Is the Letter ‘t’ in the Word ‘gourmet’? Disruption in Task-Evoked Connectivity Networks in Adults with Impaired Literacy Skills
Abstract
1. Introduction
1.1. Domain-Specific Spelling Network
1.2. From Brain Activation to Brain Connectivity
1.3. Domain-General Resting State Networks (RSN)
1.4. Goals of the Current Work
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.2.1. Behavioural Data Collection
2.2.2. fMRI Data Collection
Neuroimaging Tasks:
- (1)
- Orthographic (O) condition: This condition used words that have an irregular spelling-to-sound correspondence so the retrieval of the spelling of the words was necessary in order to make the judgment. The letter option that was given was either a) absent from the pronunciation of the word (e.g., ‘T’ in ‘gourmet’), b) ambiguous with respect to associated phonemes (e.g., ‘C’ in ‘cello’), or c) highly associated with a specific phoneme (e.g., ‘G’ associated with /g/, as in ‘get’) but was pronounced differently in a selected word (e.g., sound of ‘G’ in ‘regime’). In each case, the decision of the letter probe could not be made by the sound or pronunciation of the words alone.
- (2)
- Orthographic–Phonological (OP) condition: The words in this condition had consistent spelling-to-sound correspondence (e.g., letter ‘A’ in ‘gaze’). Thus, participants could utilize sound-based information, pronunciation-based information, or print-based information.
- (3)
- Phonological (P) condition: The stimuli in this condition were pseudowords (e.g., letter ‘N’ in ‘bint’), for which there were no stored whole-word sound, pronunciation-based representations, or print-based representations to retrieve. Therefore, participants had to generate the spelling of these stimuli to make the decision of whether the letter was in the spelling of the word or not.
2.3. Procedure
2.4. Analysis
2.4.1. Behavioural Data Analysis
2.4.2. Functional Connectivity Analyses
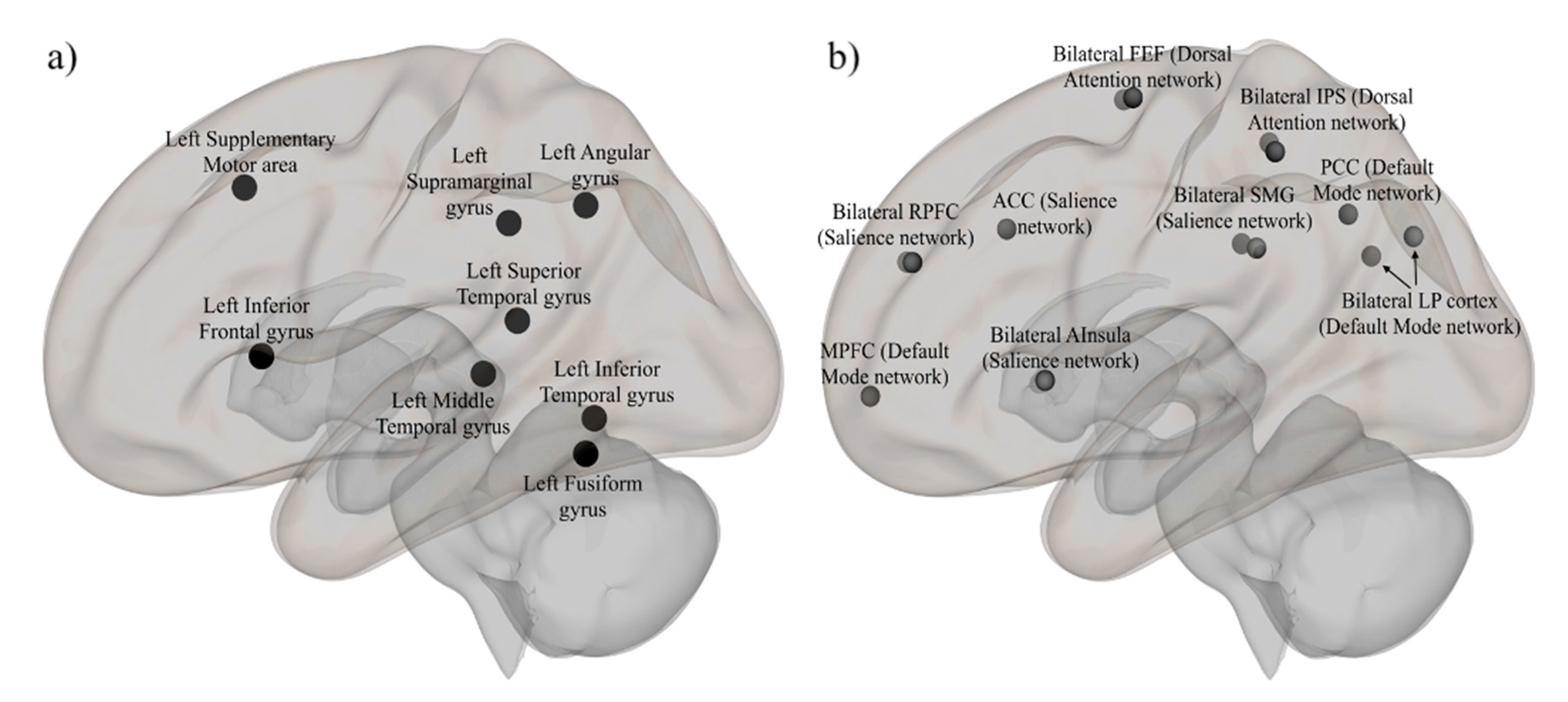
2.4.3. Brain Regions of Interests
3. Results
3.1. Behavioural Performance
3.2. Within-Network SpN Functional Connectivity
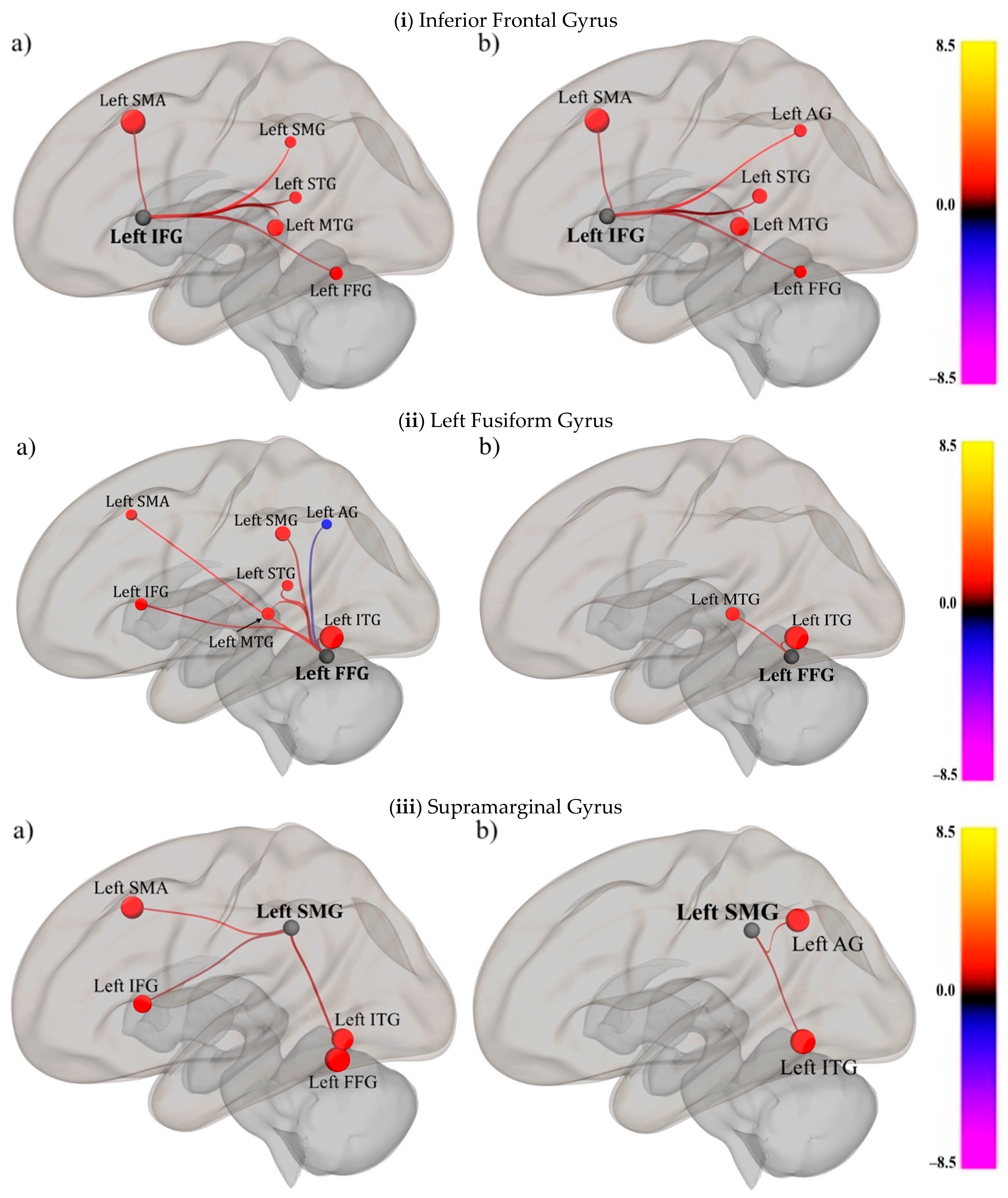
3.2.1. Characterize the Functional Connectivity of the Spelling Network (SpN) during the Three In-Task Conditions in People with and without Reading Impairments
Inferior Frontal Gyrus (Speech)
Fusiform Gyrus (Print)
Supramarginal Gyrus (Sound)
Independent Sample t-Tests:
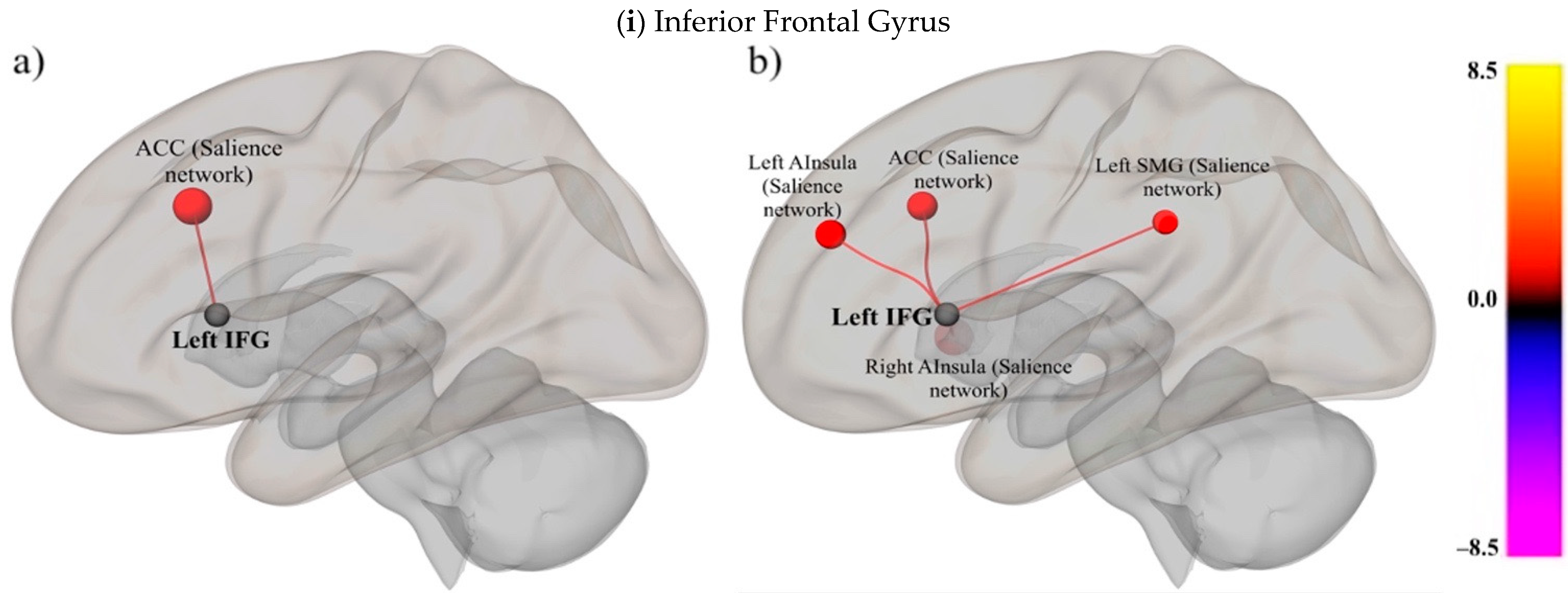
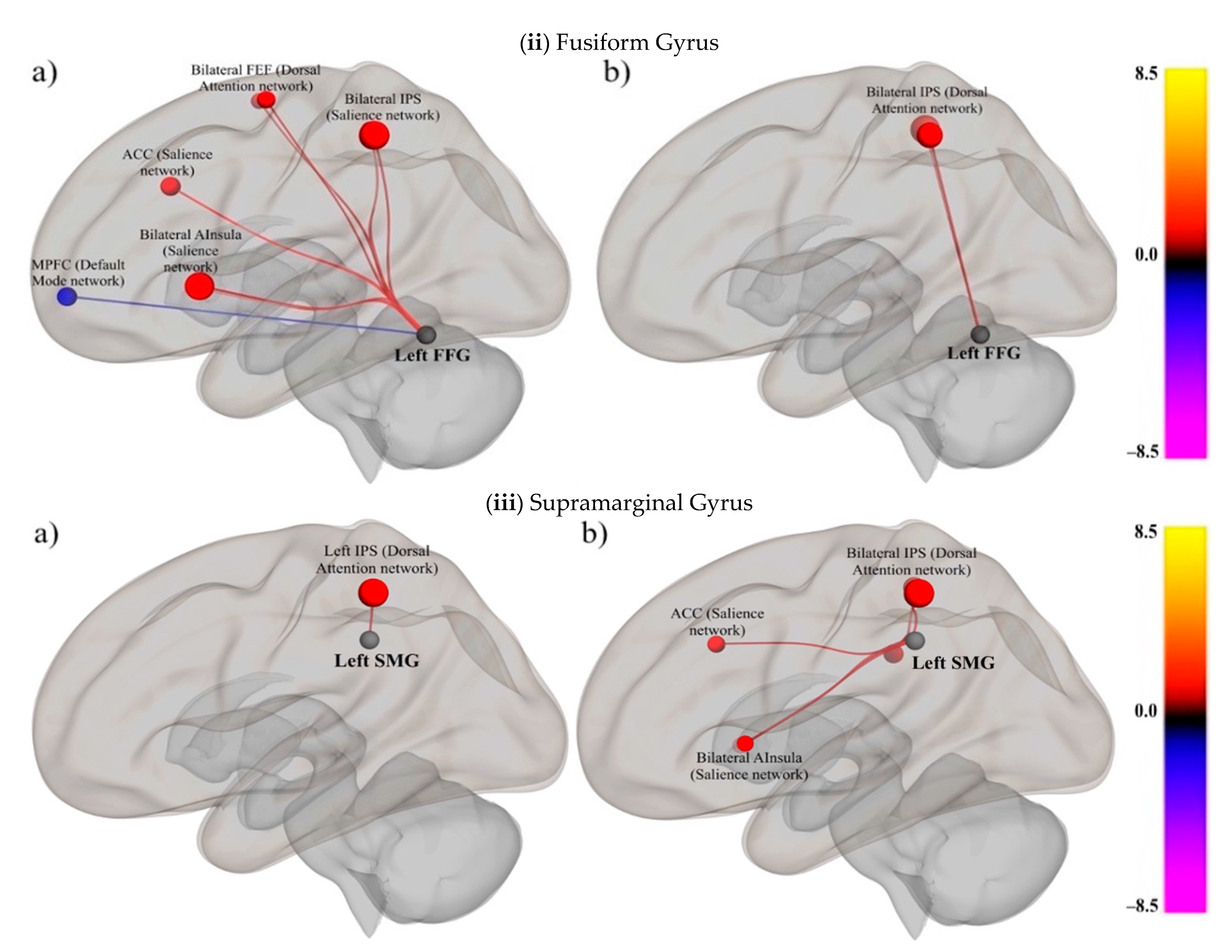
3.2.2. Examine the Relationships between Domain-Specific Networks (i.e., SpN) and Domain-General RSNs in People with and without Literacy Impairments
Inferior Frontal Gyrus (Speech)
Fusiform Gyrus (Print)
Supramarginal Gyrus (Sound)
Independent Sample t-Tests
