Cytokines in Pediatric Pilocytic Astrocytomas: A Clinico-Pathological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Demographics
2.2. Tumor Histopathological Diagnosis
2.3. Cerebrospinal Fluid Collection
2.4. Patient-Derived Tumour Cell Cultures and In Vitro Experiments
2.5. Molecular Investigations of Tumour and Patient-Derived Tumour Cell Lines
2.6. Real-Time Quantitative Polymerase Chain Reaction for mRNA Expression
2.7. Cytokine Multiplex Assay: CSF and Cell Supernatant Samples
2.8. Statistical Analysis
3. Results
3.1. Study Recruitment
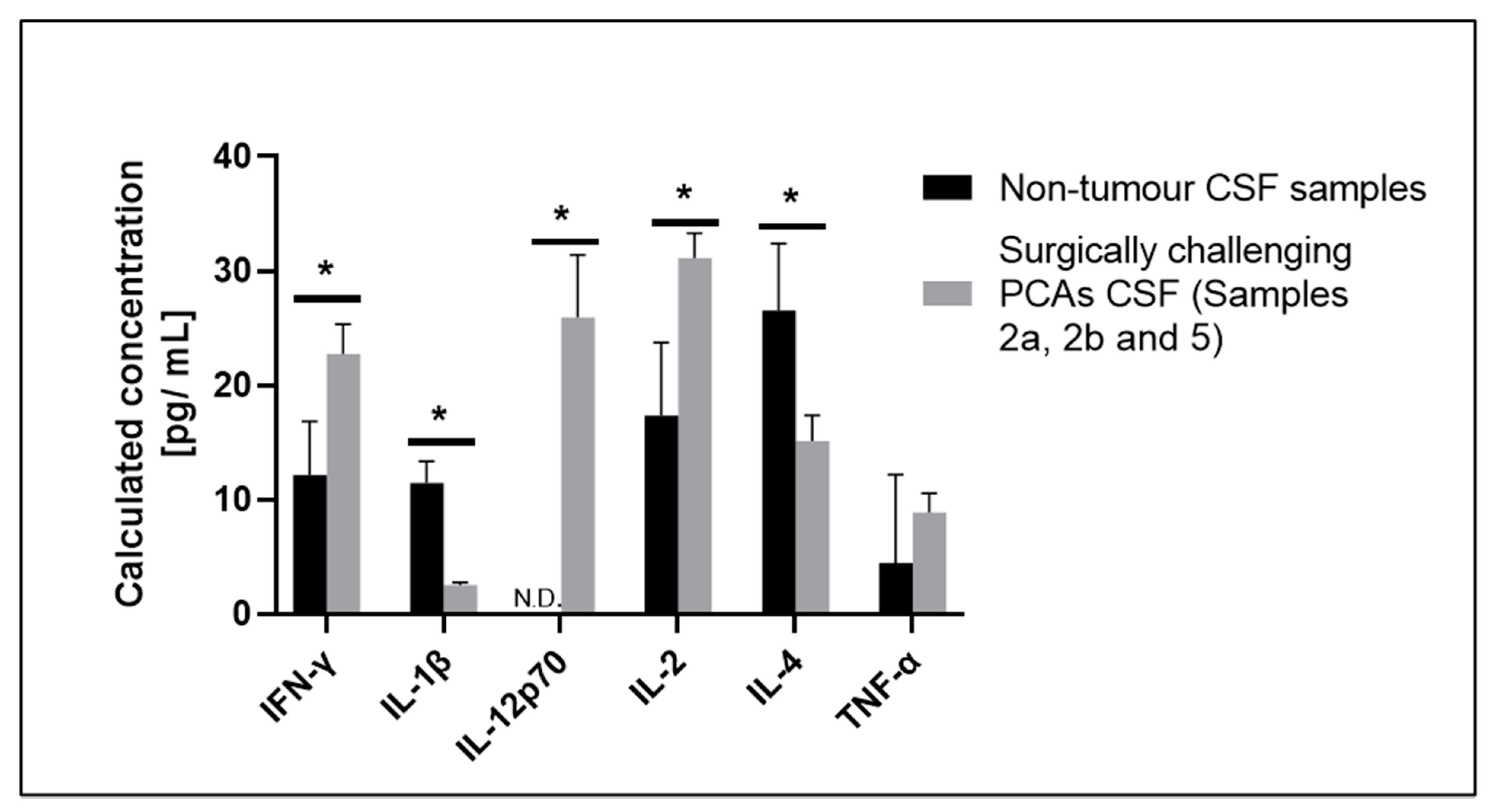
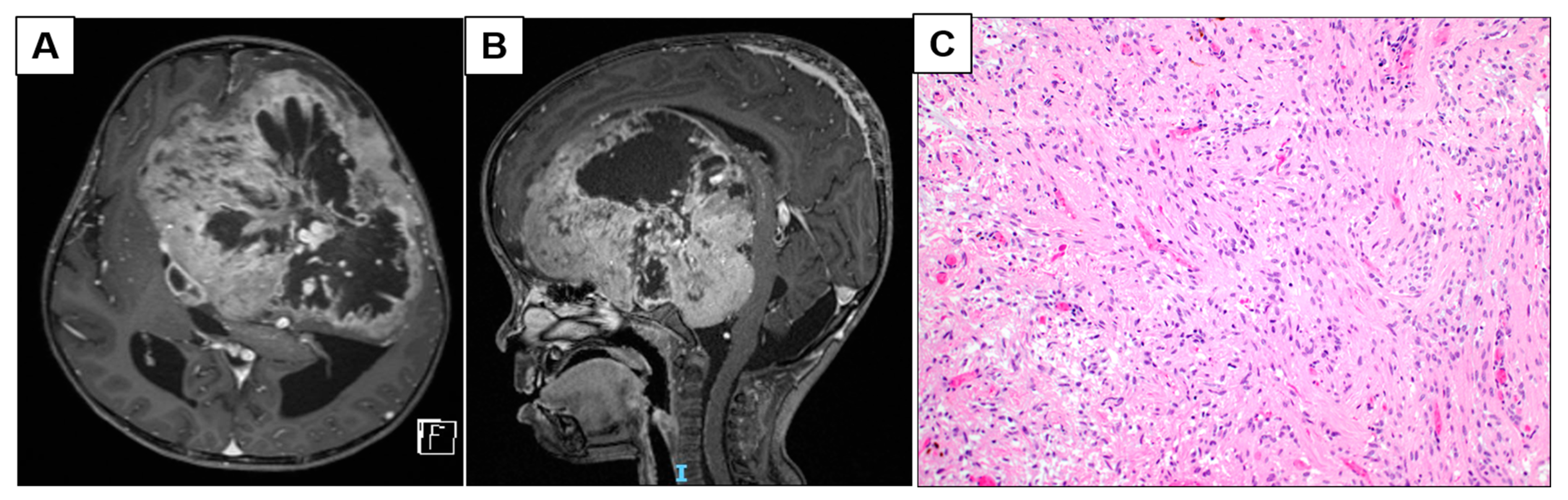
3.1.1. Patient 2: Large Frontotemporal PCA with Intratumoral Arterio-Venous Shunting
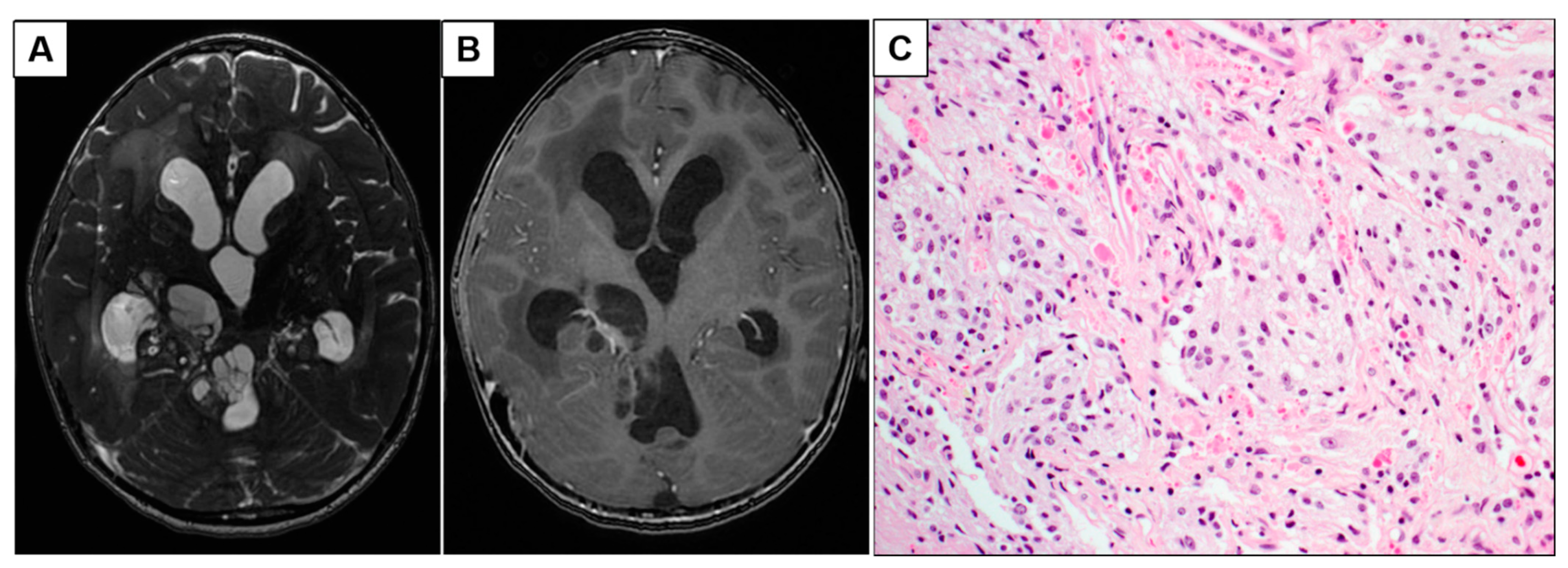
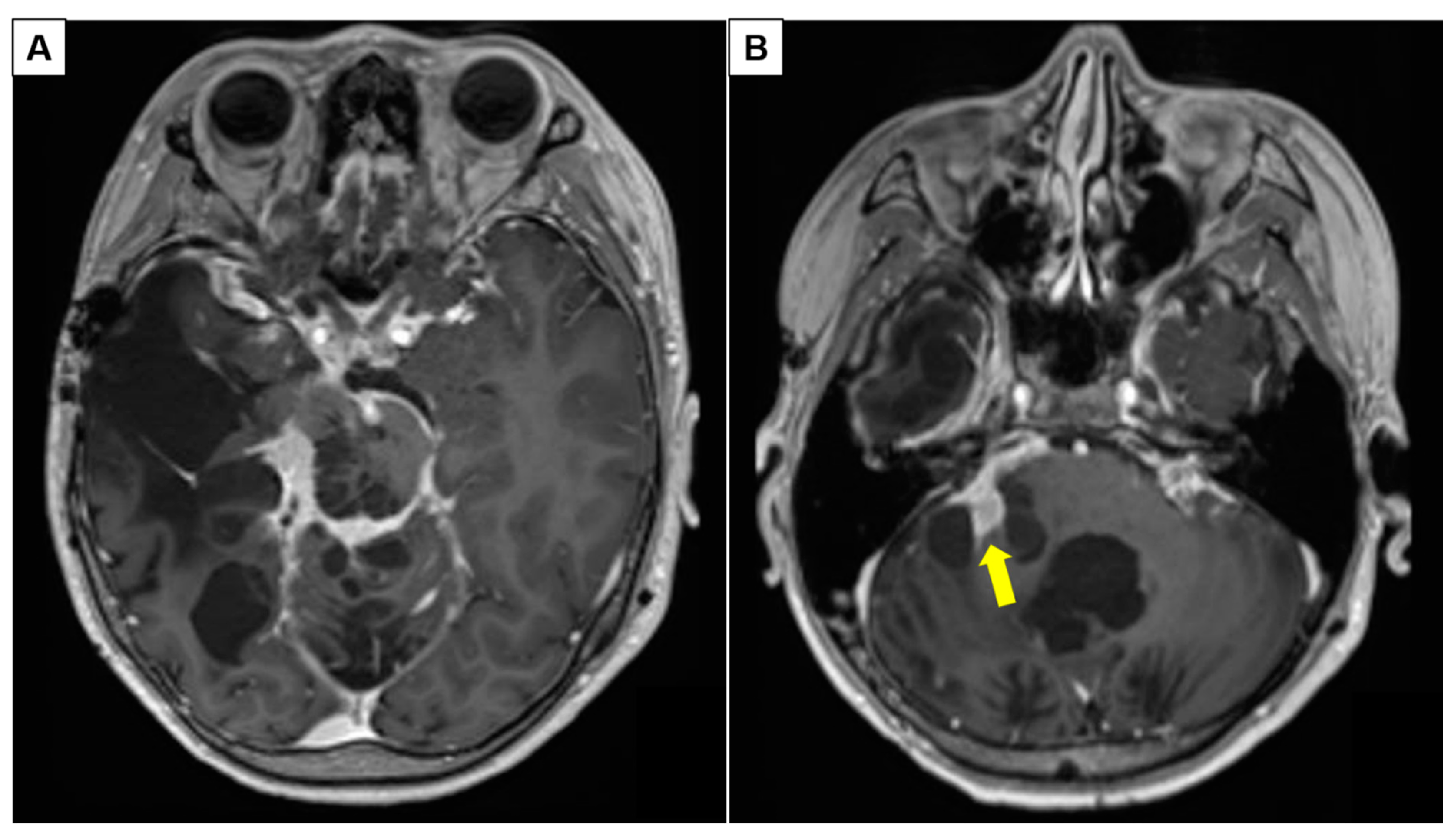
3.1.2. Patient 5: Progressive Intraventricular PCA with Leptomeningeal Disease
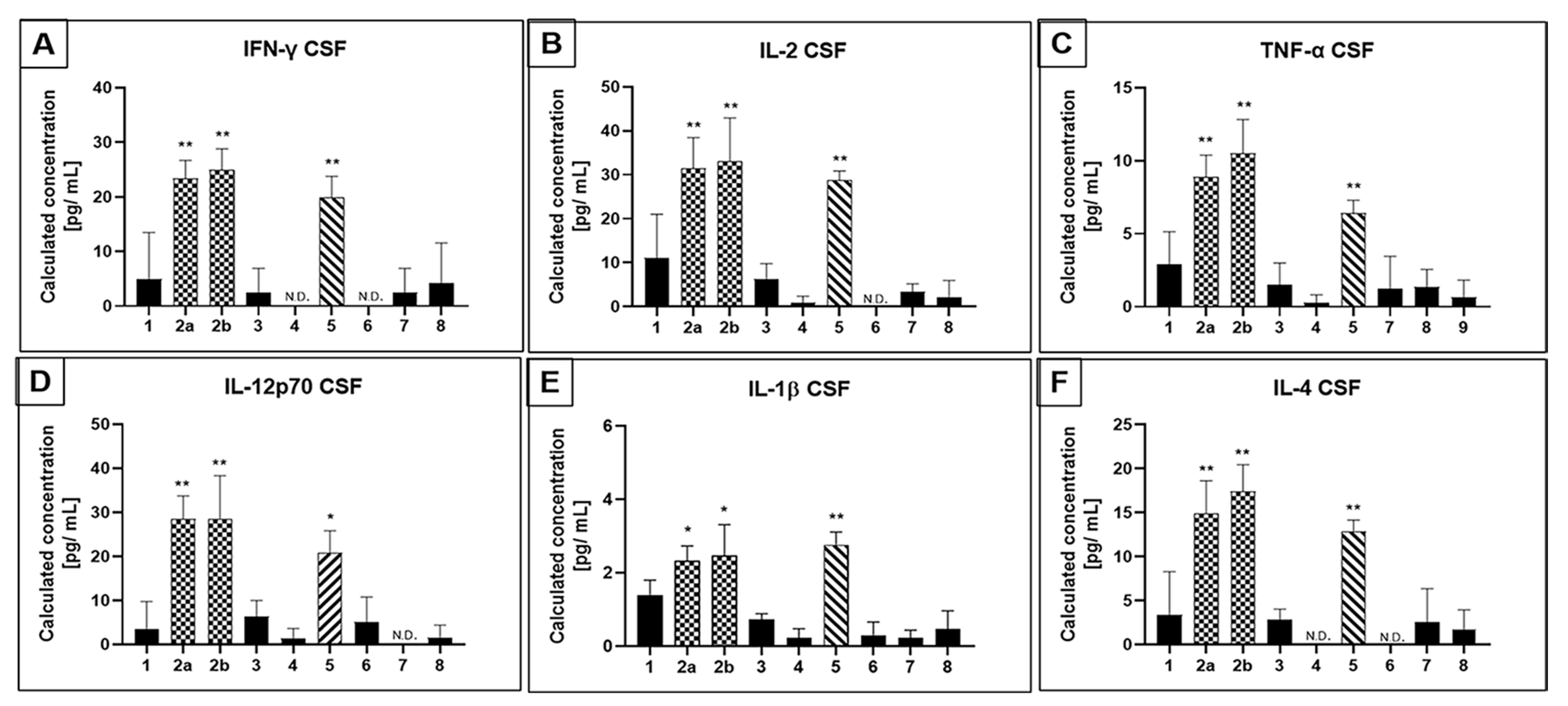
3.2. Selected CSF Cytokines Are Differentially Expressed in Therapeutically Challenging PCAs
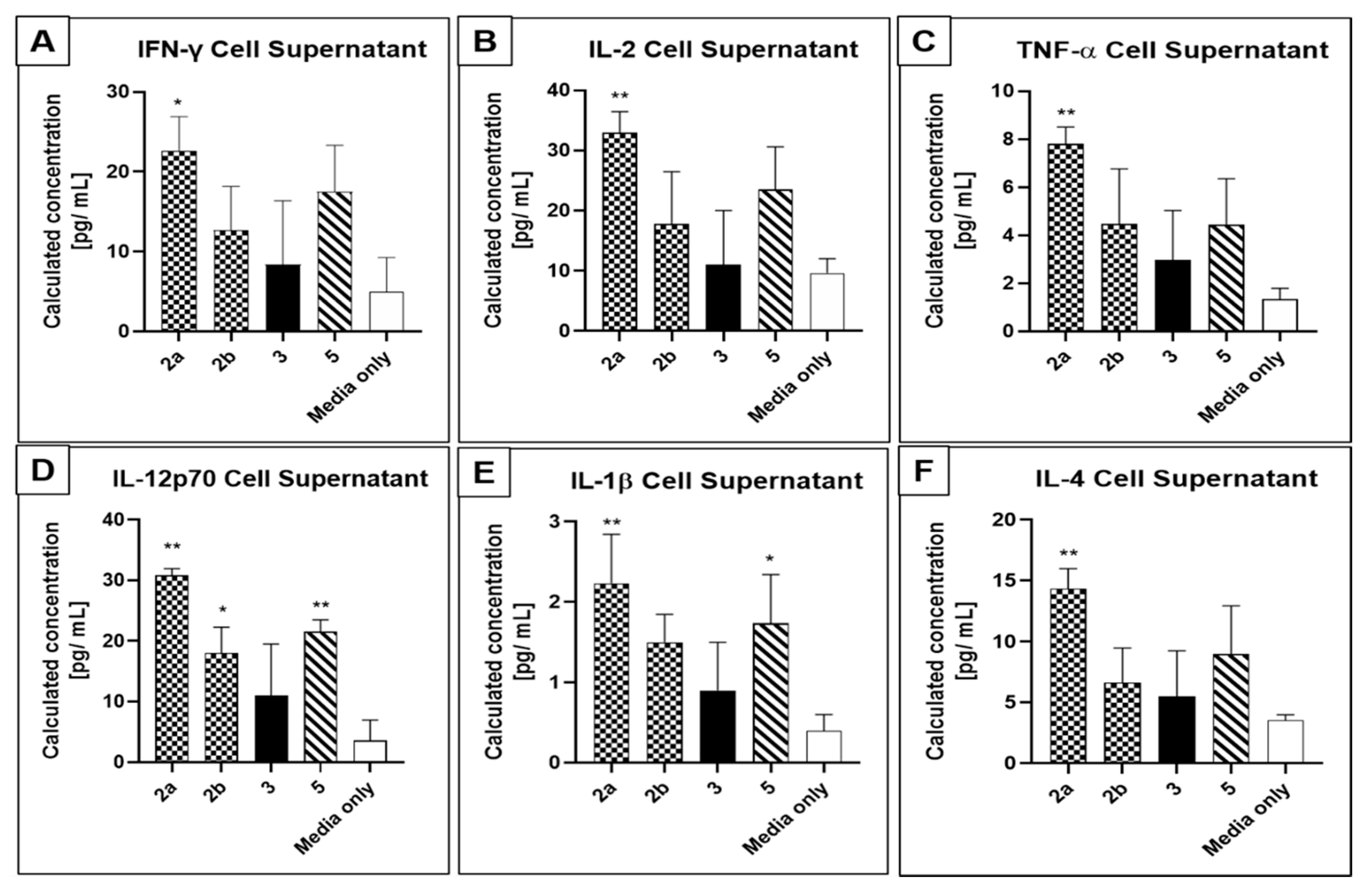
3.3. Patient-Derived Tumour Cells Express PCA Molecular Markers and Secrete Cytokines In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Baker, S.J.; Ellison, D.W.; Gutmann, D.H. Pediatric gliomas as neurodevelopmental disorders. Glia 2016, 64, 879–895. [Google Scholar] [CrossRef]
- Karajannis, M.; Allen, J.C.; Newcomb, E.W. Treatment of pediatric brain tumors. J. Cell. Physiol. 2008, 217, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Zamora, C.; Huisman, T.A.; Izbudak, I. Supratentorial Tumors in Pediatric Patients. Neuroimaging Clin. N. Am. 2017, 27, 39–67. [Google Scholar] [CrossRef]
- Pletschko, T.; Felnhofer, A.; Lamplmair, D.; Dorfer, C.; Czech, T.; Chocholous, M.; Slavc, I.; Leiss, U. Cerebellar pilocytic astrocytoma in childhood: Investigating the long-term impact of surgery on cognitive performance and functional outcome. Dev. Neurorehabil. 2018, 21, 415–422. [Google Scholar] [CrossRef]
