholar] [CrossRef]
- Gibula-Tarlowska, E.; Wydra, K.; Kotlinska, J.H. Deleterious Effects of Ethanol, Delta(9)-Tetrahydrocannabinol (THC), and Their Combination on the Spatial Memory and Cognitive Flexibility in Adolescent and Adult Male Rats in the Barnes Maze Task. Pharmaceutics 2020, 12, 654. [Google Scholar] [CrossRef]
- Galaj, E.; Kipp, B.T.; Floresco, S.B.; Savage, L.M. Persistent Alterations of Accumbal Cholinergic Interneurons and Cognitive Dysfunction after Adolescent Intermittent Ethanol Exposure. Neuroscience 2019, 404, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.G., Jr.; He, J.; Lee, J.; Styner, M.; Crews, F.T. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol. Clin. Exp. Res. 2011, 35, 671–688. [Google Scholar] [CrossRef]
- Fernandez, G.M.; Lew, B.J.; Vedder, L.C.; Savage, L.M. Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats. Neuroscience 2017, 348, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Obernier, J.A.; White, A.M.; Swartzwelder, H.S.; Crews, F.T. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol. Biochem. Behav. 2002, 72, 521–532. [Google Scholar] [CrossRef]
- Badanich, K.A.; Becker, H.C.; Woodward, J.J. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav. Neurosci. 2011, 125, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.J.; May, C.; Luikinga, S.J.; Burrows, E.L.; Hyun Kim, J.; Lawrence, A.J.; Perry, C.J. Chronic voluntary alcohol consumption causes persistent cognitive deficits and cortical cell loss in a rodent model. Sci. Rep. 2019, 9, 18651. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.P.; Bohnsack, J.P.; Kusumo, H.; Liu, W.; Pandey, S.C.; Crews, F.T. Neuroimmune and epigenetic involvement in adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons: Restoration with voluntary exercise. Addict. Biol. 2020, 25, e12731. [Google Scholar] [CrossRef]
- Badanich, K.A.; Fakih, M.E.; Gurina, T.S.; Roy, E.K.; Hoffman, J.L.; Uruena-Agnes, A.R.; Kirstein, C.L. Reversal learning and experimenter-administered chronic intermittent ethanol exposure in male rats. Psychopharmacology 2016, 233, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- DePoy, L.; Daut, R.; Brigman, J.L.; MacPherson, K.; Crowley, N.; Gunduz-Cinar, O.; Pickens, C.L.; Cinar, R.; Saksida, L.M.; Kunos, G.; et al. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc. Natl. Acad. Sci. USA 2013, 110, 14783–14788. [Google Scholar] [CrossRef]
- Fisher, H.; Bright, N.; Gallo, M.; Pajser, A.; Pickens, C.L. Relationship of low doses of alcohol voluntarily consumed during adolescence and early adulthood with subsequent behavioral flexibility. Behav. Pharmacol. 2017, 28, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Kroener, S.; Mulholland, P.J.; New, N.N.; Gass, J.T.; Becker, H.C.; Chandler, L.J. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS ONE 2012, 7, e37541. [Google Scholar] [CrossRef]
- Fernandez, G.M.; Savage, L.M. Adolescent binge ethanol exposure alters specific forebrain cholinergic cell populations and leads to selective functional deficits in the prefrontal cortex. Neuroscience 2017, 361, 129–143. [Google Scholar] [CrossRef]
- Ray, M.H.; Hite, T.; Gallo, M.; Pickens, C.L. Operant over-responding is more sensitive than reversal learning for revealing behavioral changes after withdrawal from alcohol consumption. Physiol. Behav. 2018, 196, 176–184. [Google Scholar] [CrossRef]
- Aguirre, C.G.; Stolyarova, A.; Das, K.; Kolli, S.; Marty, V.; Ray, L.; Spigelman, I.; Izquierdo, A. Sex-dependent effects of chronic intermittent voluntary alcohol consumption on attentional, not motivational, measures during probabilistic learning and reversal. PLoS ONE 2020, 15, e0234729. [Google Scholar] [CrossRef]
- Simms, J.A.; Steensland, P.; Medina, B.; Abernathy, K.E.; Chandler, L.J.; Wise, R.; Bartlett, S.E. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol. Clin. Exp. Res. 2008, 32, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 1973, 29, 203–210. [Google Scholar] [CrossRef]