Inferior Frontal Gyrus (Speech)
Fusiform Gyrus (Print)
3.2.3. Examine the Relationships between SpN Connectivity and Spelling Behaviour in People with and without Reading Impairments
3.2.4. Examine the Relationships between RSN Connectivity and Spelling Behaviour in People with and without Literacy Impairments
Fusiform Gyrus (Print)
Inferior Frontal Gyrus (Speech)
4. Discussion
4.1. Characterization of the Spelling Network (SpN)
Connectivity during Retrieval of Whole-Word Representations in the Impaired Group
4.2. Connections between SpN and Domain-General RSNs
4.2.1. Increased SpN–DMN Connections in Impaired Readers
4.2.2. Altered SpN–Attention Connections
4.3. SpN– and RSN–Behaviour Correlations.
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hampson, M.; Tokoglu, F.; Sun, Z.; Schafer, R.J.; Skudlarski, P.; Gore, J.C.; Constable, R.T. Connectivity–behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. Neuroimage 2006, 31, 513–519. [Google Scholar] [CrossRef]
- Yeo, B.T.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef] [PubMed]
- Finn, E.S.; Shen, X.; Holahan, J.M.; Scheinost, D.; Lacadie, C.; Papademetris, X.; Shaywitz, S.E.; Shaywitz, B.A.; Constable, R.T. Disruption of functional networks in dyslexia: A whole-brain, data-driven analysis of connectivity. Biol. Psychiatry 2014, 76, 397–404. [Google Scholar] [CrossRef]
- Gebauer, D.; Fink, A.; Kargl, R.; Reishofer, G.; Koschutnig, K.; Purgstaller, C.; Fazekas, F.; Enzinger, C. Differences in brain function and changes with intervention in children with poor spelling and reading abilities. PLoS ONE 2012, 7, e38201. [Google Scholar] [CrossRef] [PubMed]
- Achal, S.; Hoeft, F.; Bray, S. Individual differences in adult reading are associated with left temporo-parietal to dorsal striatal functional connectivity. Cereb. Cortex 2016, 26, 4069–4081. [Google Scholar] [CrossRef]
- Schurz, M.; Wimmer, H.; Richlan, F.; Ludersdorfer, P.; Klackl, J.; Kronbichler, M. Resting-state and task-based functional brain connectivity in developmental dyslexia. Cereb. Cortex 2014, 25, 3502–3514. [Google Scholar] [CrossRef] [PubMed]
- Van Der Mark, S.; Klaver, P.; Bucher, K.; Maurer, U.; Schulz, E.; Brem, S.; Martin, E.; Brandeis, D. The left occipitotemporal system in reading: Disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. Neuroimage 2011, 54, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Boets, B.; de Beeck, H.P.O.; Vandermosten, M.; Scott, S.K.; Gillebert, C.R.; Mantini, D.; Bulthe, J.; Sunaert, S.; Wouters, J.; Ghesquière, P. Intact but less accessible phonetic representations in adults with dyslexia. Science 2013, 342, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Norton, E.S.; Black, J.M.; Stanley, L.M.; Tanaka, H.; Gabrieli, J.D.; Sawyer, C.; Hoeft, F. Functional neuroanatomical evidence for the double-deficit hypothesis of developmental dyslexia. Neuropsychologia 2014, 61, 235–246. [Google Scholar] [CrossRef]
