Comprehensive Review of SBA-15 Mesoporous Silica: Functionalization Strategies, Diffusion Mechanisms, and Emerging Applications
Abstract
1. Introduction
1.1. Relevance and Applications of Mesoporous Materials
1.2. Review Methodology
2. Structural Characterization and Comparison Between Materials
2.1. Structure and Synthesis of SBA-15
2.2. Synthesis Methods and Their Effect on Diffusive Properties
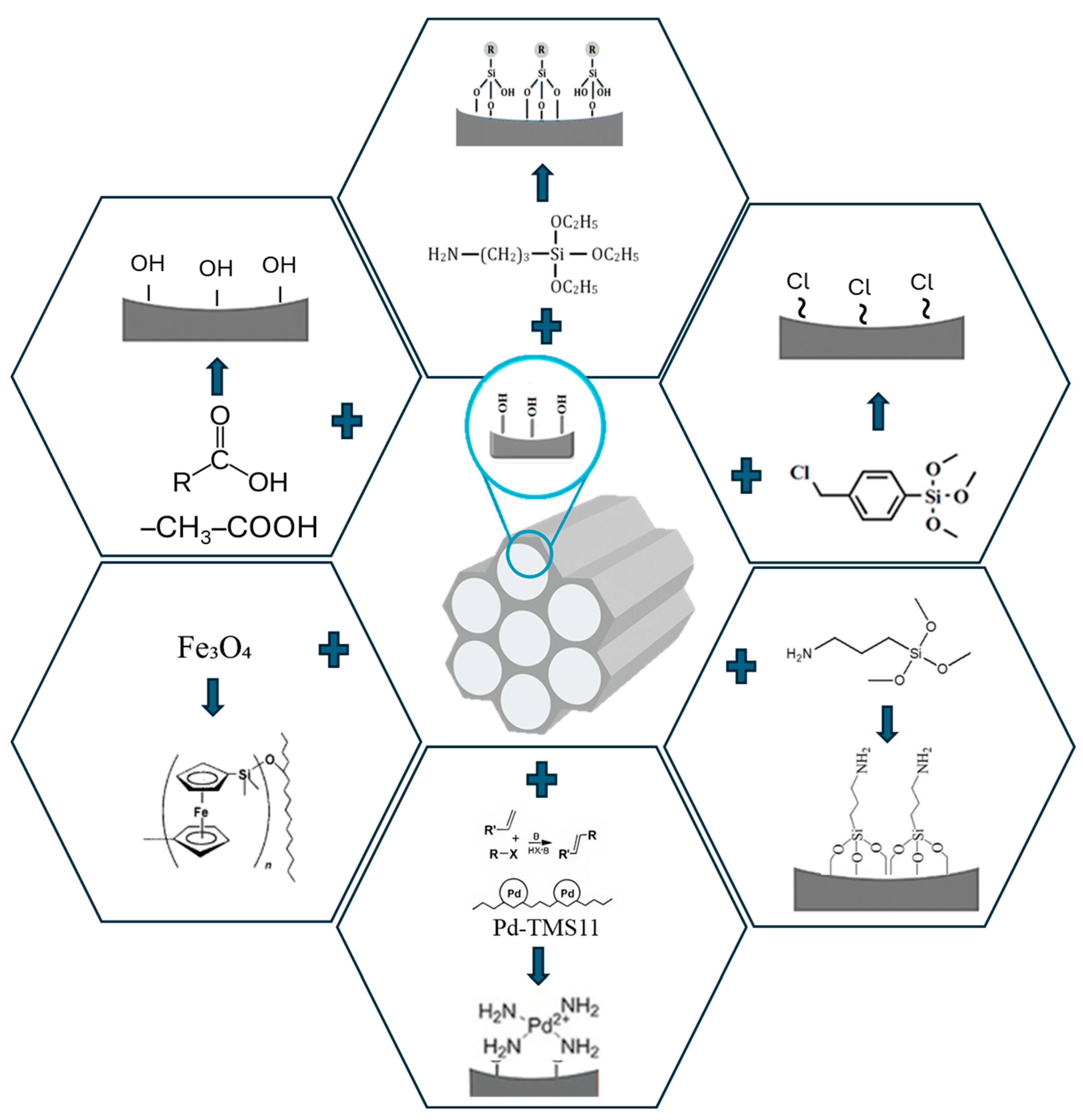
3. Functionalization of Mesoporous Materials: Strategies, Properties
4. Diffusion Mechanism of Substances in Mesopores: General Model Applied to SBA-15
4.1. Modeling Diffusion and Transport Mechanisms
4.1.1. Molecular Diffusion (Fickian)
4.1.2. Knudsen Diffusion
4.1.3. Combined Diffusion: Bosanquet Model
4.1.4. Surface Diffusion
4.1.5. Multicomponent Diffusion: Maxwell–Stefan Model
4.1.6. Adsorption–Desorption: Kinetic Mechanism
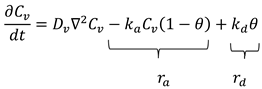

4.1.7. Contribution of Nonpolar van der Waals Forces
4.2. Effect of Functionalization on Diffusion and Transport Mechanisms
5. Challenges and Future Perspectives
6. Conclusions
7. Further Steps
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Symbol | Description | Units |
| Distance between active sites on the surface | m | |
| Attractive interaction constant between molecules or between a molecule and the surface of the material | J m6 | |
| Solute concentration in general (may vary across spatial domains) | mol m−3 | |
| Concentration at a radial distance r and time t | mol m−3 | |
| Solute concentration in the gas phase inside the porous channel | mol m−3 | |
| Initial solute concentration | mol m−3 | |
| Pore diameter | m | |
| Effective diffusion coefficient in the porous medium | m2 s−1 | |
| Diffusion coefficient in the channel volume (combined molecular and Knudsen) | m2 s−1 | |
| Molecular diffusion coefficient in the free phase (free motion between molecular collisions) | m2 s−1 | |
| Knudsen diffusion coefficient (dominated by collisions with pore walls) | m2 s−1 | |
| Surface diffusion coefficient (adsorbate migrating along the pore wall) | m2 s−1 | |
| Cross-diffusivity coefficient between species i and j | m2 s−1 | |
| Frictional diffusivity coefficient between species i and the porous solid matrix | m2 s−1 | |
| Overall effective diffusion coefficient in hierarchical materials | m2 s−1 | |
| Activation energy for surface migration (~5–30 kJ mol−1 for Van der Waals) | kJ mol−1 | |
| Adsorption energy | kJ mol−1 | |
| Total molar flux vector | mol m−2 s−1 | |
| Adsorption rate constant | s−1 | |
| Desorption rate constant | s−1 | |
| Interfacial spacing between porous domains (used in hierarchical transport) | m | |
| Distance between two particles or between an adsorbed molecule and the material surface | m | |
| Molar mass of the solute | kg mol−1 | |
| Net charge or transport factor of species i | dimensionless | |
| Radial distance from the pore axis | m | |
| Universal gas constant (8.314) | J mol−1 K−1 | |
| Absolute temperature | K | |
| Average molecular velocity of the solute | m s−1 | |
| Interfacial permeability coefficient in hierarchical transport models | m s−1 | |
| Mean free path of the molecule | m | |
| Porosity of the medium | dimensionless | |