- Collins, V.P.; Jones, D.T.; Giannini, C. Pilocytic astrocytoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015, 129, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Qaddoumi, I.; Sultan, I.; Broniscer, A. Pediatric low-grade gliomas and the need for new options for therapy: Why and how? Cancer Biol. Ther. 2009, 8, 4–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sadighi, Z.; Slopis, J. Pilocytic astrocytoma: A disease with evolving molecular heterogeneity. J. Child. Neurol. 2013, 28, 625–632. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Ellison, D.W.; Figarella-Branger, D.; Perry, A.; Reifenberger, G.; von Deimling, A. WHO Classification of Tumours of the Central Nervous System, 4th ed.; IARC: Lyon, France, 2016. [Google Scholar]
- Mueller, S.; Chang, S. Pediatric brain tumors: Current treatment strategies and future therapeutic approaches. Neurother. J. Am. Soc. Exp. Neurother. 2009, 6, 570–586. [Google Scholar] [CrossRef]
- Burkhard, C.; di Patre, P.L.; Schuler, D.; Schuler, G.; Yasargil, M.G.; Yonekawa, Y.; Lutolf, U.M.; Kleihues, P.; Ohgaki, H. A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J. Neurosurg. 2003, 98, 1170–1174. [Google Scholar] [CrossRef]
- Minehan, K.J.; Brown, P.D.; Scheithauer, B.W.; Krauss, W.E.; Wright, M.P. Prognosis and treatment of spinal cord astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Ait Khelifa-Gallois, N.; Laroussinie, F.; Puget, S.; Sainte-Rose, C.; Dellatolas, G. Long-term functional outcome of patients with cerebellar pilocytic astrocytoma surgically treated in childhood. Brain Inj. 2015, 29, 366–373. [Google Scholar] [CrossRef]