- Bain, A.M.; Bailet, L.L.; Moats, L.C. Written Language Disorders: Theory into Practice; Pro-ed: Austin, TX, USA, 1991. [Google Scholar]
- Ellis, A.W. Spelling and writing (and reading and speaking). In Normality and Pathology in Cognitive Functions; Academic Press: London, UK, 1982; pp. 113–146. [Google Scholar]
- Houghton, G.; Zorzi, M. Normal and impaired spelling in a connectionist dual-route architecture. Cogn. Neuropsychol. 2003, 20, 115–162. [Google Scholar] [CrossRef]
- Roeltgen, D.P.; Heilman, K.M. Review of agraphia and a proposal for an anatomically-based neuropsychological model of writing. Appl. Psycholinguist. 1985, 6, 205–229. [Google Scholar] [CrossRef]
- Beeson, P.; Rapcsak, S.; Plante, E.; Chargualaf, J.; Chung, A.; Johnson, S.; Trouard, T. The neural substrates of writing: A functional magnetic resonance imaging study. Aphasiology 2003, 17, 647–665. [Google Scholar] [CrossRef]
- DeMarco, A.T.; Wilson, S.M.; Rising, K.; Rapcsak, S.Z.; Beeson, P.M. Neural substrates of sublexical processing for spelling. Brain Lang. 2017, 164, 118–128. [Google Scholar] [CrossRef]
- Ellenblum, G.; Purcell, J.J.; Song, X.; Rapp, B. High-level integrative networks: A resting-state fMRI investigation of reading and spelling. J. Cogn. Neurosci. 2019, 31, 961–977. [Google Scholar] [CrossRef]
- Purcell, J.J.; Jiang, X.; Eden, G.F. Shared orthographic neuronal representations for spelling and reading. Neuroimage 2017, 147, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Rapcsak, S.Z.; Beeson, P.M. The role of left posterior inferior temporal cortex in spelling. Neurology 2004, 62, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.; Hurley, R.S.; Rogalski, E.; Mesulam, M.M. Anatomic, clinical, and neuropsychological correlates of spelling errors in primary progressive aphasia. Neuropsychologia 2012, 50, 1929–1935. [Google Scholar] [CrossRef]
- Richards, T.L.; Grabowski, T.J.; Boord, P.; Yagle, K.; Askren, M.K.; Mestre, Z.; Robinson, P.W.; Welker, O.; Gulliford, D.; Nagy, W.; et al. Contrasting brain patterns of writing-related DTI parameters, fMRI connectivity, and DTI–fMRI connectivity correlations in children with and without dysgraphia or dyslexia. Neuroimage Clin. 2015, 8, 408–421. [Google Scholar] [CrossRef]
- Steinbrink, C.; Vogt, K.; Kastrup, A.; Müller, H.P.; Juengling, F.D.; Kassubek, J.; Riecker, A. The contribution of white and gray matter differences to developmental dyslexia: Insights from DTI and VBM at 3.0 T. Neuropsychologia 2008, 46, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Arndt, E.J.; Foorman, B.R. Second graders as spellers: What types of errors are they making? Assess. Eff. Interv. 2010, 36, 57–67. [Google Scholar] [CrossRef]