| Tortuosity of the diffusive path | dimensionless | |
| Hopping frequency of adsorbate between sites (~1012 s−1) | s−1 | |
| Chemical potential of i | dimensionless | |
| Surface coverage fraction (occupied sites over total available) | dimensionless | |
| Mole fraction of species j | dimensionless | |
| Laplacian operator (gradient of the gradient) | m−2 | |
| Gradient of concentration field | mol m−4 | |
| Gradient of chemical potential of species i | J mol−1 m−1 | |
| Partial derivative related to time | s−1 | |
| Second partial derivative related to the radial coordinate | m−2 |
References
- Everett, D.H. Manual of Symbols and Terminology for Physicochemical Quantities and Units, Appendix II: Definitions, Terminology and Symbols in Colloid and Surface Chemistry. Pure Appl. Chem. 1972, 31, 577–638. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Asefa, T.; Dubovoy, V. 9.09—Ordered Mesoporous/Nanoporous Inorganic Materials via Self-Assembly. In Comprehensive Supramolecular Chemistry II; Atwood, J.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 9, pp. 157–192. [Google Scholar] [CrossRef]
- Shakeri, M.; Shal, Z.K.; Van Der Voort, P. An overview of the challenges and progress of synthesis, characterization and applications of plugged sba-15 materials for heterogeneous catalysis. Materials 2021, 14, 5082. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, M.; Liu, J.; Guo, B. Recent advances of SBA-15-based composites as the heterogeneous catalysts in water decontamination: A mini-review. J. Environ. Manag. 2020, 254, 109787. [Google Scholar] [CrossRef]
- Janus, R.; Wądrzyk, M.; Lewandowski, M.; Natkański, P.; Łątka, P.; Kuśtrowski, P. Understanding porous structure of SBA-15 upon pseudomorphic transformation into MCM-41: Non-direct investigation by carbon replication. J. Ind. Eng. Chem. 2020, 92, 131–144. [Google Scholar] [CrossRef]
- Asensio, J.; Beltrán, M.I.; Juárez-Serrano, N.; Berenguer, D.; Marcilla, A. Study of the Decomposition of N-Nitrosonornicotine (NNN) under Inert and Oxidative Atmospheres: Effect of the Addition of SBA-15 and MCM-41. Appl. Sci. 2022, 12, 9426. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y. Stabilization of Amine-Containing CO2 Adsorbents: Dramatic Effect of Water Vapor. J. Am. Chem. Soc. 2010, 132, 6312–6314. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous silica nanoparticles for drug delivery: Current insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Batonneau-Gener, I.; Ghssein, G.; Pouilloux, Y. Recent Advances in Heavy Metal Adsorption via Organically Modified Mesoporous Silica: A Review. Water 2025, 17, 669. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Dikio, E.D. Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Galarneau, A.; Cambon, H.; Di Renzo, F.; Fajula, F. True microporosity and surface area of mesoporous SBA-15 silicas as a function of synthesis temperature. Langmuir 2001, 17, 8328–8335. [Google Scholar] [CrossRef]
- Juárez-Serrano, N.; Asensio, J.; Blasco, I.; Beltrán, M.; Marcilla, A. Effect of Time, Temperature and Stirring Rate Used in the First Step of the Synthesis of SBA-15 on Its Application as Reductor of Tars in Tobacco Smoke. Catalysts 2021, 11, 375. [Google Scholar] [CrossRef]