- Gnekow, A.K.; Falkenstein, F.; von Hornstein, S.; Zwiener, I.; Berkefeld, S.; Bison, B.; Warmuth-Metz, M.; Driever, P.H.; Soerensen, N.; Kortmann, R.D.; et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012, 14, 1265–1284. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, A.; Hodges, J.C.; Teh, B.S.; Chintagumpala, M.; Paulino, A.C. Outcome of patients with pilocytic astrocytoma and leptomeningeal dissemination. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 350–354. [Google Scholar] [CrossRef]
- Jones, D.T.; Gronych, J.; Lichter, P.; Witt, O.; Pfister, S.M. MAPK pathway activation in pilocytic astrocytoma. Cell Mol. Life Sci. 2012, 69, 1799–1811. [Google Scholar] [CrossRef]
- Choudhri, A.F.; Siddiqui, A.; Klimo, P., Jr. Pediatric Cerebellar Tumors: Emerging Imaging Techniques and Advances in Understanding of Genetic Features. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Rickert, C.H.; Paulus, W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv. Syst. 2001, 17, 503–511. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Conklin, H.M.; Huang, S.; Srivastava, D.; Sanford, R.; Ellison, D.W.; Merchant, T.E.; Hudson, M.M.; Hoehn, M.E.; Robison, L.L.; et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011, 13, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.W.; Kieran, M.W.; Bouffet, E.; Alexandrescu, S.; Bandopadhayay, P.; Bornhorst, M.; Ellison, D.; Fangusaro, J.; Fisher, M.J.; Foreman, N.; et al. Pediatric low-grade gliomas: Next biologically driven steps. Neuro Oncol. 2018, 20, 160–173. [Google Scholar] [CrossRef]
- Qian, B.Z. Inflammation fires up cancer metastasis. Semin. Cancer Biol. 2017, 47, 170–176. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Zamarron, B.F.; Chen, W. Dual roles of immune cells and their factors in cancer development and progression. Int. J. Biol. Sci. 2011, 7, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Albulescu, R.; Codrici, E.; Popescu, I.D.; Mihai, S.; Necula, L.G.; Petrescu, D.; Teodoru, M.; Tanase, C.P. Cytokine patterns in brain tumour progression. Mediat. Inflamm. 2013, 2013, 979748. [Google Scholar] [CrossRef]