- Kemp, N.; Parrila, R.K.; Kirby, J.R. Phonological and orthographic spelling in high-functioning adult dyslexics. Dyslexia 2009, 15, 105–128. [Google Scholar] [CrossRef]
- Tops, W.; Callens, C.; Van Cauwenberghe, E.; Adriaens, J.; Brysbaert, M. Beyond spelling: The writing skills of students with dyslexia in higher education. Read. Writ. 2013, 26, 705–720. [Google Scholar] [CrossRef]
- Borkowska, A.R.; Francuz, P.; Soluch, P.; Wolak, T. Brain activation in teenagers with isolated spelling disorder during tasks involving spelling assessment and comparison of pseudowords. fMRI study. Brain Dev. 2014, 36, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Banfi, C.; Koschutnig, K.; Moll, K.; Schulte-Körne, G.; Fink, A.; Landerl, K. Reading-related functional activity in children with isolated spelling deficits and dyslexia. Lang. Cogn. Neurosci. 2020, 1–19. [Google Scholar] [CrossRef]
- Pugh, K.R.; Mencl, W.E.; Jenner, A.R.; Katz, L.; Frost, S.J.; Lee, J.R.; Shaywitz, S.E.; Shaywitz, B.A. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 207–213. [Google Scholar] [CrossRef]
- Riddick, B.; Sterling, C.; Farmer, M.; Morgan, S. Self-esteem and anxiety in the educational histories of adult dyslexic students. Dyslexia 1999, 5, 227–248. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef]
- Twait, E.; Farah, R.; Horowitz-Kraus, T. Decreased functional connectivity of the salience network during narrative comprehension in children with reading difficulties: An fMRI study. Neuroimage Clin. 2018, 20, 987–992. [Google Scholar] [CrossRef]
- Koyama, M.S.; Di Martino, A.; Kelly, C.; Jutagir, D.R.; Sunshine, J.; Schwartz, S.J.; Castellanos, F.X.; Milham, M.P. Cortical signatures of dyslexia and remediation: An intrinsic functional connectivity approach. PLoS ONE 2013, 8, e55454. [Google Scholar] [CrossRef]
- Chaddock-Heyman, L.; Weng, T.B.; Kienzler, C.; Erickson, K.I.; Voss, M.W.; Drollette, E.S.; Raine, L.B.; Kao, S.-C.; Hillman, C.H.; Kramer, A.F. Scholastic performance and functional connectivity of brain networks in children. PLoS ONE 2018, 13, e0190073. [Google Scholar] [CrossRef]
- Bailey, S.K.; Aboud, K.S.; Nguyen, T.Q.; Cutting, L.E. Applying a network framework to the neurobiology of reading and dyslexia. J. Neurodev. Disord. 2018, 10, 37. [Google Scholar] [CrossRef]
- Vogel, A.C.; Miezin, F.M.; Petersen, S.E.; Schlaggar, B.L. The putative visual word form area is functionally connected to the dorsal attention network. Cereb. Cortex 2012, 22, 537–549. [Google Scholar] [CrossRef]