- Grini, M.I.; Benbayer, C.; Saidi-Besbes, S.; Elaissari, A. Advances in mesoporous silica nanoparticles as carriers for drug delivery and other biomedical applications. Microporous Mesoporous Mater. 2025, 391, 113603. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Zhao, D. Functional Ordered Mesoporous Materials: Present and Future. Nano Lett. 2022, 22, 3177–3179. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T. Increasing selective CO2 adsorption on amine-grafted SBA-15 by increasing silanol density. J. Phys. Chem. C 2011, 115, 21264–21272. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. A review: Mesoporous Santa Barbara amorphous-15, types, synthesis and its applications towards biorefinery production. Am. J. Appl. Sci. 2010, 7, 1579–1586. [Google Scholar] [CrossRef]
- Pérez-Page, M.; Yu, E.; Li, J.; Rahman, M.; Dryden, D.M.; Vidu, R.; Stroeve, P. Template-based syntheses for shape controlled nanostructures. Adv. Colloid Interface Sci. 2016, 234, 51–79. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Juárez-Serrano, N.; Berenguer, D.; Martínez-Castellanos, I.; Blasco, I.; Beltrán, M.; Marcilla, A. Effect of reaction time and hydrothermal treatment time on the textural properties of SBA-15 synthesized using sodium silicate as a silica source and its efficiency for reducing tobacco smoke toxicity. Catalysts 2021, 11, 808. [Google Scholar] [CrossRef]
- Asefa, T.; MacLachlan, M.J.; Coombs, N.; Ozin, G.A. Periodic mesoporous organosilicas with organic groups inside the channel walls. Nature 1999, 402, 867–871. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Serra, E.; Blanco, R.M.; Díaz, I. Síntesis y Caracterización de Materiales Mesoporosos Ordenados y su Aplicación como Soportes en la Inmovilización de Lipasa. An. Quím. 2008, 104, 97–103. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=2662603 (accessed on 15 April 2025).
- Han, W.; Jia, Y.; Xiong, G.; Yang, W. Template-free sol-gel synthesis of mesoporous materials with ZSM-5 structure walls. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 165, pp. 515–518. [Google Scholar] [CrossRef]
- Isaacs, M.A.; Robinson, N.; Barbero, B.; Durndell, L.J.; Manayil, J.C.; Parlett, C.M.A.; D’Agostino, C.; Wilson, K.; Lee, A.F. Unravelling mass transport in hierarchically porous catalysts. J. Mater. Chem. A 2019, 7, 11814–11825. [Google Scholar] [CrossRef]
- Asfaha, Y.G.; Tekile, A.K.; Zewge, F. Hybrid process of electrocoagulation and electrooxidation system for wastewater treatment: A review. Clean. Eng. Technol. 2021, 4, 100261. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, W. Microwave-assisted Inorganic Syntheses. In Modern Inorganic Synthetic Chemistry; Elsevier: Amsterdam, The Netherlands, 2011; pp. 173–195. [Google Scholar] [CrossRef]
- Vigón, P.V. Síntesis de Materiales Mesoporosos Compuestos Sílice/Carbono y su Empleo como Plataforma para la Fabricación de Materiales con Propiedades Avanzadas. Ph.D. Thesis, Universidad de Oviedo, Oviedo, Spain, 2013. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=82387 (accessed on 17 May 2025).
- Martínez, J.G. Sólidos Ordenados Desde la Nano a la Macroestructura. An. Quim. 2006, 1, 5–12, ISSN 1575-341 (Print), 2792-5250 (Electronic). Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=1996641 (accessed on 17 May 2025).