- Shalaby, T.; Achini, F.; Grotzer, M.A. Targeting cerebrospinal fluid for discovery of brain cancer biomarkers. J. Cancer Metastasis Treat. 2016, 2, 176–187. [Google Scholar] [CrossRef]
- Corrales, L.; Scilla, K.; Caglevic, C.; Miller, K.; Oliveira, J.; Rolfo, C. Immunotherapy in Lung Cancer: A New Age in Cancer Treatment. Adv. Exp. Med. Biol. 2018, 995, 65–95. [Google Scholar] [CrossRef]
- Sambi, M.; Bagheri, L.; Szewczuk, M.R. Current Challenges in Cancer Immunotherapy: Multimodal Approaches to Improve Efficacy and Patient Response Rates. J. Oncol. 2019, 2019, 4508794. [Google Scholar] [CrossRef] [PubMed]
- Landi, D.B.; Thompson, E.M.; Ashley, D.M. Immunotherapy for pediatric brain tumors. Neuroimmunol. Neuroinflamm. 2018, 5, 29. [Google Scholar] [CrossRef][Green Version]
- Chamberlain, M.; Soffietti, R.; Raizer, J.; Ruda, R.; Brandsma, D.; Boogerd, W.; Taillibert, S.; Groves, M.D.; le Rhun, E.; Junck, L.; et al. Leptomeningeal metastasis: A Response Assessment in Neuro-Oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol. 2014, 16, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Leptomeningeal metastasis. Curr. Opin. Oncol. 2010, 22, 627–635. [Google Scholar] [CrossRef]
- Tian, Y.; Rich, B.E.; Vena, N.; Craig, J.M.; Macconaill, L.E.; Rajaram, V.; Goldman, S.; Taha, H.; Mahmoud, M.; Ozek, M.; et al. Detection of KIAA1549-BRAF fusion transcripts in formalin-fixed paraffin-embedded pediatric low-grade gliomas. J. Mol. Diagn. 2011, 13, 669–677. [Google Scholar] [CrossRef]