- Smallwood, J.; Gorgolewski, K.J.; Golchert, J.; Ruby, F.J.M.; Engen, H.G.; Baird, B.; Vinski, M.T.; Schooler, J.W.; Margulies, D.S. The default modes of reading: Modulation of posterior cingulate and medial prefrontal cortex connectivity associated with comprehension and task focus while reading. Front. Hum. Neurosci. 2013, 7, 734. [Google Scholar] [CrossRef] [PubMed]
- Buchweitz, A.; Costa, A.C.; Toazza, R.; de Moraes, A.B.; Cara, V.M.; Esper, N.B.; Aguzzoli, C.; Gregolim, B.; Dresch, L.F.; Soldatelli, M.D.; et al. Decoupling of the occipitotemporal cortex and the brain’s default-mode network in dyslexia and a role for the cingulate cortex in good readers: A brain imaging study of Brazilian children. Dev. Neuropsychol. 2019, 44, 146–157. [Google Scholar] [CrossRef]
- Manis, F.R.; Seidenberg, M.S.; Doi, L.M.; McBride-Chang, C.; Petersen, A. On the bases of two subtypes of development dyslexia. Cognition 1996, 58, 157–195. [Google Scholar] [CrossRef]
- Torgeson, J.K.; Wagner, R.K.; Rashotte, C.A. Test of Word Reading Efficiency; Pro-Ed: Austin, TX, USA, 1999. [Google Scholar]
- Woodcock, R.W. Woodcock Reading Mastery Tests: Normative Update: WRMT-R/NU: Examiner’s Manual: Forms G and H; AGS: Circle Pines, MN, USA, 1998. [Google Scholar]
- Wilkinson, G.; Robertson, G. Wide Range Achievement Test, 4th ed.; WRAT-4; Western Psychological Services: Torrance, CA, USA, 2005. [Google Scholar]
- Pennington, B.F.; McCabe, L.L.; Smith, S.D.; Lefly, D.L.; Bookman, M.O.; Kimberling, W.J.; Lubs, H.A. Spelling errors in adults with a form of familial dyslexia. Child Dev. 1986, 57, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Bruck, M. Component spelling skills of college students with childhood diagnoses of dyslexia. Learn. Disabil. Q. 1993, 16, 171–184. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence; The Psychological Corporation: New York, NY, USA, 1999. [Google Scholar]
- Hsieh, L.; Rapp, B. Functional magnetic resonance imaging of the cognitive components of the spelling process. Brain Lang. 2004, 91, 40–41. [Google Scholar] [CrossRef]
- Ludersdorfer, P.; Kronbichler, M.; Wimmer, H. Accessing orthographic representations from speech: The role of left ventral occipitotemporal cortex in spelling. Hum. Brain Mapp. 2015, 36, 1393–1406. [Google Scholar] [CrossRef]
- Balota, D.A.; Yap, M.J.; Hutchison, K.A.; Cortese, M.J.; Kessler, B.; Loftis, B.; Neely, J.H.; Nelson, D.L.; Simpson, G.B.; Treiman, R. The English lexicon project. Behav. Res. Methods 2007, 39, 445–459. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.S.; Druzgal, T.J.; Lopez-Larson, M.; Jeong, E.K.; Desai, K.; Yurgelun-Todd, D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum. Brain Mapp. 2011, 32, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Birn, R.M.; Handwerker, D.A.; Jones, T.B.; Bandettini, P.A. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage 2009, 44, 893–905. [Google Scholar] [CrossRef]