- Williams, J.H.; Zheng, Q.; Mantle, M.D.; Sederman, A.J.; Gladden, L.F. Probing the Diffusion Mechanism of n-Alkanes in Mesoporous Confinement Using Pulsed Field Gradient NMR. J. Phys. Chem. C 2023, 127, 15326–15335. [Google Scholar] [CrossRef]
- Kumar, P.; Guliants, V.V. Periodic mesoporous organic–inorganic hybrid materials: Applications in membrane separations and adsorption. Microporous Mesoporous Mater. 2010, 132, 1–14. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, Y.; Zhang, Y.; Zhu, T.; Wang, X. Functionalization of SBA-15 mesoporous silica with bis-schiff base for the selective removal of Pb(II) from water. J. Solid State Chem. 2021, 301, 122320. [Google Scholar] [CrossRef]
- Li, D.; Chai, K.; Yao, X.; Zhou, L.; Wu, K.; Huang, Z.; Yan, J.; Qin, X.; Wei, W.; Ji, H. β-Cyclodextrin functionalized SBA-15 via amide linkage as a super adsorbent for rapid removal of methyl blue. J. Colloid Interface Sci. 2021, 583, 100–112. [Google Scholar] [CrossRef]
- Chuin, L.X.; Kamaruzaman, S.; Praveena, S.M.; Yahaya, N. Recent applications of β-cyclodextrin in selective adsorption of pesticides, heavy metals, and organic pollutants from water samples: Mini review. Microchem. J. 2024, 206, 111583. [Google Scholar] [CrossRef]
- da Silva, F.d.C.M.; Costa, M.J.d.S.; da Silva, L.K.R.; Batista, A.M.; da Luz, G.E., Jr. Functionalization methods of SBA-15 mesoporous molecular sieve: A brief overview. SN Appl. Sci. 2019, 1, 654. [Google Scholar] [CrossRef]
- Shimon, D.; Chen, C.-H.; Lee, J.J.; Didas, S.A.; Sievers, C.; Jones, C.W.; Hayes, S.E. 15N Solid State NMR Spectroscopic Study of Surface Amine Groups for Carbon Capture: 3-Aminopropylsilyl Grafted to SBA-15 Mesoporous Silica. Environ. Sci. Technol. 2018, 52, 1488–1495. [Google Scholar] [CrossRef]
- Ojeda-López, R.; Pérez-Hermosillo, I.J.; Esparza-Schulz, J.M.; Cervantes-Uribe, A.; Domínguez-Ortiz, A. SBA-15 materials: Calcination temperature influence on textural properties and total silanol ratio. Adsorption 2015, 21, 659–669. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, J.; Heeres, H.J.; Melián-Cabrera, I. Thermal detemplation of SBA-15 mesophases. Effect of the activation protocol on the framework contraction. Microporous Mesoporous Mater. 2013, 176, 103–111. [Google Scholar] [CrossRef]
- Szegedi, Á.; Lázár, K.; Solt, H.; Popova, M. Peculiar redox properties of SBA-15 supported copper ferrite catalysts promoting total oxidation of a model volatile organic air pollutant. Surfaces Interfaces 2025, 56, 105498. [Google Scholar] [CrossRef]
- Gil, A.G.; Wu, Z.; Chadwick, D.; Li, K. Ni/SBA-15 Catalysts for combined steam methane reforming and water gas shift—Prepared for use in catalytic membrane reactors. Appl. Catal. A Gen. 2015, 506, 188–196. [Google Scholar] [CrossRef]
- Laskowski, Ł.; Laskowska, M.; Bałanda, M.; Fitta, M.; Kwiatkowska, J.; Dziliński, K.; Karczmarska, A. Mesoporous silica SBA-15 functionalized by nickel–phosphonic units: Raman and magnetic analysis. Microporous Mesoporous Mater. 2014, 200, 253–259. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, Y.; Guo, Y.; Miao, S.; Chen, Q.; Chen, Z. Functionalization of SBA-15 mesoporous materials with 2-acetylthiophene for adsorption of Cr(III) ions. Microporous Mesoporous Mater. 2020, 292, 109754. [Google Scholar] [CrossRef]