- Marx, V. Cell-line authentication demystified. Nat. Methods 2014, 11, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Ater, J.L.; Xia, C.; Mazewski, C.M.; Booth, T.N.; Freyer, D.R.; Packer, R.J.; Sposto, R.; Vezina, G.; Pollack, I.F. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children’s Oncology Group. Cancer 2016, 122, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Kocialkowski, S.; Liu, L.; Pearson, D.M.; Backlund, L.M.; Ichimura, K.; Collins, V.P. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008, 68, 8673–8677. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Hutter, B.; Jager, N.; Korshunov, A.; Kool, M.; Warnatz, H.J.; Zichner, T.; Lambert, S.R.; Ryzhova, M.; Quang, D.A.; et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat. Genet. 2013, 45, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.S.; Barakat, L.P.; Jones, N.L.; Ulrich, C.M.; Deatrick, J.A. Expectations for function and independence by childhood brain tumors survivors and their mothers. Narrat. Inq. Bioeth. 2014, 4, 233–251. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Kawashima, T.; Leisenring, W.; Stratton, K.; Stovall, M.; Hudson, M.M.; Sklar, C.A.; Robison, L.L.; Oeffinger, K.C. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J. Clin. Oncol. 2014, 32, 1218–1227. [Google Scholar] [CrossRef]
- Copeland, D.R.; Moore, B.D., 3rd; Francis, D.J.; Jaffe, N.; Culbert, S.J. Neuropsychologic effects of chemotherapy on children with cancer: A longitudinal study. J. Clin. Oncol. 1996, 14, 2826–2835. [Google Scholar] [CrossRef]
- Brown, R.T.; Sawyer, M.G.; Antoniou, G.; Toogood, I.; Rice, M. Longitudinal follow-up of the intellectual and academic functioning of children receiving central nervous system-prophylactic chemotherapy for leukemia: A four-year final report. J. Dev. Behav. Pediatr. 1999, 20, 373–377. [Google Scholar] [CrossRef]
- Mahone, E.M.; Prahme, M.C.; Ruble, K.; Mostofsky, S.H.; Schwartz, C.L. Motor and perceptual timing deficits among survivors of childhood leukemia. J. Pediatr. Psychol. 2007, 32, 918–925. [Google Scholar] [CrossRef]