- Saad, Z.S.; Gotts, S.J.; Murphy, K.; Chen, G.; Jo, H.J.; Martin, A.; Cox, R.W. Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012, 2, 25–32. [Google Scholar] [CrossRef]
- Norton, E.S.; Kovelman, I.; Petitto, L. Are There Separate Neural Systems for Spelling? New Insights into the Role of Rules and Memory in Spelling from Functional Magnetic Resonance Imaging. Mind Brain Educ. 2007, 1, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Rapp, B.; Lipka, K. The literate brain: The relationship between spelling and reading. J. Cogn. Neurosci. 2011, 23, 1180–1197. [Google Scholar] [CrossRef] [PubMed]
- Richlan, F. Developmental dyslexias: Dysfunction of a left hemisphere reading network. Front. Hum. Neurosci. 2012, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Shirer, W.R.; Ryali, S.; Rykhlevskaia, E.; Menon, V.; Greicius, M.D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex 2012, 22, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Spreng, R.N.; Stevens, W.D.; Chamberlain, J.P.; Gilmore, A.W.; Schacter, D.L. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 2010, 53, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Bitan, T.; Booth, J.R.; Choy, J.; Burman, D.D.; Gitelman, D.R.; Mesulam, M.M. Shifts of effective connectivity within a language network during rhyming and spelling. J. Neurosci. 2005, 25, 5397–5403. [Google Scholar] [CrossRef]
- Banfi, C.; Koschutnig, K.; Moll, K.; Schulte-Körne, G.; Fink, A.; Landerl, K. White matter alterations and tract lateralization in children with dyslexia and isolated spelling deficits. Hum. Brain Mapp. 2019, 40, 765–776. [Google Scholar] [CrossRef]
- Lee, J.C.; Dick, A.S.; Tomblin, J.B. Altered brain structures in the dorsal and ventral language pathways in individuals with and without developmental language disorder (DLD). Brain Imaging Behav. 2020, 14, 2569–2586. [Google Scholar] [CrossRef] [PubMed]
- Vandermosten, M.; Boets, B.; Poelmans, H.; Sunaert, S.; Wouters, J.; Ghesquiere, P. A tractography study in dyslexia: Neuroanatomic correlates of orthographic, phonological and speech processing. Brain 2012, 135, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The restless brain. Brain Connect. 2011, 1, 3–12. [Google Scholar] [CrossRef]
- Kelly, A.C.; Uddin, L.Q.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage 2008, 39, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, G.K.; Dougherty, R.F.; Bammer, R.; Siok, W.T.; Gabrieli, J.D.; Wandell, B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex 2005, 41, 354–363. [Google Scholar] [CrossRef]