- Xia, X.; Jin, Y.; Zhao, H.; Wang, G.; Huang, D. Optimization and Experiment of Hot Air Drying Process of Cyperus esculentus Seeds. Agriculture 2023, 13, 617. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.T. Template Removal from SBA-15 by Ionic Liquid for Amine Grafting: Applications to CO2 Capture and Natural Gas Desulfurization. ACS Sustain. Chem. Eng. 2020, 8, 8295–8304. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic–inorganic hybrid materials. Angew. Chem. Int. Ed. Engl. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, Z.-F.; Wen, Z.-H.; Yuan, A.-H.; Liu, X.-Q.; Zhang, Z.-Z.; Zhou, H. Modification of as Synthesized SBA-15 with Pt nanoparticles: Nanoconfinement Effects Give a Boost for Hydrogen Storage at Room Temperature. Sci. Rep. 2017, 7, 4509. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, T.; Xu, X.; Xiao, P.; Li, J. Pt nanoparticles supported on SBA-15: Synthesis, characterization and applications in heterogeneous catalysis. Appl. Catal. B Environ. 2013, 130–131, 197–217. [Google Scholar] [CrossRef]
- Laskowski, Ł.; Laskowska, M.; Jelonkiewicz, J.; Galkowski, T.; Pawlik, P.; Piech, H.; Doskocz, M. Iron Doped SBA-15 Mesoporous Silica Studied by Mössbauer Spectroscopy. J. Nanomater. 2016, 2016, 1256851. [Google Scholar] [CrossRef]
- Dudarko, O.; Kobylinska, N.; Mishra, B.; Kessler, V.G.; Tripathi, B.P.; Seisenbaeva, G.A. Facile strategies for synthesis of functionalized mesoporous silicas for the removal of rare-earth elements and heavy metals from aqueous systems. Microporous Mesoporous Mater. 2021, 315, 110919. [Google Scholar] [CrossRef]
- Larki, A.; Saghanezhad, S.J.; Ghomi, M. Recent advances of functionalized SBA-15 in the separation/preconcentration of various analytes: A review. Microchem. J. 2021, 169, 106601. [Google Scholar] [CrossRef]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; García-Martínez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef]
- Albayati, T.M.; Sabri, A.A.; Abed, D.B. Adsorption of binary and multi heavy metals ions from aqueous solution by amine functionalized SBA-15 mesoporous adsorbent in a batch system. Desalination Water Treat. 2019, 151, 315–321. [Google Scholar] [CrossRef]
- Kärger, J.; Ruthven, D.M. Diffusion in nanoporous materials: Fundamental principles, insights and challenges. New J. Chem. 2016, 40, 4027–4048. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Kärger, J.; Valiullin, R. Mass transfer in mesoporous materials: The benefit of microscopic diffusion measurement. Chem. Soc. Rev. 2013, 42, 4172–4197. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, S. The Method of Volume Averaging; Theory and Applications of Transport in Porous Media; Springer: Dordrecht, The Netherlands, 1999; Volume 13. [Google Scholar] [CrossRef]
- Bird, R.B.; Earl, S.W.; Lightfoot, E.N. Fenómenos de Transporte, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; Available online: https://books.google.com/books/about/Transport_Phenomena.html?id=L5FnNlIaGfcC (accessed on 17 May 2025).
- Kärger, J.; Freude, D.; Haase, J. Diffusion in nanoporous materials: Novel insights by combining MAS and PFG NMR. Processes 2018, 6, 147. [Google Scholar] [CrossRef]