- Pickens, C.L.; Kallenberger, P.; Pajser, A.; Fisher, H. Voluntary alcohol access during adolescence/early adulthood, but not during adulthood, causes faster omission contingency learning. Behav. Brain Res. 2019, 370, 111918. [Google Scholar] [CrossRef]
- Pajser, A.; Breen, M.; Fisher, H.; Pickens, C.L. Individual differences in conditioned fear are associated with levels of adolescent/early adult alcohol consumption and instrumental extinction. Behav. Brain Res. 2018, 349, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Pajser, A.; Fisher, H.; Pickens, C.L. Pre-training naltrexone increases conditioned fear learning independent of adolescent alcohol consumption history. Physiol. Behav. 2021, 229, 113212. [Google Scholar] [CrossRef] [PubMed]
- Pajser, A.; Limoges, A.; Long, C.; Pickens, C.L. Individual differences in voluntary alcohol consumption are associated with conditioned fear in the fear incubation model. Behav. Brain Res. 2019, 362, 299–310. [Google Scholar] [CrossRef]
- Pickens, C.L.; Fisher, H.; Bright, N.; Gallo, M.; Ray, M.H.; Anji, A.; Kumari, M. Prior alcohol consumption does not impair go/no-go discrimination learning, but causes over-responding on go trials, in rats. Behav. Brain Res. 2016, 312, 272–278. [Google Scholar] [CrossRef]
- Borlikova, G.G.; Elbers, N.A.; Stephens, D.N. Repeated withdrawal from ethanol spares contextual fear conditioning and spatial learning but impairs negative patterning and induces over-responding: Evidence for effect on frontal cortical but not hippocampal function? Eur. J. Neurosci. 2006, 24, 205–216. [Google Scholar] [CrossRef]
- Tombaugh, T.N. Ethanol-Metrecal diets: II. Failure to obtain impaired performance on a series of appetitively and aversively motivated tasks. Pharmacol. Biochem. Behav. 1981, 15, 463–469. [Google Scholar] [CrossRef]
- Marszalek-Grabska, M.; Gibula-Bruzda, E.; Bodzon-Kulakowska, A.; Suder, P.; Gawel, K.; Talarek, S.; Listos, J.; Kedzierska, E.; Danysz, W.; Kotlinska, J.H. ADX-47273, a mGlu5 receptor positive allosteric modulator, attenuates deficits in cognitive flexibility induced by withdrawal from ‘binge-like’ ethanol exposure in rats. Behav. Brain Res. 2018, 338, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.G., Jr.; Liu, W.; Oguz, I.; Styner, M.; Crews, F.T. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol. Biochem. Behav. 2014, 116, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Ripley, T.L.; O’Shea, M.; Stephens, D.N. Repeated withdrawal from ethanol impairs acquisition but not expression of conditioned fear. Eur. J. Neurosci. 2003, 18, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.N.; Brown, G.; Duka, T.; Ripley, T.L. Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. Eur. J. Neurosci. 2001, 14, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Vetter-O’Hagen, C.; Varlinskaya, E.; Spear, L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol. Alcohol. 2009, 44, 547–554. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, M.L.; Escarabajal, M.D.; Aguero, A. Sex differences in adult Wistar rats in the voluntary consumption of ethanol after pre-exposure to ethanol-induced flavor avoidance learning. Pharmacol. Biochem. Behav. 2015, 37, 7–15. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pickens, C.L.; Gallo, M.; Fisher, H.; Pajser, A.; Ray, M.H. Alcohol Consumption during Adulthood Does Not Impair Later Go/No-Go Reversal Learning in Male Rats. NeuroSci 2021, 2, 166-176. https://doi.org/10.3390/neurosci2020012
Pickens CL, Gallo M, Fisher H, Pajser A, Ray MH. Alcohol Consumption during Adulthood Does Not Impair Later Go/No-Go Reversal Learning in Male Rats. NeuroSci. 2021; 2(2):166-176. https://doi.org/10.3390/neurosci2020012
Chicago/Turabian StylePickens, Charles L., Mark Gallo, Hayley Fisher, Alisa Pajser, and Madelyn H. Ray. 2021. "Alcohol Consumption during Adulthood Does Not Impair Later Go/No-Go Reversal Learning in Male Rats" NeuroSci 2, no. 2: 166-176. https://doi.org/10.3390/neurosci2020012
APA StylePickens, C. L., Gallo, M., Fisher, H., Pajser, A., & Ray, M. H. (2021). Alcohol Consumption during Adulthood Does Not Impair Later Go/No-Go Reversal Learning in Male Rats. NeuroSci, 2(2), 166-176. https://doi.org/10.3390/neurosci2020012