- Richards, T.L.; Berninger, V.W.; Yagle, K.J.; Abbott, R.D.; Peterson, D.J. Changes in DTI Diffusivity and fMRI connectivity cluster coefficients for students with and without specific learning disabilities in written language: Brain’s response to writing instruction. J. Nat. Sci. 2017, 3, e350. [Google Scholar] [PubMed]
| Skilled Group Mean (SD) | Impaired Group Mean (SD) | t Values | p Values | |
|---|---|---|---|---|
| Age (years) | 21.58 (2.04) | 24.36 (5.36) | −2.08 | 0.46 |
| Gender (% female) | 73 | 71 | 0.14 | 0.89 |
| TOWRE-SWE fluency (raw scores) | 2.10 (0.26) | 1.81 (0.27) | 3.15 | 0.004 * |
| TOWRE-SWE fluency (standardized scores) | 96.63 (11.29) | 83.36 (8.78) | 3.59 | 0.001 * |
| TOWRE-PDE fluency (raw scores) | 1.36 (0.16) | 0.90 (0.23) | 6.73 | <0.001 ** |
| TOWRE-PDE fluency (standardized scores) | 105.53 (8.74) | 84.43 (9.42) | 6.63 | <0.001 ** |
| Word identification | 95 (0.05) | 79 (0.11) | 5.76 | <0.001 ** |
| Word Attack | 91 (0.07) | 72 (0.11) | 6.04 | <0.001 ** |
| Spelling (raw score out of 42) | 35.47 (2.89) | 28.29 (6.60) | 4.24 | <0.001 ** |
| Spelling (standardized score) | 84.58 (4.81) | 72.71 (10.22) | 4.45 | 0.001 * |
| Non-verbal IQ | 82 (0.07) | 81 (0.06) | 0.60 | 0.554 |
| O condition | ||||
| Reaction time | 861.24 (120.81) | 980.09 (161.74) | −2.42 | 0.022 * |
| Accuracy | 0.78 (0.09) | 0.69 (0.14) | 2.33 | 0.052 |
| OP condition | ||||
| Reaction time | 810.30 (125.17) | 927.22 (135.18) | −2.56 | 0.015 * |
| Accuracy | 0.86 (0.07) | 0.79 (0.11) | 2.40 | 0.046 * |
| P condition | ||||
| Reaction time | 846.19 (125.53) | 946.85 (125.83) | −2.30 | 0.029 * |
| Accuracy | 0.83 (0.10) | 0.80 (0.13) | 1.15 | 0.337 |
| (i) Inferior Frontal Gyrus | |||||||
| Skilled Group | Impaired Group | ||||||
| Targets | Beta | t(18) | p-FDR | Targets | Beta | t(13) | p-FDR |
| SMA | 0.27 | 5.44 | 0.000255 | SMA | 0.17 | 6.11 | 0.000262 |
| MTG | 0.17 | 3.6 | 0.007215 | MTG | 0.14 | 4.19 | 0.003689 |
| SMG | 0.11 | 2.94 | 0.013688 | STG | 0.14 | 3.12 | 0.018907 |
| STG | 0.11 | 2.91 | 0.013688 | AG | 0.13 | 2.48 | 0.046241 |
| FFG | 0.13 | 2.89 | 0.013688 | FFG | 0.09 | 2.38 | 0.046241 |
| (ii) Left Fusiform Gyrus | |||||||
| Skilled Group | Impaired Group | ||||||
| Targets | Beta | t(18) | p-FDR | Targets | Beta | t(13) | p-FDR |
| ITG | 0.42 | 5.65 | 0.000164 | ITG | 0.39 | 10.91 | < 0.0001 |
| SMG | 0.15 | 4.15 | 0.0021 | MTG | 0.19 | 4.54 | 0.001948 |
| IFG | 0.13 | 2.89 | 0.019768 | ||||
| MTG | 0.21 | 2.8 | 0.019768 | ||||
| STG | 0.11 | 2.72 | 0.019768 | ||||
| SMA | 0.21 | 2.37 | 0.034309 | ||||
| AG | −0.14 | −2.05 | 0.054903 | ||||
| (iii) Supramarginal Gyrus | |||||||
| Skilled Group | Impaired Group | ||||||