- Zhokh, A. Size-controlled non-Fickian diffusion in a combined micro- and mesoporous material. Chem. Phys. 2019, 520, 27–31. [Google Scholar] [CrossRef]
- Duong, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998; Volume 1, p. 916. [Google Scholar] [CrossRef]
- Hoang, V.-T.; Huang, Q.; Eić, M.; Do, T.-O.; Kaliaguine, S. Structure and diffusion characterization of SBA-15 materials. Langmuir 2005, 21, 2051–2057. [Google Scholar] [CrossRef]
- Rusinque, H.; Brenner, G. Mass transport in porous media at the micro- and nanoscale: A novel method to model hindered diffusion. Microporous Mesoporous Mater. 2019, 280, 157–165. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, K.; Du, S.-W.; Wu, L.-G.; Li, C.-J.; Chen, H.L.; Guo, H.-C. Transformation from Knudsen diffusion to facilitated diffusion for CO2 in confined mass transfer channels of polyimide mixed matrix membranes. Chem. Eng. J. 2023, 477, 147280. [Google Scholar] [CrossRef]
- Vignes, A. Diffusion in Binary Solutions Variation of Diffusion Coefficient with Composition. Ind. Eng. Chem. Fundam. 1966, 5, 189–199. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, Y.; Wang, L.; Gong, H. A theoretical insight into diffusion mechanism of aldol condensation of acetaldehyde in Zr-BEA zeolite. Mol. Catal. 2025, 572, 114770. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–676. [Google Scholar] [CrossRef]
- Subagyono, R.R.D.J.N.; Chaffee, A.L. CO2 adsorption on SBA-15: A molecular modelling. IOP Conf. Ser. Earth Environ. Sci. 2018, 144, 012045. [Google Scholar] [CrossRef]
- Obeso, J.L.; López-Cervantes, V.B.; Ojeda-López, R.; Yañez-Aulestia, A.; Cordero-Sánchez, S.; Sánchez-González, E.; Novelo-Peralta, O.; Morales, K.E.R.; Solís-Ibarra, D.; Ibarra, I.A.; et al. SO2 Capture and Detection by SBA-15 and Amine-Functionalized SBA-15. Ind. Eng. Chem. Res. 2024, 63, 2223–2230. [Google Scholar] [CrossRef]
- Lacarra-Etxarri, I.; De La Torre, U.; González-Marcos, J.A.; González-Velasco, J.R.; Pereda-Ayo, B. Polyethylenimine-functionalized SBA-15 mesoporous silica for CO2 direct air capture and conversion to methane in a coupled catalytic reactor. J. CO2 Util. 2025, 97, 103134. [Google Scholar] [CrossRef]
- Niu, Y.; Zou, S.; Liu, H.; Hu, M.; Cai, J.; Li, C.; Mumford, K.A.; Alivand, M.S.; Barzagli, F.; Zhang, R. Functionalized SBA-15-based catalysts for energy-efficient CO2 desorption: Bridging experimentation and machine learning to enhance amine sorbents regeneration. Chem. Eng. J. 2025, 522, 167944. [Google Scholar] [CrossRef]
- Yang, W.; Shirazian, S.; Soltani, R.; Zare, M.H. Bio-originated mesosilicate SBA-15: Synthesis, characterization, and application for heavy metal removal. npj Clean Water 2024, 7, 49. [Google Scholar] [CrossRef]
- García-Martínez, J.; Serrano-Torregrosa, E. Chemistry Education: Best Practices, Opportunities and Trends; Wiley: Hoboken, NJ, USA, 2015; ISBN 978-3-527-33605-0. [Google Scholar]
- Liang, B.; Zhu, P.; Gu, J.; Yuan, W.; Xiao, B.; Hu, H.; Rao, M. Advancing Adsorption and Separation with Modified SBA-15: A Comprehensive Review and Future Perspectives. Molecules 2024, 29, 3543. [Google Scholar] [CrossRef] [PubMed]