- Espy, K.A.; Moore, I.M.; Kaufmann, P.M.; Kramer, J.H.; Matthay, K.; Hutter, J.J. Chemotherapeutic CNS prophylaxis and neuropsychologic change in children with acute lymphoblastic leukemia: A prospective study. J. Pediatr. Psychol. 2001, 26, 1–9. [Google Scholar] [CrossRef]
- Packer, R.J.; Pfister, S.; Bouffet, E.; Avery, R.; Bandopadhayay, P.; Bornhorst, M.; Bowers, D.C.; Ellison, D.; Fangusaro, J.; Foreman, N.; et al. Pediatric low-grade gliomas: Implications of the biologic era. Neuro Oncol. 2017, 19, 750–761. [Google Scholar] [CrossRef]
- Habiyaremye, G.; Morales, D.M.; Morgan, C.D.; McAllister, J.P.; CreveCoeur, T.S.; Han, R.H.; Gabir, M.; Baksh, B.; Mercer, D.; Limbrick, D.D., Jr. Chemokine and cytokine levels in the lumbar cerebrospinal fluid of preterm infants with post-hemorrhagic hydrocephalus. Fluids Barriers CNS 2017, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; Morales, D.M.; Arshad, R.; McAllister, J.P., 2nd; Limbrick, D.D., Jr. Cerebrospinal fluid biomarkers of neuroinflammation in children with hydrocephalus and shunt malfunction. Fluids Barriers CNS 2021, 18, 4. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in cancer immunotherapy. Oncoimmunology 2016, 5, e1163462. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Wang, L.; Zhang, C. Interplay between inflammatory tumor microenvironment and cancer stem cells (Review). Oncol. Lett. 2018, 16, 679–686. [Google Scholar] [CrossRef]
- Lemos, H.; Huang, L.; Chandler, P.R.; Mohamed, E.; Souza, G.R.; Li, L.; Pacholczyk, G.; Barber, G.N.; Hayakawa, Y.; Munn, D.H.; et al. Activation of the STING adaptor attenuates experimental autoimmune encephalitis. J. Immunol. 2014, 192, 5571–5578. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.Z.; Chouaib, S. Targeting hypoxia at the forefront of anticancer immune responses. Oncoimmunology 2014, 3, e954463. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Ealick, S.E.; Cook, W.J.; Vijay-Kumar, S.; Carson, M.; Nagabhushan, T.L.; Trotta, P.P.; Bugg, C.E. Three-dimensional structure of recombinant human interferon-gamma. Science 1991, 252, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.P.; Goncalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Mojic, M.; Takeda, K.; Hayakawa, Y. The Dark Side of IFN-gamma: Its Role in Promoting Cancer Immunoevasion. Int. J. Mol. Sci. 2017, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Hamanishi, J.; Abiko, K.; Matsumura, N.; Baba, T.; Konishi, I. Dual Faces of IFNgamma in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clin. Cancer Res. 2016, 22, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Nakamura, S.; Toki, M.; Motoda, R.; Kurimoto, M.; Orita, K. Identification of IFN-gamma-producing cells in IL-12/IL-18-treated mice. Cell Immunol. 1999, 198, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Cacalano, N.A.; Johnston, J.A. Interleukin-2 signaling and inherited immunodeficiency. Am. J. Hum. Genet. 1999, 65, 287–293. [Google Scholar] [CrossRef][Green Version]
- Choudhry, H.; Helmi, N.; Abdulaal, W.H.; Zeyadi, M.; Zamzami, M.A.; Wu, W.; Mahmoud, M.M.; Warsi, M.K.; Rasool, M.; Jamal, M.S. Prospects of IL-2 in Cancer Immunotherapy. BioMed Res. Int. 2018, 2018, 9056173. [Google Scholar] [CrossRef] [PubMed]
| Patient Number | Location of Tumor | Presence of KIAA-BRAF Fusion Gene (Yes/No) | BRAF V600E Mutant Positive (Yes/No) | LMD (Yes/No) | Adjuvant Treatment before Surgery (Yes/No) | CSF Cytology Positive for Tumour Cells (Yes/No) |
|---|---|---|---|---|---|---|
| 1 | Suprasellar | No | No | No | No | No |
| 2a^ | Left frontotemporal | Yes (15-9) | Yes | Yes | No | No |
| 2b^ | Left frontotemporal | Yes (15-9) | Yes | Yes | Yes (Chemotherapy) | No |