| Targets | Beta | t(18) | p-FDR | Targets | Beta | t(13) | p-FDR |
| FFG | 0.15 | 4.15 | 0.0042 | ITG | 0.14 | 3.02 | 0.048354 |
| SMA | 0.14 | 3.73 | 0.005043 | AG | 0.16 | 2.84 | 0.048354 |
| ITG | 0.2 | 3.58 | 0.005043 | ||||
| IFG | 0.11 | 2.94 | 0.015243 | ||||
| (i) Inferior Frontal Gyrus | |||||||
| Skilled Group | Impaired Group | ||||||
| Targets | Beta | t(18) | p-FDR | Targets | Beta | t(13) | p-FDR |
| ACC | 0.22 | 3.82 | 0.017542 | Right AInsula | 0.27 | 6.02 | 0.000601 |
| ACC | 0.22 | 5.53 | 0.000682 | ||||
| Left SMG | 0.21 | 4.96 | 0.001218 | ||||
| Left RPFC | 0.17 | 4.58 | 0.001806 | ||||
| Left IPS | 0.15 | 2.72 | 0.049002 | ||||
| (ii) Fusiform Gyrus | |||||||
| Skilled Group | Impaired Group | ||||||
| Targets | Beta | t(18) | p-FDR | Targets | Beta | t(13) | p-FDR |
| Left IPS | 0.26 | 5.82 | 0.000225 | Right IPS | 0.20 | 6.62 | 0.000248 |
| Left AInsula | 0.15 | 5.53 | 0.000225 | Left IPS | 0.23 | 5.54 | 0.000719 |
| MPFC | −0.13 | 3.52 | 0.009241 | ||||
| ACC | 0.16 | 3.52 | 0.009241 | ||||
| Left FEF | 0.15 | 3.13 | 0.017402 | ||||
| Right AInsula | 0.09 | 2.83 | 0.022545 | ||||
| Right IPS | 0.14 | 2.81 | 0.022545 | ||||
| Right FEF | 0.14 | 2.79 | 0.022545 | ||||
| (iii) Supramarginal Gyrus | |||||||
| Skilled Group | Impaired Group | ||||||
| Targets | Beta | t(18) | p-FDR | Targets | Beta | t(13) | p-FDR |
| Left IPS | 0.26 | 6.04 | 0.000146 | Left IPS | 0.31 | 6.92 | 0.000147 |
| Right IPS | 0.21 | 4.52 | 0.003995 | ||||
| Right SMG | 0.18 | 3.87 | 0.009018 | ||||
| ACC | 0.15 | 3.29 | 0.020636 | ||||
| Right AInsula | 0.15 | 3.07 | 0.023493 | ||||
| Left AInsula | 0.21 | 3.01 | 0.023493 | ||||
| Left RPFC | 0.14 | 2.52 | 0.050879 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheema, K.; Hodgetts, W.E.; Cummine, J. Is the Letter ‘t’ in the Word ‘gourmet’? Disruption in Task-Evoked Connectivity Networks in Adults with Impaired Literacy Skills. NeuroSci 2021, 2, 75-94. https://doi.org/10.3390/neurosci2010005
Cheema K, Hodgetts WE, Cummine J. Is the Letter ‘t’ in the Word ‘gourmet’? Disruption in Task-Evoked Connectivity Networks in Adults with Impaired Literacy Skills. NeuroSci. 2021; 2(1):75-94. https://doi.org/10.3390/neurosci2010005
Chicago/Turabian StyleCheema, Kulpreet, William E. Hodgetts, and Jacqueline Cummine. 2021. "Is the Letter ‘t’ in the Word ‘gourmet’? Disruption in Task-Evoked Connectivity Networks in Adults with Impaired Literacy Skills" NeuroSci 2, no. 1: 75-94. https://doi.org/10.3390/neurosci2010005
APA StyleCheema, K., Hodgetts, W. E., & Cummine, J. (2021). Is the Letter ‘t’ in the Word ‘gourmet’? Disruption in Task-Evoked Connectivity Networks in Adults with Impaired Literacy Skills. NeuroSci, 2(1), 75-94. https://doi.org/10.3390/neurosci2010005