- Gatti, G.; Costenaro, D.; Vittoni, C.; Paul, G.; Crocellà, V.; Mangano, E.; Brandani, S.; Bordiga, S.; Cossi, M.; Marchese, L. CO2 adsorption on different organo-modified SBA-15 silicas: A multidisciplinary study on the effects of basic surface groups. Phys. Chem. Chem. Phys. 2017, 19, 14114–14128. [Google Scholar] [CrossRef]
- Wawrzyńczak, A.; Nowak, I.; Feliczak-Guzik, A. SBA-15- and SBA-16-Functionalized Silicas as New Carriers of Niacinamide. Int. J. Mol. Sci. 2023, 24, 17567. [Google Scholar] [CrossRef]
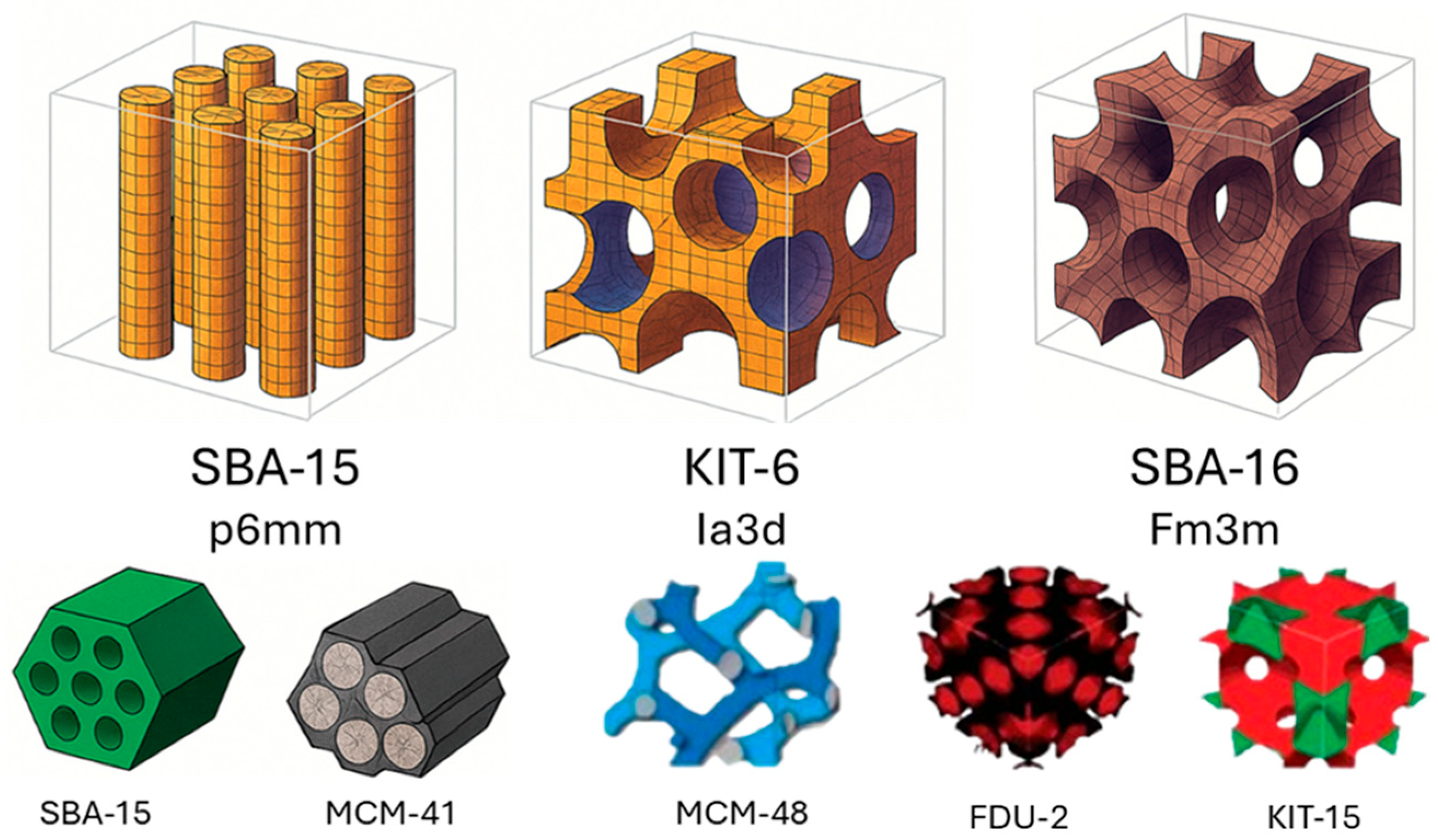
| Mesoporous Material | Structure | Pore Size (nm) | Surface Area (m2 g−1) | Typical Synthesis Method | Ref. |
|---|---|---|---|---|---|
| MCM-41 | Hexagona lp6mm | 2–10 | 800–1000 | CTAB-assisted sol–gel | [4,25] |
| SBA-15 | Hexagonal p6mm | 5–30 | 500–1000 | P123-templated sol–gel | [20,25] |
| KIT-6 | Cubic Ia3d | 6–12 | 400–900 | P123/butanol cosurfactant system | [27,29] |
| FDU-12 | Cubic Fm3m | 5–15 | 600–900 | Modified triblock copolymers | [27,30] |
| MSU-X | Disordered | 2–12 | 500–700 | Nonionic self-assembly | [29] |
| TUD-1 | Interconnected | 3–10 | 600–850 | Surfactant-assisted sol–gel | [29] |
| MCM-48 | Cubic Ia3d | 2–4 | 800–1100 | CTAB-templated sol–gel | [4,25] |
| FSM-16 | Lamellar | 2–3 | 900–1000 | Surfactant intercalation | [4] |
| Synthesis Method | Conditions | Pore Size (nm) | (m2 s−1) | Key Advantages | Ref. |
|---|---|---|---|---|---|
| Conventional sol–gel | HCl, 35–40 °C, 24–72 h | 6–8 | ~1.0 × 10−8 | High ordering, easy scalability | [22] |
| Hydrothermal | 100–130 °C, 24–48 h | 6–10 | 1.2 × 10−8 | Enhanced crystallinity, structural stability | [8,27] |
| Microwave-assisted | 2.45 GHz, 80–100 °C, 1–2 h | 5–8 | 3.5 × 10−7 | Rapid synthesis, uniform particle size control | [3,30] |
| Sonochemical | Ultrasonic frequency, low T | 5–9 | 1.5 × 10−7 | Improved homogeneity, morphological dispersion | [21] |
| Solvothermal | Organic solvents, high pressure | 5–7 | ~1.8 × 10−8 | Precise shape/crystallinity/particle size control | [4] |
| Dual-template (P123/CTAB) | Mixed surfactants | 4–12 (bimodal) | ~1.0 × 10−8 | Hierarchical meso-macroporous structures | [15] |
| Post-synthesis grafting | Grafting con –NH2, tioles, etc. | 4–7 | 2.8 × 10−7 | Tailored surfaces for selective adsorption/catalysis | [10,19] |
| Base Material | Functional Group/Modifier | Incorporation Method | Conditions | Application | Ref. |
|---|---|---|---|---|---|
| SBA-15 | –NH2 (aminopropyl, APTES) | Grafting | EtOH, 60–80 °C, 12–24 h | CO2 capture, drug immobilization, VOCs | [10,39,47,48] |
| SBA-15 | Bis-Schiff base | 3-step anchoring (silanization + condensation) | Organic solvent, RT–80 °C | Selective removal of Pb(II) and other metals | [12,35] |
| SBA-15 | –COOH (carboxylic acid) | Post-synthesis oxidation | HNO3, 50–80 °C, 6–12 h | Adsorption of dyes, metals | [17,45] |
| SBA-15 | –SH (tiol) | Co-condensation or grafting | pH acid, 25–50 °C | Adsorption of noble metals (Au, Ag, Pt) | [17,45] |
| SBA-15 | Fe3O4 (magnetic oxides) | Coprecipitation in mesostructure | 60–90 °C, pH 8–9 | Magnetic separation, reuse | [17,41,49] |
| SBA-15 | Pt/Pd o Ni, Cu | Impregnation + reduction | 200–300 °C, H2O o Ar | Heterogeneous catalysis (hydrogenation, oxidation) | [35,42,44,50,51] |