| 3 | Third ventricle | No | Yes | No | No | No |
| 4 | Third ventricle | No | No | No | No | No |
| 5* | Right thalamus, brainstem, and temporoparietal | No | Yes | Yes | Yes (Radiation) | No |
| 6 | Posterior fossa | No | No | No | No | No |
| 7 | Posterior fossa | Yes (15-9) | No | No | No | No |
| 8 | Posterior fossa | Yes (16-9) | No | No | No | No |
| Patient Number | Primary Tumor Positive for KIAA-BRAF (Yes/No) | Patient-Derived Cells Positive for KIAA-BRAF (Yes/No) | Primary Tumor Expresses GFAP (Yes/No) | Patient-Derived Cells Express GFAP (Yes/No) |
|---|---|---|---|---|
| 2a | Yes (15-9) | Yes (15-9) | Yes | Yes |
| 2b | Yes (15-9) | Yes (15-9) | Yes | Yes |
| 3 | No | No | Yes | Yes |
| 5 | No | No | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bte Syed Sulaiman, N.; Kuick, C.H.; Chang, K.T.E.; Wan, K.R.; Looi, W.S.; Low, D.C.Y.; Seow, W.T.; Low, S.Y.Y. Cytokines in Pediatric Pilocytic Astrocytomas: A Clinico-Pathological Study. NeuroSci 2021, 2, 95-108. https://doi.org/10.3390/neurosci2010006
Bte Syed Sulaiman N, Kuick CH, Chang KTE, Wan KR, Looi WS, Low DCY, Seow WT, Low SYY. Cytokines in Pediatric Pilocytic Astrocytomas: A Clinico-Pathological Study. NeuroSci. 2021; 2(1):95-108. https://doi.org/10.3390/neurosci2010006
Chicago/Turabian StyleBte Syed Sulaiman, Nurfarhanah, Chik Hong Kuick, Kenneth T. E. Chang, Kai Rui Wan, Wen Shen Looi, David C. Y. Low, Wan Tew Seow, and Sharon Y. Y. Low. 2021. "Cytokines in Pediatric Pilocytic Astrocytomas: A Clinico-Pathological Study" NeuroSci 2, no. 1: 95-108. https://doi.org/10.3390/neurosci2010006
APA StyleBte Syed Sulaiman, N., Kuick, C. H., Chang, K. T. E., Wan, K. R., Looi, W. S., Low, D. C. Y., Seow, W. T., & Low, S. Y. Y. (2021). Cytokines in Pediatric Pilocytic Astrocytomas: A Clinico-Pathological Study. NeuroSci, 2(1), 95-108. https://doi.org/10.3390/neurosci2010006