| SBA-15 | Organic groups (alkyls, phenyls) | Grafting or co-condensation | RT–120 °C, organic solvent | Hydrophobicity tuning, drug anchoring | [11,52] |
| SBA-15 | β-Cyclodextrin (β-CD) | Modification by supramolecular anchoring | RT–50 °C, aqueous solvent | Adsorption of organic contaminants | [36,37] |
| SBA-15 | Azobenzene | Photoactive modification | RT–60 °C Organic solvent UV (365 nm) or visible (>450 nm) light | Photoactivated diffusion control, sensors | [3,40] |
| MCM-41 | –COOH (carboxylic acid | Post-synthesis oxidation | APS, HNO3, 50–80 °C | Adsorption of dyes | [45] |
| KIT-6 | –SH (tiol) | Co-condensation | pH acid, 25–50 °C | Adsorption of heavy metals (Hg2+, Cd2+) | [17] |
| CMK-3 | –SO3H (sulfonic acid) | Reflux with H2SO4 | 80–120 °C, 6–12 h | Esterification, acid catalysis | [34] |
| MOF-5 | –NH2, –COOH | Reflux with H2SO4 | Solvothermal tempering | Selective adsorption, sensors, catalysis | [4] |
| Strategy | Surface Coverage (Groups nm−2) | ΔBET (%) | Thermal Stability (°C) | Key Application | Mechanistic Trade-off |
|---|---|---|---|---|---|
| Grafting –NH2 (APTES, MPTMS) | 0.5–3.0 | −20 to −30 | ≈250 | CO2 capture, heavy-metal and VOC adsorption | Heterogeneous coverage in narrow pores; possible diffusion constriction |
| Co-condensation (–NH2–SH) | 1.0–3.5 | ≈−20 | <200 | Uniform functional sites, solid-phase extraction | Slight pore shrinkage; lower thermal stability of organic groups |
| Metal impregnation (Pt, Cu, Ni) | — | ≈−10 | ≈600 | Redox catalysis | Agglomeration or leaching if anchoring is weak; partial pore blocking |
| Isomorphic substitution (Si → Al) | — | ≈−10 | ≈600 | Brønsted acidity, selective adsorption | Reduced surface area but enhanced acid strength and gas-phase reactivity |
| Supramolecular (β-CD, azobenzene) | — | −15 to −20 | <200 | Host–guest adsorption, photo-responsive control | Lower thermal robustness; potential desorption of guest molecules |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, M.; Flores, D.; Morillo, G.; Narváez, R.; Marcilla, A.; Rosero, M. Comprehensive Review of SBA-15 Mesoporous Silica: Functionalization Strategies, Diffusion Mechanisms, and Emerging Applications. Sustain. Chem. 2025, 6, 42. https://doi.org/10.3390/suschem6040042
Muñoz M, Flores D, Morillo G, Narváez R, Marcilla A, Rosero M. Comprehensive Review of SBA-15 Mesoporous Silica: Functionalization Strategies, Diffusion Mechanisms, and Emerging Applications. Sustainable Chemistry. 2025; 6(4):42. https://doi.org/10.3390/suschem6040042
Chicago/Turabian StyleMuñoz, Morayma, Diego Flores, Grace Morillo, Ricardo Narváez, Antonio Marcilla, and Marco Rosero. 2025. "Comprehensive Review of SBA-15 Mesoporous Silica: Functionalization Strategies, Diffusion Mechanisms, and Emerging Applications" Sustainable Chemistry 6, no. 4: 42. https://doi.org/10.3390/suschem6040042
APA StyleMuñoz, M., Flores, D., Morillo, G., Narváez, R., Marcilla, A., & Rosero, M. (2025). Comprehensive Review of SBA-15 Mesoporous Silica: Functionalization Strategies, Diffusion Mechanisms, and Emerging Applications. Sustainable Chemistry, 6(4), 42. https://doi.org/10.3390/suschem6040042








